Published online Mar 7, 2006. doi: 10.3748/wjg.v12.i9.1356
Revised: September 10, 2005
Accepted: October 10, 2005
Published online: March 7, 2006
AIM: To study the apoptosis induced by preoperative oral 5’-DFUR administration in gastric adenocarcinoma and its mechanism of action.
METHODS: Sixty gastric cancer patients were divided randomly into three groups (20 each group) before operation: group one: 5’-DFUR oral administration at the dose of 800-1200mg/d for 3 - 5 d, group two: 500mg 5-FU + 200 mg/d CF by venous drip for 3 - 5 d, group three (control group). One or two days after chemotherapy, the patients were operated. Fas/FasL, PD-ECGF and PCNA were examined by immunohistochemistry and apoptotic tumor cells were detected by in situ TUNEL method. Fifty-four patients received gastrectomy, including 12 palliative resections and 42 radical resections. Six patients were excluded. Finally 18 cases in 5’-DFUR group, 16 cases in CF + 5-FU group, and 20 cases in control group were analyzed.
RESULTS: There was no significant difference in patient mean age, gender, white blood cell count, haematoglobin (HB), thromboplastin, perioperative complication incidence, radical or palliation resection, invasion depth (T), lymphonode involvement (N), metastasis (M) and TNM staging among the three groups. However, the PCNA index (PI) in 5’-DFUR group (40.51 ± 12.62) and 5-FU + CF group (41.12 ± 15.26) was significantly lower than that in control group (58.33 ± 15.69) (F = 9.083, P = 0.000). The apoptotic index (AI) in 5’-DFUR group (14.39 ± 9.49) and 5-FU + CF group (14.11±9.68) was significantly higher than that in control group (6.88 ± 7.37) (F = 4.409, P = 0.017). The expression rates of Fas and FasL in group one and group three were 66.7% (12/18) and 50% (9/18), 43.8% (7/16) and 81.3% (13/16), 45.0% (9/20) and 85% (17/20), respectively. The expression rate of FasL in 5’-DFUR group was significantly lower than that in the other two groups (χ2=6.708, P = 0.035). Meanwhile, the expression rate of PD-ECGF was significantly lower in 5’-DFUR group (4/18,28.6%) than in CF + 5-FU group(9/16,56.3%)and control group (13/20,65.0%) (χ2 = 7.542, P = 0.023). The frequency of Fas expression was significantly correlated with palliative or radical resection (χ2 = 7.651, P = 0.006), invasion depth (χ2 = 8.927, P = 0.003), lymphatic spread (χ2 = 4.488, P = 0.034) and UICC stages (χ2 = 8.063, P = 0.045) respectively. By the end of March 2005, 45 patients were followed up. The 0.5-, 1-, 2-, 3-year survival rates were 96%,73%,60%,48%, respectively, which were related with T, N, M and Fas expression, but not with PD-ECGF and FasL expression.
CONCLUSION: Preoperative oral 5’-DFUR administration may induce apoptosis of gastric carcinoma cells and decrease tumor cell proliferation index, but cannot improve the prognosis of patients with gastric cancer. Down-regulation of FasL and PD-ECGF expression mediated by 5’-DFUR may be one of its anti-cancer mechanisms. Fas expression correlates with the progression of gastric carcinoma and may be an effective prognostic factor.
- Citation: Zhao WH, Wang SF, Ding W, Sheng JM, Ma ZM, Teng LS, Wang M, Wu FS, Luo B. Apoptosis induced by preoperative oral 5’-DFUR administration in gastric adenocarcinoma and its mechanism of action. World J Gastroenterol 2006; 12(9): 1356-1361
- URL: https://www.wjgnet.com/1007-9327/full/v12/i9/1356.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i9.1356
Cell apoptosis and proliferation regulate homeostasis. Disordered balance results in tumorigenesis. Cell apoptosis can hinder tumor growth[1-3]. Therefore,induction of tumor apoptosis is a good anti-cancer therapy. At present, the relationship between chemotherapy and cancer cell apoptosis has drawn more and more attention. However, studies on induction of apoptosis in gastric carcinoma are performed in vitro. Inducers reported include γ-ray, beta-ionone, biological response modifiers, chemotherapeutics, etc[4-8]. Chemotherapeutics-induced apoptosis is one of its anti-cancer mechanisms. Arsenic trioxide[9], hydroxycamptothecin[10], cisplatin[11] , paclitaxel[12], fluorouracil and its derivant [13,14] , oxaliplatin[15] can induce apoptosis in human gastric carcinoma cells. 5’-deoxy-5-flurouridine (doxifluridine or 5’-DFUR (known as Furtulon) is a selective anti-cancer medicine, which can be converted into 5-FU by thymidine phosphorylase (the same substance as traversing platelet-derived endothelial cell growth factor, PD-ECGF)[14,19,20]. Accordingly, the concentration of 5-FU is high in tumor tissue[19,21]. The therapeutic index of doxifluridine is ten times that of 5-FU[19]. Doxifluridine has been widely used in treatment of breast cancer, colorectal carcinoma, ovarian adenocarcinoma, bladder cancer and gastric carcinoma[22-26].
In this study, we used doxifluridine as an apoptosis inducer to study the change of apoptosis and expression of proliferative cell nuclear antigen (PCNA), Fas and Fas ligand (FasL), and PD-ECGF in gastric adenocarcinoma and its mechanism of action.
Patients who were diagnosed as malignant gastric neoplasm (age ≤ 70 years, Karnofsky’s scale >90) and could endure chemotherapy and operation,were enrolled in this study. The patients were divided into three groups (20 each): group 1: 800 mg - 1 200 mg/d 5’-DFUR for 3-5 days, group 2: 500 mg 5-FU + 200 mg/d CF by venous drip for 3-5 d and group 3 (control group). One or two days after chemotherapy, the patients underwent surgery. From Oct. 2001 to Oct. 2003, 60 gastric cancer patients (20 in each group) were enrolled (37 males and 17 female, mean age 57.5 years, range 32-70 years).
Of the 60 patients, 54 underwent gastrectomy (including 12 palliative resections and 42 radical resections, and 6 were excluded. No perioperative mortality occurred during the first 30 days after surgery. Complications were recorded in four of 54 patients resected, one gastric perforation on the fourth day after 5’-DFUR oral chemotherapy, one preoperative gastrorrhagia and one postoperative cerebral infarction in CF+5-FU group, one venous thrombosis of lower extremities in control group complicated by cerebral hemorrhage during thrombolysis treatment. The patients with gastric perforation and gastrorrhagia required emergency laparotomy.
PCNA, Fas/FasL and PD-ECGF expression was determined by En Vision immunohistochemistry[27] .The kit was purchased from DAKO Co., USA. Apoptotic cells were detected by terminal deoxynucleotidyl transferase (TdT)- mediated dUTP nick end labeling (TUNEL) method. The kit was purchased from Bochringer Mannheim Co., Germany. The proliferation index (PI) and apoptotic index (AI) of gastric carcinoma cells were evaluated by PCNA immunohistochemical staining and in situ TUNEL.
The stained cells had Fas/FasL or PD-ECGF positive expression. Brown-stained nuclei were considered as positive cells of PCNA and apoptosis, brown cellular membrane and cytoplasm were considered as Fas or FasL positive cells (Figures 1A and 1B), brown or yellow staining in cytoplasm and/or cell nuclei was considered as PD-ECGF positive cells (Figure 1C). PCNA index (PI) and apoptotic index (AI) of positive cells in 1000 tumor cells were calculated under high power field (*400) of microscope.
AI = TUNEL mark cells/tumor cells (more than one thousand)*100%
PI = PCNA mark cells/tumor cells (more than one thousand)*100%
All data were analyzed by ANOVA and chi square test. P < 0.05 was considered statistically significant.
Fifty-four patients underwent surgical resection, including 18 cases in 5’-DFUR group, 16 cases in CF+5-FU group, and 20 cases in control group. There was no significant differences in patient mean age, gender, white blood cell count, haematoglobin (HB), thromboplastin, perioperative complication incidence, radical or palliation resection, invasion depth(T), lymphonode involvement(N), metastasis(M)and TNM staging among the three groups.
The expression of PI, AI, FasL and PD-ECGF was not statistically significant as compared to the operation procedure (radical or palliation resection), early or advanced tumor, T, N, M and UICC stages. In contrast, positive staining of Fas was closely related to radical resection but not with serosal invasion, lymphnode metastasis and early UICC stages (Table 1, Figure 1).
| PI AI | Fas | FasL | PD-ECGF | |
| (%) (%) | + - | + - | + - | |
| Way of resection | ||||
| radical | 46.31±15.30 12.13±9.65 | 26 16 | 29 13 | 19 23 |
| Palliation | 50.74±21.27 9.40±8.35 | 2 10 | 10 2 | 7 5 |
| P/χ2 Value | 0.654 0.791 | 7.651 | 0.949 | 0.641 |
| P Value | 0.422 0.378 | 0.006 | 0.33 | 0.423 |
| Early cancer | ||||
| Yes | 45.29±12.34 14.54±12.38 | 7 2 | 6 3 | 3 6 |
| No | 47.70±17.51 10.92±8.70 | 21 24 | 33 12 | 23 22 |
| F/χ2 Value | 0.154 1.123 | 2.908 | 0.166 | 0.949 |
| P Value | 0.697 0.294 | 0.088 | 0.684 | 0.33 |
| Invasion depth | ||||
| T1-2 | 46.65±15.76 11.96±9.81 | 20 8 | 19 9 | 12 16 |
| T3-4 | 47.99±17.92 11.06±9.04 | 8 18 | 20 6 | 14 12 |
| F/χ2 Value | 0.086 0.121 | 8.927 | 0.552 | 0.652 |
| P Value | 0.770 0.729 | 0.003 | 0.457 | 0.419 |
| Lymphonode | ||||
| N0 | 50.37±16.34 10.51±9.89 | 13 5 | 14 4 | 9 9 |
| N1-4 | 45.76±16.87 12.03±9.20 | 15 21 | 25 11 | 17 19 |
| F/χ2 value | 0.917 0.312 | 4.488 | 0.415 | 0.037 |
| P value | 0.343 0.579 | 0.034 | 0.519 | 0.847 |
| Metastasis | ||||
| M0 | 46.73±15.79 11.51±9.10 | 26 19 | 33 12 | 22 23 |
| M1 | 50.10±21.5 11.60±11.24 | 7 2 | 6 3 | 4 5 |
| F/χ2 value | 0.301 0.001 | 3.798 | 0.166 | 0.059 |
| P value | 0.585 0.980 | 0.051 | 0.684 | 0.808 |
| UICC stage | ||||
| I | 50.42±16.84 10.98±9.99 | 13 4 | 13 4 | 9 8 |
| II | 42.69±13.24 11.78±10.65 | 5 4 | 6 3 | 2 7 |
| III | 46.03±16.2 12.73±10.10 | 7 9 | 11 5 | 7 9 |
| IV | 48.01±20.18 10.52±7.25 | 3 9 | 9 3 | 8 4 |
| F/χ2 value | 0.452 0.148 | 8.063 | 0.434 | 4.352 |
| P value | 0.717 0.931 | 0.045 | 0.933 | 0.226 |
Either 5’-DFUR or CF+5-FU preoperative chemotherapy could significantly inhibit cell proliferation and induce apoptosis as compared to control group. The frequency of Fas expression had no significant difference among three groups. However, the frequency of FasL expression was significantly lower in 5’-DFUR group (50%) than those in CF+5-FU group(81.3%)and control group(85%). There was no significant difference between groups two and three. The PD-ECGF expression was detected in 28.6% of 5’-DFUR group, 56.3% of CF+5-FU group and 65.0% of control group (Table 2).
| Group N | Fas | FasL | PI | AI | PD-ECGF + - |
| + - | + - | (%) | (%) | ||
| 5’-DFUR 18 | 12 6 | 9 9 | 40.51 ± 12.62 | 14.39 ± 9.49 | 4 14 |
| CF+5-FU 16 | 7 9 | 13 3 | 41.12 ± 15.26 | 14.11 ± 9.68 | 9 7 |
| Control 20 | 9 11 | 17 3 | 58.33 ± 15.69 | 6.88 ± 7.37 | 13 7 |
| X2/F value | 2.379 | 6.708 | 9.083 | 4.409 | 7.542 |
| P value | 0.304 | 0.035 | 0 | 0.017 | 0.023 |
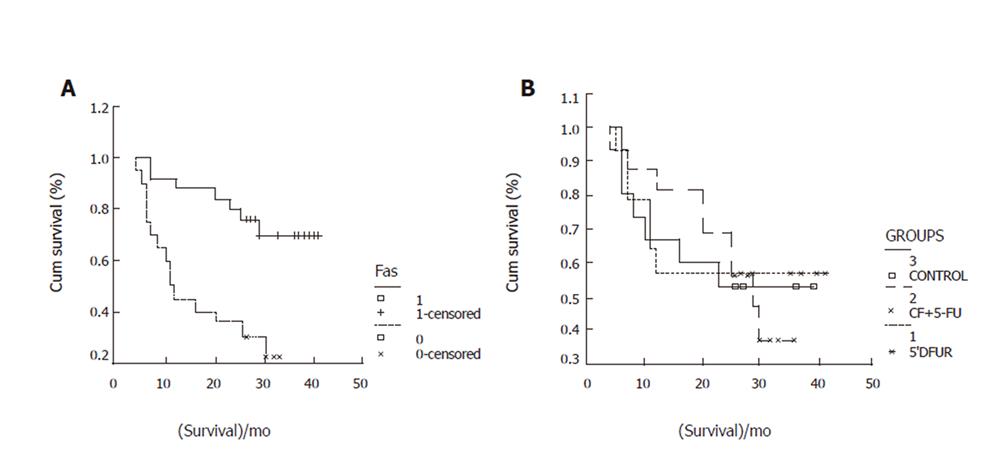
By the end of March 2005, 45 patients were followed up. During the follow-up, 22 cases died, 23 remained alive (including one patient with cerebral hemorrhage after operation). The 0.5-,1-,2-, 3-year survival rates were 96%,73%,60%,48% respectively, which were related with T (χ2=30.32,P=0.0000), N (χ2=22.10,P=0.0000), M (χ2=17.04,P=0.0000) and Fas expression (χ2=12.24,P=0.0005, Figure 2), but not with PD-ECGF (χ2=0.78,P=0.3775) and FasL expression (χ2=0.7,P=0.7967).
Gastric carcinoma is one of the most common malignant tumors and remains a leading cause of cancer-related death worldwide [15,16,28-30]. In China, it still ranks the first[31,32]. Operative resection is the most effective treatment nowadays. However, the 5-year survival rate was 25%~60% even after radical resection[33-36], micrometastasis is the major reason for recurrence[29,37,38]. Preoperation chemotherapy can inhibit micrometastasis, but strong chemotherapy may potentially increase operation complications in resectable patients[30,35,36]. It was reported that surgical resection may serve as a stimulus for the growth of residual tumor[30]. Inada et al13] have reported the effectiveness of preoperative 5-Fu venous chemotherapy, which can induce cancer cell apoptosis in vivo. Our study showed that preoperative venous CF+5-Fu chemotherapy and 5’-DFUR oral administration could induce gastric carcinoma cell apoptosis and inhibit cancer cell proliferation. Wang et al[22] reported that preoperative 5’-DFUR chemotherapy is able to partially inhibit the expression of extracellular signal-regulated kinase(ERK)which is closely related with cell proliferation in breast cancer. PCNA index decrease in our studies might be related with inhibition of ERK-1 and ERK-2 expression. Liang et al[39] studied apoptosis in ovarian cancer and found that apoptosis induced by chemotherapy is decreased, suggesting that apoptosis induced by chemotherapy has a time limit. Therefore, preoperative chemotherapy-induced tumor cell apoptosis can inhibit malignant behavior in some degree.
Fas(CD95/APO-1)/Fas ligand (FasL) system is one of the major apoptotic pathways and plays an important role in maintenance of cell colony, elimination of malignant transformation cells and regulation of immune system[1-3,40]. In general, Fas/FasL system plays an important role in prognosis and immune escape. Fas of activated T lymphocytes and tumor-infiltrating lymphocytes(TIL) can increase apoptosis[1-3,40-45]. Tumor cells combined with activated T lymphocytes and TIL can kill surrounding normal infiltrating lymphocytes, escape immune system[1,2,22,23,43,44]. Our investigation showed that preoperative 5’-DFUR oral administration chemotherapy could down regulate FasL expression, which may be one of its mechanisms underlying tumor cell apoptosis. But CF+5-FU venous chemotherapy had no influence on Fas/FasL expression, suggesting that 5’-DFUR and 5-FU have a different mechanism of action. Induction of 5-FU is relevant with activation of apoptosis gene bax[45], and expression of bcl-2 oncogene[13].
PD-ECGF, one of the angiogenesis factors, extracted from fresh thrombocytolysis matters is the same substance as thymidine phosphorylase(TP), PyNPase[20,46-49]. It can promote angiogenesis, cell proliferation and inhibit apoptosis[46-49]. Konno et al[47] reported that expression of PD-ECGF in gastric carcinoma has a positive correlation with PCNA, and PD-ECGF can also promote tumor growth. Osaki et al[20] found that increased PD-ECGF expression is closely related with decreased apoptosis in gastric carcimoma. The mean apoptotic index in early and advanced gastric cancer is significantly lower in positive PD-ECGF than in negative PD-ECGF. Koizumi et al[21] determined PD-ECGF expression in gastric carcinoma and found that the responsive rate to chemotherapy is 56.8% in positive PD-ECGF group and 0% in negative group, and 82.4% in PD-ECGF over expression group, indicating that chemosensitivity is closely related with PD-ECGF expression. PD-ECGF can increase the activity of 5-FU and other anti-cancer medicines[50]. Cytokine such as interferon, can up-regulate PD-ECGF and cytokine levels, thus detecting PD-ECGF level in tumor tissue can predict chemotherapy sensitivity and its efficacy[18,26,51]. PD-ECGF inhibitors can decrease tumor angiogenesis, prevent infiltration and metastasis[52]. PD-ECGF is the key enzyme for the metabolism of Fortulon. Satoh B et al[14] reported that by oral administration of 5’-DFUR, 1 200 mg per day for seven days significantly decreases PD-ECGF activity and acidic protein. Our results showed that preoperative oral administration of Fortulon for 3 - 5 d can down-regulate PD-ECGF expression and induce cancer cell apoptosis in gastric carcinoma. Over expression of PD-ECGF is an important factor of tumor metastasis [53]. Our results also showed that patients with PD-ECGF overexpression had a higher tendency toward liver metastasis. For this reason, down regulation of PD-ECGF expression by preoperative 5’-DFUR chemotherapy plays a certain role in preventing postoperative recurrence and metastasis of gastric carcinoma. It was reported that PD-ECGF level is closely related with tumor metastasis[24,25].
Kabayashi and kimur[8] performed a multicenter clinical trial of preoperative chemotherapy for gastric cancer and found that oral administration of 5’-DFUR may induce apoptosis of gastric carcinoma and decrease proliferation index in old patients with advanced gastric cancer, but cannot improve their prognosis. However, Fas expression is often presented in early stage of tumors and shows a better prognosis. Thus, Fas expression in gastric carcinoma may be an effective prognostic factor for survival.
In conclusion, 5’-DFUR or CF+5-FU chemotherapy can induce apoptosis and inhibit proliferation of tumor cells. Down regulation of FasL and PD-ECGF induced by 5’-DFUR may be one of its anti-cancer mechanisms. Fas expression is correlated with progression of gastric carcinoma and may be an effective prognostic factor.
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH
| 1. | Pinti M, Troiano L, Nasi M, Moretti L, Monterastelli E, Mazzacani A, Mussi C, Ventura P, Olivieri F, Franceschi C. Genetic polymorphisms of Fas (CD95) and FasL (CD178) in human longevity: studies on centenarians. Cell Death Differ. 2002;9:431-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Chang YC, Xu YH. Expression of Bcl-2 inhibited Fas-mediated apoptosis in human hepatocellular carcinoma BEL-7404 cells. Cell Res. 2000;10:233-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Eichhorst ST, Müller M, Li-Weber M, Schulze-Bergkamen H, Angel P, Krammer PH. A novel AP-1 element in the CD95 ligand promoter is required for induction of apoptosis in hepatocellular carcinoma cells upon treatment with anticancer drugs. Mol Cell Biol. 2000;20:7826-7837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Ramp U, Caliskan E, Mahotka C, Krieg A, Heikaus S, Gabbert HE, Gerharz CD. Apoptosis induction in renal cell carcinoma by TRAIL and gamma-radiation is impaired by deficient caspase-9 cleavage. Br J Cancer. 2003;88:1800-1807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Yamamoto M, Maehara Y, Sakaguchi Y, Kusumoto T, Ichiyoshi Y, Sugimachi K. Transforming growth factor-beta 1 induces apoptosis in gastric cancer cells through a p53-independent pathway. Cancer. 1996;77:1628-1633. [PubMed] [Cited in This Article: ] |
| 6. | Timmermann W, Illert B, Vollmers HP, Krenn V, Rückle-Lanz H, Wilhelm M, Thiede A. [Induction of apoptosis by preoperative passive immunotherapy in resectable stomach carcinoma]. Kongressbd Dtsch Ges Chir Kongr. 2002;119:396-397. [PubMed] [Cited in This Article: ] |
| 7. | Yan J, Xu YH. Tributyrin inhibits human gastric cancer SGC-7901 cell growth by inducing apoptosis and DNA synthesis arrest. World J Gastroenterol. 2003;9:660-664. [PubMed] [Cited in This Article: ] |
| 8. | Kobayashi T, Kimura T. [Long-term outcome of preoperative chemotherapy with 5'-deoxy-5-fluorouridine (5'-DFUR) for gastric cancer]. Gan To Kagaku Ryoho. 2000;27:1521-1526. [PubMed] [Cited in This Article: ] |
| 9. | Xing M, Zhang EJ, Ye X. Investigation of the pathway of apoptosis induced by arsenic trioxide in cancer cells. Zhongguo Yaolixue Tongbao. 2002;18:87-90. [Cited in This Article: ] |
| 10. | Tu SP, Jiang SH, Tan JH, Zhong J, Qiao MM, Jiang XH, Zhang YP, Yuan YZ, Wu YL, Wu YY. The mechanism of apoptosis induced by hydroxycamptothecin in gastric cancer cells. Zhonghua Xiaohua Zazhi. 1999;19:19-21. [Cited in This Article: ] |
| 11. | Jones NA, Turner J, McIlwrath AJ, Brown R, Dive C. Cisplatin- and paclitaxel-induced apoptosis of ovarian carcinoma cells and the relationship between bax and bak up-regulation and the functional status of p53. Mol Pharmacol. 1998;53:819-826. [PubMed] [Cited in This Article: ] |
| 12. | Zhou HB, Zhu JR. Paclitaxel induces apoptosis in human gastric carcinoma cells. World J Gastroenterol. 2003;9:442-445. [PubMed] [Cited in This Article: ] |
| 13. | Inada T, Ichikawa A, Igarashi S, Kubota T, Ogata Y. Effect of preoperative 5-fluorouracil on apoptosis of advanced gastric cancer. J Surg Oncol. 1997;65:106-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 14. | Satoh B, Ohtoshi M, Ishida Y, Hen-mi K, Kaneko I, Soda M, Sugihara J, Shibagaki F, Iwai N, Nakamura T. [Correlation between pyrimidine nucleoside phosphorylase (PyNPase)/thymidine phosphorylase/platelet-derived endothelial cell growth factor and histological prognostic factor, and influence of 5'-deoxy-5-fluorouridine (5'-DFUR) administration on PyNPase activities and serum immunosuppressive acidic protein levels. A study group of oral anti-cancer drugs in Seiban/Tajima area]. Gan To Kagaku Ryoho. 1998;25:359-364. [PubMed] [Cited in This Article: ] |
| 15. | Lin WL, Li DG, Chen Q, Lu HM. Clinical and experimental study of oxaliplatin in treating human gastric carcinoma. World J Gastroenterol. 2004;10:2911-2915. [PubMed] [Cited in This Article: ] |
| 16. | Ji YB, Gao SY, Ji HR, Kong Q, Zhang XJ, Yang BF. Anti-neoplastic efficacy of Haimiding on gastric carcinoma and its mechanisms. World J Gastroenterol. 2004;10:484-490. [PubMed] [Cited in This Article: ] |
| 17. | Liu JR, Chen BQ, Yang BF, Dong HW, Sun CH, Wang Q, Song G, Song YQ. Apoptosis of human gastric adenocarcinoma cells induced by beta-ionone. World J Gastroenterol. 2004;10:348-351. [PubMed] [Cited in This Article: ] |
| 18. | Fukui T, Matsui K, Kato H, Takao H, Sugiyama Y, Kunieda K, Saji S. Significance of apoptosis induced by tumor necrosis factor-alpha and/or interferon-gamma against human gastric cancer cell lines and the role of the p53 gene. Surg Today. 2003;33:847-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Sun XN, Yang QC, Hu JB. Pre-operative radiochemotherapy of locally advanced rectal cancer. World J Gastroenterol. 2003;9:717-720. [PubMed] [Cited in This Article: ] |
| 20. | Osaki M, Sakatani T, Okamoto E, Goto E, Adachi H, Ito H. Thymidine phosphorylase expression results in a decrease in apoptosis and increase in intratumoral microvessel density in human gastric carcinomas. Virchows Arch. 2000;437:31-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Koizumi W, Saigenji K, Nakamaru N, Okayasu I, Kurihara M. Prediction of response to 5'-deoxy-5-fluorouridine (5'-DFUR) in patients with inoperable advanced gastric cancer by immunostaining of thymidine phosphorylase/platelet-derived endothelial cell growth factor. Oncology. 1999;56:215-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Wang Z, Wang S, Zhu F, Ye Y, Yu Y, Qiao X. Expression of extracellular signal-regulated kinase and its relationship with clinicopathological characteristics of breast cancer. Zhonghua Zhong Liu Za Zhi. 2002;24:360-363. [PubMed] [Cited in This Article: ] |
| 23. | Xu L, Zhan YQ, Li W, Sun XW. Influence of preoperative chemotherapy with 5-fluoro uracilum and citrovorum factor on apoptosis and proliferation of human gastric carcinoma cells. Zhonghua Weichang Waike Zazhi. 2003;6:47-49. [Cited in This Article: ] |
| 24. | Ueda M, Fujii H, Yoshizawa K, Kumagai K, Ueki K, Terai Y, Yanagihara T, Ueki M. Effects of sex steroids and growth factors on invasive activity and 5'-deoxy-5-fluorouridine sensitivity in ovarian adenocarcinoma OMC-3 cells. Jpn J Cancer Res. 1998;89:1334-1342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Nishimura G, Izumi R, Matsuki N, Takeyama S, Konishi K, Fujita H, Miyata R, Sasaki T, Kojima Y, Takada M. [Effect on 5'-deoxy-5-fluorouridine (5'-DFUR) of pyrimidine nucleoside phosphorylase (PyNPase), matrix metalloprotease and serum IAP values. Hokuriku Colorectal Cancer Chemotherapy Study Group]. Gan To Kagaku Ryoho. 1997;24:1947-1952. [PubMed] [Cited in This Article: ] |
| 26. | Li G, Kawakami S, Kageyama Y, Yan C, Saito K, Kihara K. IFN gamma-induced up-regulation of PD-ECGF/TP enhances the cytotoxicity of 5-fluorouracil and 5'-deoxy-5-fluorouridine in bladder cancer cells. Anticancer Res. 2002;22:2607-2612. [PubMed] [Cited in This Article: ] |
| 27. | Li L, Zhang WY. Expression and clinical significance of p27(kip1), p16 and proliferating cell nuclear antigen in nasopharyngeal carcinoma. Zhonghua Binglixue Zazhi. 2003;32:347-349. [Cited in This Article: ] |
| 28. | Bani-Hani KE, Yaghan RJ, Heis HA, Shatnawi NJ, Matalka II, Bani-Hani AM, Gharaibeh KA. Gastric malignancies in Northern Jordan with special emphasis on descriptive epidemiology. World J Gastroenterol. 2004;10:2174-2178. [PubMed] [Cited in This Article: ] |
| 29. | Chen XM, Chen GY, Wang ZR, Zhu FS, Wang XL, Zhang X. Detection of micrometastasis of gastric carcinoma in peripheral blood circulation. World J Gastroenterol. 2004;10:804-808. [PubMed] [Cited in This Article: ] |
| 30. | Fink U, Stein HJ, Schuhmacher C, Wilke HJ. Neoadjuvant chemotherapy for gastric cancer: update. World J Surg. 1995;19:509-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Sun X, Mu R, Zhou Y, Dai X, Qiao Y, Zhang S, Huangfu X, Sun J, Li L, Lu F. [1990-1992 mortality of stomach cancer in China]. Zhonghua Zhong Liu Za Zhi. 2002;24:4-8. [PubMed] [Cited in This Article: ] |
| 32. | Sun XD, Mu R, Zhou YS, Dai XD, Zhang SW, Huangfu XM, Sun J, Li LD, Lu FZ, Qiao YL. [Analysis of mortality rate of stomach cancer and its trend in twenty years in China]. Zhonghua Zhong Liu Za Zhi. 2004;26:4-9. [PubMed] [Cited in This Article: ] |
| 33. | Noguchi Y, Imada T, Matsumoto A, Coit DG, Brennan MF. Radical surgery for gastric cancer. A review of the Japanese experience. Cancer. 1989;64:2053-2062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 34. | Nakamura K, Ueyama T, Yao T, Xuan ZX, Ambe K, Adachi Y, Yakeishi Y, Matsukuma A, Enjoji M. Pathology and prognosis of gastric carcinoma. Findings in 10,000 patients who underwent primary gastrectomy. Cancer. 1992;70:1030-1037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 35. | Ajani JA, Ota DM, Jessup JM, Ames FC, McBride C, Boddie A, Levin B, Jackson DE, Roh M, Hohn D. Resectable gastric carcinoma. An evaluation of preoperative and postoperative chemotherapy. Cancer. 1991;68:1501-1506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 36. | Ajani JA, Mayer RJ, Ota DM, Steele GD, Evans D, Roh M, Sugarbaker DJ, Dumas P, Gray C, Vena DA. Preoperative and postoperative combination chemotherapy for potentially resectable gastric carcinoma. J Natl Cancer Inst. 1993;85:1839-1844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 98] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Zhao A, Li J, Sun W. [Detection and significance of lymph node micrometastases in patients with histologically node-negative gastric carcinoma]. Zhonghua Zhong Liu Za Zhi. 2000;22:222-224. [PubMed] [Cited in This Article: ] |
| 38. | Zhang XW, Fan P, Yang HY, Yang L, Chen GY. [Significance of detecting disseminated tumor cells in peripheral blood of gastric and colorectal cancer patients]. Zhonghua Zhong Liu Za Zhi. 2003;25:66-69. [PubMed] [Cited in This Article: ] |
| 39. | Liang JF, Liu LY, Cheng SJ. Relation between apoptosis and proliferation or genes expression after chemotherapy in overian epithelial carcinoma. Zhongguo Aizheng Zazhi. 2002;12:26-28. [Cited in This Article: ] |
| 40. | Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305-1308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4271] [Cited by in F6Publishing: 4145] [Article Influence: 159.4] [Reference Citation Analysis (0)] |
| 41. | Nagashima H, Mori M, Sadanaga N, Mashino K, Yoshikawa Y, Sugimachi K. Expression of Fas ligand in gastric carcinoma relates to lymph node metastasis. Int J Oncol. 2001;18:1157-1162. [PubMed] [Cited in This Article: ] |
| 42. | Liu HF, Liu WW, Fang DC, Men RF. Relationship between Fas antigen expression and apoptosis in human gastric carcinoma and adjacent noncancerous tissues. Huaren Xiaohua Zazhi. 1998;6:321-322. [Cited in This Article: ] |
| 43. | Koyama S, Koike N, Adachi S. Fas receptor counterattack against tumor-infiltrating lymphocytes in vivo as a mechanism of immune escape in gastric carcinoma. J Cancer Res Clin Oncol. 2001;127:20-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Zheng HC, Sun JM, Wei ZL, Yang XF, Zhang YC, Xin Y. Expression of Fas ligand and caspase-3 contributes to formation of immune escape in gastric cancer. World J Gastroenterol. 2003;9:1415-1420. [PubMed] [Cited in This Article: ] |
| 45. | Pan C, Feng ZQ, Peng T, Liu NB. Induction of Apoptosis of Gastric Carcinoma Cell Line SGC-7901 by 5-Fluorouracil in Vitro. Nanjing Yike Daxue Xuebo. 2000;20:163-165. [Cited in This Article: ] |
| 46. | Ogawa K, Konno S, Takebayashi Y, Miura K, Katsube T, Kajiwara T, Aiba M, Aikou T, Akiyama S. Clinicopathological and prognostic significance of thymidine phosphorylase expression in gastric carcinoma. Anticancer Res. 1999;19:4363-4367. [PubMed] [Cited in This Article: ] |
| 47. | Konno S, Takebayashi Y, Aiba M, Akiyama S, Ogawa K. Clinicopathological and prognostic significance of thymidine phosphorylase and proliferating cell nuclear antigen in gastric carcinoma. Cancer Lett. 2001;166:103-111 doi: 10.1016/S0304-3835(01)00432-3. [Cited in This Article: ] |
| 48. | Kimura H, Konishi K, Kaji M, Maeda K, Yabushita K, Miwa A. Correlation between expression levels of thymidine phosphorylase (dThdPase) and clinical features in human gastric carcinoma. Hepatogastroenterology. 2002;49:882-886. [PubMed] [Cited in This Article: ] |
| 49. | Yoshikawa T, Suzuki K, Kobayashi O, Sairenji M, Motohashi H, Tsuburaya A, Nakamura Y, Shimizu A, Yanoma S, Noguchi Y. Thymidine phosphorylase/platelet-derived endothelial cell growth factor is upregulated in advanced solid types of gastric cancer. Br J Cancer. 1999;79:1145-1150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Katayanagi S, Aoki T, Takagi Y, Ito K, Sudo H, Tsuchida A, Koyanagi Y. Measurement of serum thymidine phosphorylase levels by highly sensitive enzyme-linked immunosorbent assay in gastric cancer. Oncol Rep. 2003;10:115-119. [PubMed] [Cited in This Article: ] |
| 51. | Hotta T, Taniguchi K, Kobayashi Y, Johata K, Sahara M, Naka T, Watanabe T, Ochiai M, Tanimura H, Tsubota YT. Preoperative endoscopic analysis of thymidine phosphorylase and dihydropyrimidine dehydrogenase in gastrointestinal cancer. Oncol Rep. 2004;11:1233-1239. [PubMed] [Cited in This Article: ] |
| 52. | Matsushita S, Nitanda T, Furukawa T, Sumizawa T, Tani A, Nishimoto K, Akiba S, Miyadera K, Fukushima M, Yamada Y. The effect of a thymidine phosphorylase inhibitor on angiogenesis and apoptosis in tumors. Cancer Res. 1999;59:1911-1916. [PubMed] [Cited in This Article: ] |
| 53. | Suda Y, Kuwashima Y, Shioya T, Uchida K, Tanaka Y. [The expression of thymidylate synthase and thymidine phosphorylase in the early-stage of gastric cancer]. Gan To Kagaku Ryoho. 1999;26:321-327. [PubMed] [Cited in This Article: ] |