Published online Feb 21, 2006. doi: 10.3748/wjg.v12.i7.1086
Revised: July 2, 2005
Accepted: July 20, 2005
Published online: February 21, 2006
AIM: To determine whether increased blood flow of the liver can cause oxidative stress and hepatocyte damage, and to elaborate methods suitable for measuring the antioxidant defence during hepatic surgery on rat model.
METHODS: In nembutal narcosis, the left lateral and the medial lobes of the liver were clipped for 45 min to make the total blood supply flow through the other lobes. Total antioxidant status, glutathione peroxidase and superoxide dysmutase activity, as well as the concentrations of diene conjugates and free sulphydril groups, H-donating ability and reducing power of the liver samples were determined. Chemiluminescent intensity of the liver was also measured. Metal ions (Al, Ca, Cu, Fe, Mg, Mn, Zn) and P and S concentrations of the liver were determined with an inductively coupled plasma optical emission spectrometer and Se content was measured by cathodic stripping voltammetry.
RESULTS: Glutathione peroxidase and superoxide dysmutase activities of the liver decreased significantly in the hyperemia group compared to those observed in the sham operated group. The level of total antioxidant status was also significantly lower in the hyperemia group. H-donating ability, reducing power and free sulphydril group concentration showed the same tendency. A significant correlation (P<0.05) was found between the changes in non-specific antioxidant activities. This pointed to simultaneous activity of the antioxidant defence system. Al, Cu, Mn, Zn, and S were lower in the hyperemia group than in the sham operated group when the levels of Ca, Fe, Mg, Se and P ions were higher during hyperemia.
CONCLUSION: Oxidative stress is one of the main factors for the injury of intact liver lobes during ischaemia-reperfusion.
- Citation: Váli L, Taba G, Szentmihályi K, Fébel H, Kurucz T, Pallai Z, Kupcsulik P, Blázovics A. Reduced antioxidant level and increased oxidative damage in intact liver lobes during ischaemia-reperfusion. World J Gastroenterol 2006; 12(7): 1086-1091
- URL: https://www.wjgnet.com/1007-9327/full/v12/i7/1086.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i7.1086
Acute hyperemia (hyperperfusion) occurs during operations of the liver when the blood flow of part of the liver is selectively occluded by the surgeon, therefore the non-occluded lobes have to deal with a greater amount of blood. This type of operation is relatively rare, but the mechanism of this kind of damage can be of great interest since very few data on this topic are available in the literature. After liver resection the non-resected liver tissue receives the whole blood volume of the portal vein and the hepatic artery, therefore it may be assumed that hepatic overflow is related to these operations. The same mechanism occurs during liver transplantation from a living donor both in the liver of the donor and in the recipient. Owing to the relatively small size of the liver it is rather difficult to handle hemodynamic changes[1].
Hemodynamic changes due to the regeneration of the liver may have effect on the above mentioned hepatic hyperperfusion, therefore long-term investigation on this model may be an interesting field of research in the future[2-4].
Along with the increased blood flow, oxygen tension of the hepatic tissue is also high. High oxygen tension is connected to the generation of oxygen-related free radicals, such as hydroxyl radical, superoxide-anion or hydrogen peroxyde. Oxidative stress causes major disturbances in the redox-homeostasis of the liver cells, therefore it also affects the main cellular functions through signal transduction[5].
Especially in liver tissue, oxidative stress activates the Kupffer cells and Ito cells first, which are responsible for most of the liver damage[6]. Great amounts of free radicals are generated by Kupffer cells during their respiratory burst, which is a most important source of reactive oxygen species. Kupffer cells generate other mediators (cytokines, prostaglandines) as well, and these agents increase the damage of the liver tissue[7].
Oxidative stress causes lipid peroxidation of the cellular membranes, and diene conjugates and malondialdehyde are generated[8]. The injury of the cellular membranes destroys the homeostasis of hepatocytes, which leads finally to apoptosis or necrosis[9].
The great amount of free radicals and fast blood flow increase the risk of thrombosis, which can be related to greater liver cell damage[10]. Platelet aggregation is also affected by superoxide anions and hydroxyl radicals[11,12]. The disturbance in the circulation of the liver may cause endothelial dysfunction by changing the ratio of vasoconstrictor and vasodilatator agents[13,14]. The role of the white blood cells in thrombotic mechanisms related to ischaemia-reperfusion is well known. The same reactions may occur in intact liver lobes [15,16].
Oxidative stress and the generation of free radicals are closely related to transition metals and the antioxidant defence system is dependent on metal elements and selenium[17,18]. Therefore, determination of the element content in liver is relevant.
The aim of this study was to determine whether increased blood flow of the liver may cause oxidative stress of hepatocytes and to find methods suitable for measuring antioxidant defence during hepatic surgery on rat model.
Luminol, microperoxidase, hydrogen peroxide, and 1,1-diphenyl-2-picryl-hydrasyl radical were obtained from SIGMA (St.Louis). TAS (NX2332), SOD (SD 125) and GSHPx (RS 506) kits were bought from Randox (Crumlin, UK). The standard solution for ICP measurements was made from Merck ICP standards, and other chemical reagents were purchased from Reanal Chemical Company (Budapest).
Male Wistar rats (250 g) were purchased from Charles River Hungary and housed in a temperature- and humidity-controlled room under a constant 12-h light/dark cycle. Animals had free access to water and standard rat chow. All experiments were performed with rats fasted for 12 h prior to operations. All studies were performed with the permission of the Animal Health and Food Control Station (770/004/04).
Surgery was performed under deep nembutal narcosis (35 mg/body weight kg). Body temperature was kept between 36.5 °C and 37.5 °C. After laparotomy, circulation occlusion of the left lateral and the medial liver lobes was induced. Reflow was initiated by the removal of microclips, which selectively clamped the branches of the portal vein and hepatic artery.
For evaluation of the hepatic owerflow injury, a control group and a sham operated group were compared with two intervention groups respectively.
The rats were divided into control group (n=6) in which rats were not operated; sham operated group (n=6) in which laparotomy was performed for 45 min without damage to the liver; hyperemia (hyperperfusion) group (n=6) in which the peduncules of the right, left medial and lateral lobes were clipped to make the total blood volume of the portal vein and the hepatic artery flow through the lesser lobes of the liver for 45 min and then liver samples were taken as reperfusion group (n=6) in which the clips were removed from the peduncules of the ligated lobes to restore the original hemodynamic conditions of the liver after the 45-min-hyperemia period, modelling selectively induced ischaemic resection type of the liver. After 15 min of reperfusion the livers were stored at -20 °C for further examinations. The rats were exsanguinated from the arterial vein after the experiments.
Concentration of the diene-conjugates was measured based on the method of AOAC[19]. Free SH-groups were determined by the Sedlack method based on the Ellmann reaction[20]. The H-donating ability of the samples was determined by Blois’s method modified by Blázovics et al[21] in the presence of a 1,1-diphenyl-2-picryl-hydrasyl radical. Absorbance of the methanolic DPPH-dye was detected spectrophotometrically at 517 nm. For characterization of the ability, inhibition (%) was given to the DPPH degradation[22,23]. Oyaizu’s method was adopted for the determination of the reducing power (RP) of the samples. The change in absorbance was measured, which accompanied Fe3+-Fe2+ transformation at 700 nm, and the RP was compared to that of ascorbic acid[24]. All spectrophotometric measurements were carried out with a Jasco V 550 instrument.
A recently developed chemiluminescence assay adapted to a Berthold Lumat 9501 instrument was applied. The procedure was carried out by the method of Blázovics et al [21]. The volume of liver homogenate samples (protein content was 10 mg/mL) was 0.06 mL. Chemiluminescent intensity of the samples was expressed in RLU% (relative light unit %) of the standard (basic chemical reaction means: H2O2-luminol-microperoxidase reaction).
The levels of total antioxidant status (TAS), superoxide dysmutase (SOD) and glutathione peroxidase (GSHPx) were determined from the supernatants of liver homogenates (10 mg/mL protein content) with Randox kits. The results were expressed in arbitrary units calculated from the absorbance detected by spectrophotometry. Protein content was measured by the method of Lowry[25].
Element concentration in the pooled liver homogenate samples was determined in three parallel measurements with an inductively coupled plasma optical emission spectrometer (ICP-OES). Type of instrument used was Atom Scan 25 (Thermo Jarrell Ash Co.). After digestion of the liver samples (1 mL) with a mixture of nitric acid (5 mL) and hydrogen peroxide (2 mL) diluted to 10 mL with deionised water, the concentration of 9 elements (Al, Ca, Cu, Fe, Mg, Mn, P, S, Zn) was determined three times, three seconds each time. Integration time, blank subtraction and background correction were applied during the measurements[26].
Se content in the liver was determined by a cathodic stripping voltammatric method with hanging mercury drop electrode (instrument: Trace Lab 50). The digested samples for the element measurement (previous paragraph) was measured in 1 mol/L HCl as supporting electrolyte by preconcentration at - 350 mV. Electrolysis time: 100; potential: -300 mV up to -900 mV; step duration; -50 mV/s.
One-way ANOVA statistical analysis was performed to evaluate significant levels between the different groups of rats. Pearson’s correlation matrix was used to evaluate the correlation between measured parameters. The data were expressed as present mean ± SD. Five parallel measures were carried out from each of the pooled samples, in this case the data showed mean values when c.v. was below 5.00 %. P<0.05 was considered statistically significant.
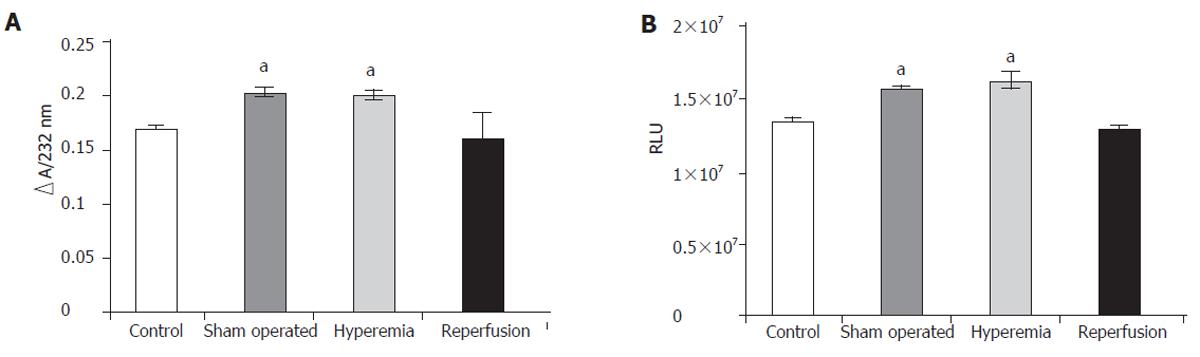
The amount of generated diene conjugates is shown in Figure 1A. The concentration of diene conjugates increased significantly in the sham operated rats, which was significantly higher in the hyperemia group than that (0.171±0.001) in the control group and (0.201±0.007) in the hyperemia group ∆E232nm. When the circulation of the liver returned to normal, the level of diene conjugates also tended to return to the normal value.
Chemiluminescent intensity was significantly higher in the sham operated group (1.339 x 10 ± 1.42 x 104 RLU) (as well as during hyperemia) than in the control group (1.612 x 107±1.03 x 106 RLU). Chemiluminescent intensity was normalized by reperfusion of the ligated lobes (Figure 1B). The concentration of diene conjugates as well as the chemiluminescent intensity pointed to oxidative damage to the liver tissue.
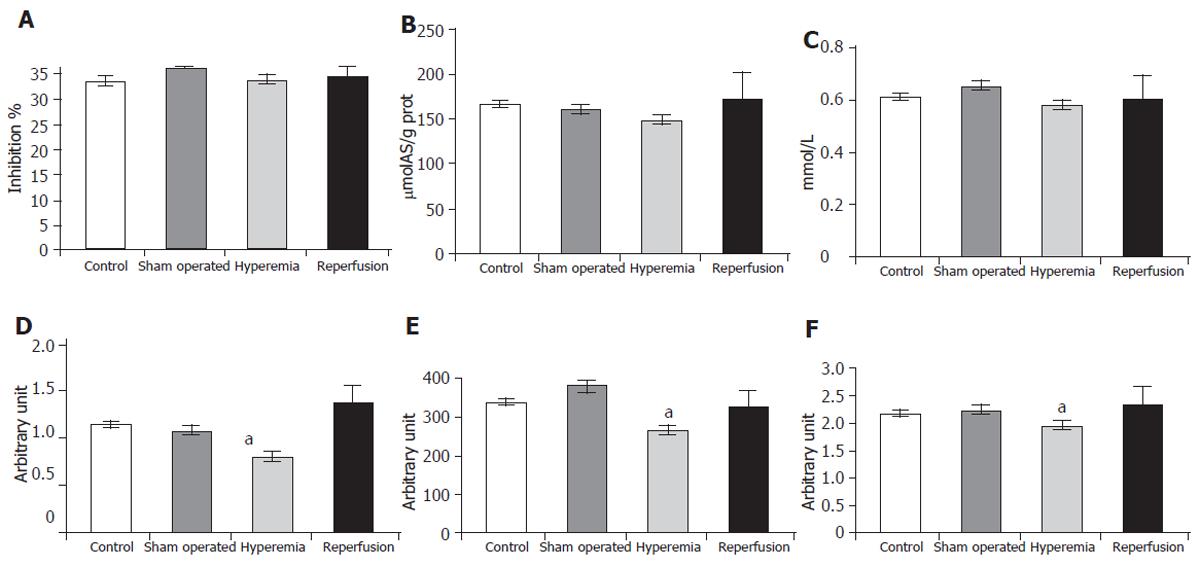
H-donating ability was lower in the hyperemia group (36.05± 0.12) than in the sham operated group (33.53± 2.85). The changes in reducing power (159.36 ± 5.76 versus 140.16± 13.68 µmolAS/g protein) and in the concentration of free SH-groups (0.649± 0.054 versus 0.579± 0.042 mmol/L) showed the same tendency, although the changes were not significant (Figures 2A, 2B, 2C), indicating a decrease in non specific antioxidant defence. In the reperfusion group these parameters showed enormous deviation, suggesting that the antioxidant system had to exert a great ability.
A positive correlation (P < 0.05) was found between the changes in H-donating ability, reducing power and the concentration of free SH-groups (r = 0.81 between H-donating ability and reducing power, r = 0.82 between H-donating ability and SH-groups and r = 0.56 between reducing power and SH-groups), indicating the parallel work of the antioxidant defence system.
The SOD activity (in arbitrary unit) of the liver decreased significantly in the hyperemia group (0.634± 0.133) compared to that in the sham operated group (1.073± 0.122) (Figure 2D). The glutathione-peroxidase (GSHPx) activity (in arbitrary unit) was significantly lower in the hyperemia group (263.11± 45.17) than in the sham operated group (380.70± 33.88). When the hepatic flow became normal, the activity of the enzyme approached the basic state (328.38± 51.87) (Figure 2E). The level of TAS (in arbitrary unit) was also significantly lower in the hyperemia group (1.936± 0.027) than in the sham operated group (2.214± 0.165) (Figure 2F).
The concentration of elements (Al, Ca, Cu, Fe, Mg, Mn, P, S, Se and Zn) was also determined from pooled liver samples by ICP-OES and polarography. The concentration of Al, Cu, Mn, Zn, and S was lower in the hyperemia group than in the sham operated group, while the level of Ca, Fe, Mg, Se and P ions increased during hyperemia. The tendency was the same in the case of Cu, Mn, Se and Zn level during reperfusion, which were essential for the activity of SOD and GSHPx enzymes (Table 1).
| Control | Sham-operated | Hyperemia | Reperfusion | |
| Al | 0.501±0.04 | 0.736±0.03 = | 0.484±0.03 a | 0.643±0.035 ace |
| Ca | 22.43±0.31 | 22.79±0.04 | 35.05±0.29 ae | 23.72±0.17 ac |
| Cu | 1.628±0.02 | 1.671±0.02 | 1.651±0.01 | 1.595±0.02 ac |
| Fe | 52.04±1.21 | 46.81±0.62 e | 58.18±1.08 ae | 59.90±1.03 ae |
| Mg | 81.40±0.29 | 84.60±1.07 | 89.76±0.49 ae | 81.87±1.03 ac |
| Mn | 1.260±0.87 | 1.314±0.02 | 1.300±0.01 | 1.279±0.01 |
| P | 1680±119 | 1714±41 | 1799±68 | 1751±40 a |
| Se | 54.6±16.8 | 107.3±28.3 e | 129.8±38.9 e | 60.9±10.9 ac |
| S | 1273±22 | 1562±14 e | 1404±11 ae | 1345±24 ace |
| Zn | 14.79±0.04 | 14.33±0.02 | 13.96±0.12 a | 13.49±0.10 ac |
The correlations between the changes in metal ion content are listed in Table 2.
| Metal ions | Correlations | Metal ions | Correlations |
| Al-Fe | r = -0.92; P < 0.05 | P-Zn | r = -0.73; P < 0.05 |
| Ca-Fe | r = 0.85; P < 0.05 | P-S | r = 0.99; P < 0.05 |
| Ca-Mg | r = 0.92; P < 0.05 | Se-Cu | r = 0.75; P < 0.05 |
| Ca-S | r = 0.86; P < 0.05 | Se-Mn | r = 0.83; P < 0.05 |
| Ca-P | r = 0.87; P < 0.05 | Se-Ca | r = 0.75; P < 0.05 |
| Cu-Mn | r = 0.72; P < 0.05 | Se-Mg | r = 0.95; P < 0.05 |
| Mg-S | r = 0.85; P < 0.05 | Se-S | r = 0.77; P < 0.05 |
| Mg-P | r = 0.79; P < 0.05 |
Hepatic overflow has not been studied thoroughly so far. The intact liver lobes of the rat ischaemia-reperfusion model are generally considered unharmed, whereas oxidative damage has been verified in our investigations. The injury is probably due to the generation of free radicals. The presence of oxidative stress causes disturbances in the cell cycle and initiates apoptosis[27]. Hepatic apoptosis is related to the generation of interferon-γ, which is able to produce oxygen-related free radicals as well[28].
The production of reactive oxygen substances leads to hepatocyte damage, therefore, poor liver function becomes even worse[29]. A better understanding of these mechanisms is important, since the generation of free radicals and the decrease in antioxidant level can be compensated with scavenger molecules such as glutathione and multivitamin infusion[30-32]. Moreover, aged patients after liver surgery have significantly weaker antioxidant defence than young ones[33].
Compared to the control group, the level of diene conjugates and the chemiluminescent intensity were significantly higher in the sham operated animals as well as in the animals with liver overflow. The high level of lipid peroxidation is one of the markers of oxidative stress, which along with the changes in chemiluminescent intensity point to hepatocyte injury. Chemiluminescent intensity is considered as the amount of generated free radicals. The generation of free radicals is related to the production of cytokines and other inflammatory agents, and NFκ-B is also activated in the process[34], which finally leads to apoptosis and/or necrosis.
Apoptosis may occur in a physiological setting without inflammation. In pathophysiological settings apoptosis frequently induces inflammation because of the onset of secondary necrosis and stimulation of cytokine and chemokine formation. In liver, mitochondrial permeability transition represents a shared pathway that leads to both necrosis and apoptosis (adenosine triphosphate depletion, cytochrome C release), which makes discrimination between the two forms of cell death difficult sometimes[35].
Signal transducer and activator of transcription-3 (Stat3) are the most important molecules involved in the initiation of liver regeneration. Stat3 activation protects against Fas-mediated liver injury by inhibiting caspase activities in redox-dependent and -independent mechanisms[36]. Stat3 provides hepatoprotection against Fas-mediated apoptotic liver damage by two mechanisms: direct inactivation of caspases and reduction of reactive oxygen species[37].
During hepatic overflow the antioxidant system is injured as well. The non-enzymatic defence system can be described by determining the values of H-donating ability, reducing power and the concentration of free SH-groups, which were found to be lower in this experiment. When the defence activity is lower, free radicals induce severer damage, suggesting that these factors are of great importance. The changes in enzymatic defence (TAS, SOD, GSHPx) show the same tendency, as observed in non-enzymatic defence. These changes may be suitable markers of hepatocyte damage. The joint effect of the high amount of reactive oxygen species produced and the decrease in the defence system may be the key to a better understanding of the damage during hepatic hyperemia.
In the present study, the high amount of blood flow caused the transfer of some metal ions into the liver cells. Especially the Ca, Fe, Mg, and P content in the liver increased during hyperemia, which can be explained by the injury of cellular membranes. The amount of the generated diene conjugates also pointed to the injury of cellular membranes. The concentration of most of the essential metal ions increased in the liver of sham operated rats. The accumulation of Fe during hepatic owerflow and after surgery is important, since this element can induce the production of free radicals through the Fenton reaction. A strong positive correlation was found between the changes in concentrations of Ca-Fe, Ca-Mg, Ca-S, Ca-P, Cu-Mn, Mg-S, Mg-P, and P-S, while there was a strong negative correlation between the levels of Al-Fe and P-Zn. The accumulation of certain elements in the liver during surgery may be harmful.
Since the presence of Mn, Zn and Cu is essential for the function of SOD enzymes, their lower ability may be attributed to the loss of the above elements[38]. Se is relevant to the action of glutation-peroxidase, which is also important in the antioxidant defence of liver cells. During our experiment the behaviour of Se was suprising. Therefore, measuring Se concentration under these conditions may lead to a new and important field of research[39]. These findings also underline the changes in the homeostasis of liver cells due to hyperperfusion.
Compared to the ischaemia-reperfusion damage[40], the injury caused by hyperemia is related to less hepatocyte dysfunction, an important risk factor for liver surgery.In conclusion, the methods applied by us are suitable for measuring compensatory mechanisms in hyperemia and ischaemia-reperfusion.
The authors thank Bárkovits Sarolta and Pintér Edina for their great help.
S- Editor Guo SY L- Editor Wang XL E- Editor Liu WF
| 1. | Wu Y, Campbell KA, Sitzmann JV. Hormonal and splanchnic hemodynamic alterations following hepatic resection. J Surg Res. 1993;55:44-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Minuk GY. Hepatic regeneration: If it ain't broke, don't fix it. Can J Gastroenterol. 2003;17:418-424. [PubMed] [Cited in This Article: ] |
| 3. | Ott R, Schuppan D, Tannapfel A, Wittekind C, Erhardt W, Henke J, Kilic N, Köckerling F, Reck T, Hohenberger W. Portal vein arterialisation as a technical option in liver transplantation: impact on function, regeneration, and morphology of the liver following hemihepatectomy in pigs. Liver Int. 2003;23:54-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Kaya Y, Aral E, Coskun T, Erkasap N, Var A. Increased intraabdominal pressure impairs liver regeneration after partial hepatectomy in rats. J Surg Res. 2002;108:250-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Oguro T, Takahashi Y, Ashino T, Takaki A, Shioda S, Horai R, Asano M, Sekikawa K, Iwakura Y, Yoshida T. Involvement of tumor necrosis factor alpha, rather than interleukin-1alpha/beta or nitric oxides in the heme oxygenase-1 gene expression by lipopolysaccharide in the mouse liver. FEBS Lett. 2002;516:63-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Cutrín JC, Llesuy S, Boveris A. Primary role of Kupffer cell-hepatocyte communication in the expression of oxidative stress in the post-ischaemic liver. Cell Biochem Funct. 1998;16:65-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 7. | Guidi L, Tricerri A, Costanzo M, Adducci E, Ciarniello M, Errani AR, De Cosmo G, Barattini P, Frasca D, Bartoloni C. Interleukin-6 release in the hepatic blood outflow during normothermic liver ischaemia in humans. Dig Liver Dis. 2003;35:409-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Molina MF, Sanchez-Reus I, Iglesias I, Benedi J. Quercetin, a flavonoid antioxidant, prevents and protects against ethanol-induced oxidative stress in mouse liver. Biol Pharm Bull. 2003;26:1398-1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 208] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis. Hepatology. 2001;33:397-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 293] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 10. | Weigel G, Griesmacher A, Toma CD, Baecker C, Heinzl H, Mueller MM. Endothelial eicosanoid metabolism and signal transduction during exposure to oxygen radicals injury. Thromb Res. 1997;87:363-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Salvemini D, Botting R. Modulation of platelet function by free radicals and free-radical scavengers. Trends Pharmacol Sci. 1993;14:36-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Caccese D, Praticò D, Ghiselli A, Natoli S, Pignatelli P, Sanguigni V, Iuliano L, Violi F. Superoxide anion and hydroxyl radical release by collagen-induced platelet aggregation--role of arachidonic acid metabolism. Thromb Haemost. 2000;83:485-490. [PubMed] [Cited in This Article: ] |
| 13. | Chen T, Zamora R, Zuckerbraun B, Billiar TR. Role of nitric oxide in liver injury. Curr Mol Med. 2003;3:519-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Graupera M, García-Pagán JC, Parés M, Abraldes JG, Roselló J, Bosch J, Rodés J. Cyclooxygenase-1 inhibition corrects endothelial dysfunction in cirrhotic rat livers. J Hepatol. 2003;39:515-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Bridges AB, Scott NA, McNeill GP, Pringle TH, Belch JJ. Circadian variation of white blood cell aggregation and free radical indices in men with ischaemic heart disease. Eur Heart J. 1992;13:1632-1636. [PubMed] [Cited in This Article: ] |
| 16. | Belch J, McLaren M, Hanslip J, Hill A, Davidson D. The white blood cell and plasma fibrinogen in thrombotic stroke. A significant correlation. Int Angiol. 1998;17:120-124. [PubMed] [Cited in This Article: ] |
| 17. | Santon A, Sturniolo GC, Albergoni V, Irato P. Metallothionein-1 and metallothionein-2 gene expression and localisation of apoptotic cells in Zn-treated LEC rat liver. Histochem Cell Biol. 2003;119:301-308. [PubMed] [Cited in This Article: ] |
| 18. | Cheng WH, Quimby FW, Lei XG. Impacts of glutathione peroxidase-1 knockout on the protection by injected selenium against the pro-oxidant-induced liver aponecrosis and signaling in selenium-deficient mice. Free Radic Biol Med. 2003;34:918-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | AOAC Official Methods of Analysis 28054 B. 14th edition, Arlington, USA: 1984. . [Cited in This Article: ] |
| 20. | Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5089] [Cited by in F6Publishing: 5142] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 21. | Blázovics A, Kovács A, Lugasi A, Hagymási K, Bíró L, Fehér J. Antioxidant defense in erythrocytes and plasma of patients with active and quiescent Crohn disease and ulcerative colitis: a chemiluminescent study. Clin Chem. 1999;45:895-896. [PubMed] [Cited in This Article: ] |
| 22. | Blois MS. Antioxidant determination by the use of stable free radicals. Nature. 1958;4617:1199-2000. [DOI] [Cited in This Article: ] [Cited by in Crossref: 7010] [Cited by in F6Publishing: 7054] [Article Influence: 106.9] [Reference Citation Analysis (0)] |
| 23. | Hatano T, Kagawa H, Yasuhara T, Okuda T. Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem Pharm Bull (Tokyo). 1988;36:2090-2097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 788] [Cited by in F6Publishing: 665] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 24. | Oyaizu M. Studies on products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307-315. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4390] [Cited by in F6Publishing: 4446] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 25. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] [Cited in This Article: ] |
| 26. | Szentmihályi K, Blázovics A, Kocsis I, Fehér E, Lakatos B, Vinkler P. The effect of fat rich diet and alcohol on ion concentration in bile in rats. Acta Alim. 2000;29:359-366. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Albright CD, Salganik RI, Craciunescu CN, Mar MH, Zeisel SH. Mitochondrial and microsomal derived reactive oxygen species mediate apoptosis induced by transforming growth factor-beta1 in immortalized rat hepatocytes. J Cell Biochem. 2003;89:254-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Watanabe Y, Suzuki O, Haruyama T, Akaike T. Interferon-gamma induces reactive oxygen species and endoplasmic reticulum stress at the hepatic apoptosis. J Cell Biochem. 2003;89:244-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Rutkowski T, Plewka A, Kaminski M. Study on ischemic/reperfusion damage to the liver: Hitherto existing successes and defeats. Acta Polo Toxicol. 2000;8:1-15. [Cited in This Article: ] |
| 30. | Cerwenka H, Bacher H, Werkgartner G, El-Shabrawi A, Quehenberger F, Hauser H, Mischinger HJ. Antioxidant treatment during liver resection for alleviation of ischemia-reperfusion injury. Hepatogastroenterology. 1998;45:777-782. [PubMed] [Cited in This Article: ] |
| 31. | Rhoden E, Pereira-Lima L, Lucas M, Mauri M, Rhoden C, Pereira-Lima JC, Zettler C, Petteffi L, Belló-Klein A. The effects of allopurinol in hepatic ischemia and reperfusion: experimental study in rats. Eur Surg Res. 2000;32:215-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Schauer RJ, Gerbes AL, Vonier D, Meissner H, Michl P, Leiderer R, Schildberg FW, Messmer K, Bilzer M. Glutathione protects the rat liver against reperfusion injury after prolonged warm ischemia. Ann Surg. 2004;239:220-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Gasbarrini A, Simoncini M, Di Campli C, De Notariis S, Colantoni A, Pola P, Bernardi M, Gasbarrini G. Ageing affects anoxia/reoxygenation injury in rat hepatocytes. Scand J Gastroenterol. 1998;33:1107-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Tapia G, Fernández V, Varela P, Cornejo P, Guerrero J, Videla LA. Thyroid hormone-induced oxidative stress triggers nuclear factor-kappaB activation and cytokine gene expression in rat liver. Free Radic Biol Med. 2003;35:257-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246-1257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 444] [Cited by in F6Publishing: 453] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 36. | Haga S, Terui K, Zhang HQ, Enosawa S, Ogawa W, Inoue H, Okuyama T, Takeda K, Akira S, Ogino T. Stat3 protects against Fas-induced liver injury by redox-dependent and -independent mechanisms. J Clin Invest. 2003;112:989-998. [PubMed] [Cited in This Article: ] |
| 37. | Taub R. Hepatoprotection via the IL-6/Stat3 pathway. J Clin Invest. 2003;112:978-980. [PubMed] [Cited in This Article: ] |
| 38. | Chavez-Cartaya R, Jamieson NV, Ramirez P, Marin J, Pino-Chavez G. Free radical scavengers to prevent reperfusion injury following experimental warm liver ischaemia. Is there a real physiological benefit. Transpl Int. 1999;12:213-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Batcioglu K, Ozturk C, Karagozler A, Karatas F. Comparison of the selenium level with GSH-Px activity in the liver of mice treated with 7,12 DMBA. Cell Biochem Funct. 2002;20:115-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Váli L, Fébel H, Fehér J, Blázovics A. Changes of the redox-homeostasis during hepatic ischaemia-reperfusion of the rat. 46th Annual Meeting of the Hungarian Society of Gastroenterology. 2004;1417-7013, p: 147. [Cited in This Article: ] |