Published online Feb 28, 2006. doi: 10.3748/wjg.v12.i8.1270
Revised: July 11, 2005
Accepted: July 20, 2005
Published online: February 28, 2006
AIM: To observe if the total amount of platelet P-selectin (tP-selectin) in patients with inflammatory bowel disease (IBD) was related to disease entity or activity, 5-aminosalicylic acid (5-ASA) medication or gender.
METHODS: tP-selectin was measured by immunoassay in seventeen IBD patients and twelve healthy controls.
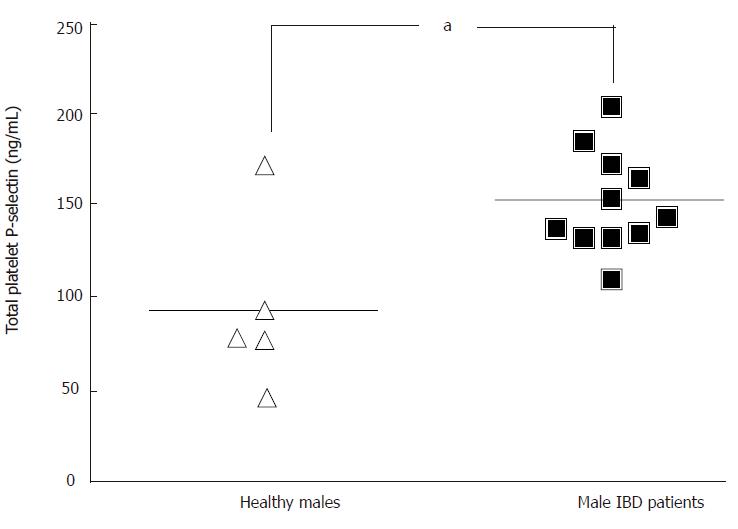
RESULTS: Compared to controls, there was no difference of tP-selectin in patients related to disease entity or activity and 5-ASA medication. When the groups were split according to gender the male patient group showed higher levels of tP-selectin compared to male controls (153 ng/mL vs 94 ng/mL, P< 0.05).
CONCLUSION: Increased tP-selectin levels may alter the inflammatory response and susceptibility to thromboembolic disease. As previously shown with soluble P-selectin, tP-selectin shows gender dependent differences important to consider in future studies.
- Citation: Fägerstam JP, Whiss PA. Higher platelet P-selectin in male patients with inflammatory bowel disease compared to healthy males. World J Gastroenterol 2006; 12(8): 1270-1272
- URL: https://www.wjgnet.com/1007-9327/full/v12/i8/1270.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i8.1270
Upon platelet activation, intracellular P-selectin is expressed on the membrane surface and released from the platelets[1]. In addition to P-selectin, platelets release various inflammatory mediators that regulate both hemostasis and inflammation[2].
Ulcerative colitis (UC) and Crohn´s disease (CD) are disorders of unknown aetiology, jointly referred to as inflammatory bowel disease (IBD). Current research suggests platelet dysfunction as a contributor to the disease since increased risk of thromboembolic disease and abnormal platelet activity have been found. Increased surface expression of platelet P-selectin in patients with IBD did not appear to reflect disease activity[3,4], whereas soluble P-selectin in plasma recently was suggested to be parellel to the severity of UC[5]. Using a new technique to quantify the total amount of P-selectin in platelets, patients with hypertension have been reported to have higher levels of platelet P-selectin[6]. Our aim of this study was to observe if total amount of platelet P-selectin (tP-selectin) differs between IBD patients and healthy controls and if there is a difference regarding disease entity, disease activity, 5-aminosalicylic acid (5-ASA) medication and gender.
Controls and patients with intake of anti-platelet drugs or steroids two weeks prior to sample collection were excluded. Seven female and five male healthy blood-donors served as a controls (mean age 44.9, range 25-65 years). The patient group consisted of six female and eleven male IBD patients (mean age 44.4, range 19-69 years). Diagnosis was verified with histological examination (biopsy and colonoscopy); twelve patients had UC and five patients had CD. Patients were sub-grouped according to disease activity, 5-ASA medication and gender. In UC eight patients were in relapse and in CD all patients were in remission. Ten patients had 5-ASA medication.
Isolated platelets (IP) were prepared as previously described[7]. Frozen IP samples were thawed and incubated with 0.5% Triton X-100 (Sigma Chemical Co., St. Louis, MO, USA) at 37 ˚C for 1 h. Thereafter, the permeabilised IP samples were gently mixed prior to measurement of tP-selectin levels according to standard procedures using an immunoassay from R&D Systems (Abingdon, Oxfordshire, UK).
All values are presented as mean ± standard error of the mean (SE). Statistical analyses were calculated by unpaired t test with Welch’s correction for samples with different variances. Differences were considered significant when P < 0.05.
All values are in ng/mL. There was no significant difference of tP-selectin between controls, 133 ± 20.1 (n = 12), and patients, 154 ± 8.1 (n = 17). When samples were sub-grouped according to gender the male patients showed significant higher levels compared to the male controls; 153 ± 8.2 and 94.1 ± 21.1 (Figure 1). In the sub-grouped male group the mean and range of age were 46.8 and 32-55 years in the control group and 46.4 and 24-65 years in the male patient group, denoting that age is not the cause of the different levels. Both the female controls and the female patients tended to have higher levels compared to male controls, although insignificantly. There were no significant differences between the female controls and female patients; 161 ± 27.7 (n = 7) and 156 ± 18.7 (n = 6). The levels of tP-selectin in UC compared to CD were 151 ± 9.6 (n = 12) and 163 ± 16.3 (n = 5). Values regarding disease activity showed following results; remission, 157 ± 11.2 (n = 9) and relapse, 152 ± 11.7 (n = 8). None of the groups showed significant difference in comparison with the controls. The levels in male patients in remission compared to male patients in relapse were; 155 ± 13.7 (n = 6) and 152 ± 9.2 (n = 5). Patients with 5-ASA compared to patients without 5-ASA had tP-selectin levels of 152 ± 9.1 (n = 10) and 159 ± 15.6 (n = 7), respectively. There was no significant difference between the male patients with 5-ASA, 151 ± 8.4 (n = 6) compared to male patients without 5-ASA, 156 ± 16.0 (n = 5).
Plasma levels of soluble P-selectin has recently been suggested to relate to the severity of UC[5] and increased tP-selectin has been found in hypertensive patients[6]. Our previous study showed that patients with IBD in remission had higher basal platelet P-selectin surface expression[4]. To investigate if this was related to higher platelet P-selectin content we analysed tP-selectin in patients with IBD. There was no difference of tP-selectin in patients taken as one group compared to controls, irrespectively of disease entity or activity and 5-ASA medication. When the groups were split according to gender the male patient group showed significantly higher levels compared to male controls. Both healthy and patient females showed greater variance compared to the male groups. This may be caused by a hormonal influence on tP-selectin, since sP-selectin fluctuates during the menstrual cycle[8] and age (pre- and post-menopausal). Other sex-based differences that influence the disease activity in IBD have recently been reported[9]. In the present study, male patients had higher tP-selectin levels regardless of disease entity, disease activity and 5-ASA medication. Taken together with our previous findings[4], we suggest that male IBD patients have higher levels of tP-selectin and exhibit higher basal platelet P-selectin expression when in remission. This could have an exaggerated influence on the inflammatory response and susceptibility to thromboembolic disease.
S- Editor Guo SY L- Editor Zhang JZ E- Editor Cao L
| 1. | Whiss PA, Andersson RG, Srinivas U. Kinetics of platelet P-selectin mobilization: concurrent surface expression and release induced by thrombin or PMA, and inhibition by the NO donor SNAP. Cell Adhes Commun. 1998;6:289-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Mannaioni PF, Di Bello MG, Masini E. Platelets and inflammation: role of platelet-derived growth factor, adhesion molecules and histamine. Inflamm Res. 1997;46:4-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 129] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Collins CE, Cahill MR, Newland AC, Rampton DS. Platelets circulate in an activated state in inflammatory bowel disease. Gastroenterology. 1994;106:840-845. [PubMed] [Cited in This Article: ] |
| 4. | Fägerstam JP, Whiss PA, Ström M, Andersson RG. Expression of platelet P-selectin and detection of soluble P-selectin, NPY and RANTES in patients with inflammatory bowel disease. Inflamm Res. 2000;49:466-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Dong WG, Liu SP, Zhu HH, Luo HS, Yu JP. Abnormal function of platelets and role of angelica sinensis in patients with ulcerative colitis. World J Gastroenterol. 2004;10:606-609. [PubMed] [Cited in This Article: ] |
| 6. | Nadar SK, Blann AD, Kamath S, Beevers DG, Lip GY. Platelet indexes in relation to target organ damage in high-risk hypertensive patients: a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT). J Am Coll Cardiol. 2004;44:415-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Whiss PA, Andersson RG, Srinivas U. Modulation of P-selectin expression on isolated human platelets by an NO donor assessed by a novel ELISA application. J Immunol Methods. 1997;200:135-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Jilma B, Hildebrandt J, Kapiotis S, Wagner OF, Kitzweger E, Müllner C, Monitzer B, Krejcy K, Eichler HG. Effects of estradiol on circulating P-selectin. J Clin Endocrinol Metab. 1996;81:2350-2355. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Cosnes J. Tobacco and IBD: relevance in the understanding of disease mechanisms and clinical practice. Best Pract Res Clin Gastroenterol. 2004;18:481-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 175] [Article Influence: 8.8] [Reference Citation Analysis (0)] |