Published online Oct 28, 2006. doi: 10.3748/wjg.v12.i40.6500
Revised: August 12, 2006
Accepted: September 15, 2006
Published online: October 28, 2006
AIM: To establish and characterize a new cell line derived from peripheral cholangiocarcinoma of a Thai patient.
METHODS: The peripheral cholangiocarcinoma specimen surgically obtained from the patient was aseptically processed by washing and mincing before culturing in Ham’s F12 medium containing 10% fetal bovine serum. After 3 mo, when the cell line has become homogeneous and stabilized, several features were investigated, including growth characteristics, immunofluorescence staining for cytokeratins, expression of tumor markers, chromosomal analysis by G-banding and multicolour fluorescence in situ hybridization (mFISH), in vitro migration and invasion characteristics.
RESULTS: The RMCCA-1 cell line has been established. These cells proliferated as a monolayer with a population doubling time of 48 h. Immunofluorescence staining showed positive staining for human cytokeratin 7 and 19 verifying the biliary epithelial origin. RMCCA-1 secreted carbohydrate antigen 19-9 (CA19-9), but insignificant levels of carcinoembryonic antigen (CEA) and α-fetoprotein (AFP). Chromosome analysis identified aneuploidy karyotypes with a modal chromosome number of 59. RMCCA-1 exhibited a low level of in vitro invasiveness, but a high degree of motility. The cell line exhibited a significant number of chromosomal aberrations as shown by mFISH and G-banding methods.
CONCLUSION: A new cell line derived from peripheral cholangiocarcinoma of a Thai patient has been established. This cell line shows a low level of in vitro invasiveness, but a high degree of motility. It will serve as a valuable tool for further studies on tumor biology, molecular pathogenesis, metastatic mechanism and response to therapeutic drugs of cholangiocarcinoma.
- Citation: Rattanasinganchan P, Leelawat K, Treepongkaruna SA, Tocharoentanaphol C, Subwongcharoen S, Suthiphongchai T, Tohtong R. Establishment and characterization of a cholangiocarcinoma cell line (RMCCA-1) from a Thai patient. World J Gastroenterol 2006; 12(40): 6500-6506
- URL: https://www.wjgnet.com/1007-9327/full/v12/i40/6500.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i40.6500
Cholangiocarcinoma is a highly malignant epithelial neoplasm that arises within the intrahepatic and extrahepatic biliary tract[1]. The pathogenesis of this disease has been strongly associated with chronic inflammation and cellular injury within bile ducts, as well as partial obstruction of bile flow, manifested by various high risk conditions such as PSC (primary sclerosing chloangitis), hepatolithiasis and infestation by liver fluke (Ophisthorchis viverrini or Clonorchis sinensis)[2,3]. Although considered as a rare disease, cholangiocarcinoma occurs at a particularly high rate in Northeastern Thailand, with 84.6:100 000 males and 36.8:100 000 females affected by the disease. This is the area where the incidence rate of cholangiocarcinoma is the highest in the world, largely accounted by the habit of consuming uncooked cyprinoid fish, which are infected with the liver fluke[4].
Cholangiocarcinoma has become a serious threat to public health due to increasing worldwide incidence and mortality rates associated with lack of early detection and limited therapeutic options. At diagnosis, most patients are presented with advanced disease, possibly with undetected metastasis, resulting in less than 12 mo survival. Even those with operable tumor, the recurrence rate was extremely high, with a 5-year survival rate of less than 40%[2,5]. Various routes of tumor spreading have been reported in cholangiocarcinoma, including direct invasion, infiltration along the biliary tree, vascular and lymphatic permeation and perineural or intraneural invasion[6].
Progress in understanding the molecular mechanisms governing cholangiocarcinoma invasion and metastasis has been limited by the lack of suitable cell lines and experimental models. Here, we described an establishment and preliminary characterization of a human cell line originated from a Thai patient presented with peripheral cholangiocarcinoma. This new cell line, which we named RMCCA-1, exhibits various characteristics typical of the biliary epithelial cells, as well as invasiveness and motility as shown by the in vitro assay. Thus this cell line will be useful for the studies of not only the tumor biology, molecular pathogenesis and drug response, but also the molecular mechanisms governing the metastatic spread of cholangiocarcinoma.
A 40-year-old male patient was admitted to Rajavithi Hospital, Bangkok, Thailand with a professional diagnosis of peripheral cholangiocarcinoma. The patient’s serum was analyzed for alkaline phosphatase (ALP), total bilirubin and direct bilirubin using a COBAS Integra 800 instrument (Roche, USA), whereas CA19-9, carcinoembryonic antigen (CEA), and α-fetoprotein (AFP) were analyzed on a ELECHYS 2012 instrument (Roche, USA). Significant laboratory analysis at admission showed elevated serum levels of ALP (374 U/L, normal 39-117 U/L), total bilirubin (19.73 mg/dL, normal 0.0-1.5 mg/dL), direct bilirubin (15.45 mg/dL, normal 0-0.5 mg/dL), CA19-9 (85.05 U/mL, normal 0-39 U/mL) and CEA (7.56 ng/mL, normal 0.0-3.4 ng/mL), whereas the AFP (2.24 U/mL, normal 0.0-5.8 U/mL), and CA125 (19.71 U/mL, normal 0.0-35 U/mL) levels were normal.
The CT examination evaluated for this study was performed on a helical CT scanner (Somatom Plus 4, Siemens Medical Solutions) using the following parameters: 5-mm collimation, 5-mm reconstruction interval, and a 1:1 table pitch. Both unenhanced and contrast-enhanced CT scans were obtained. With intravenous injection of 120 mL of nonionic contrast material [iopromide (Ultravist 370, Schering)], both hepatic artery phase (HAP) and portal venous phase (PVP) images were obtained with a scanning delay of 30 and 65 s, respectively. The results showed an ill defined hypo-density mass occupying the whole left lobe of the liver. At laparotomy, no ascites was found, and the surface of liver was smooth. Intraoperative ultrasonography revealed a solitary mass at left lobe of liver with moderate dilatation of left intrahepatic duct. The patient was then subjected to left hepatectomy and lymph node dissection. The tumor specimen was removed and subjected to histopathological study and to tissue culture under the approval of the Ethics Committee of Rajavithi Hospital.
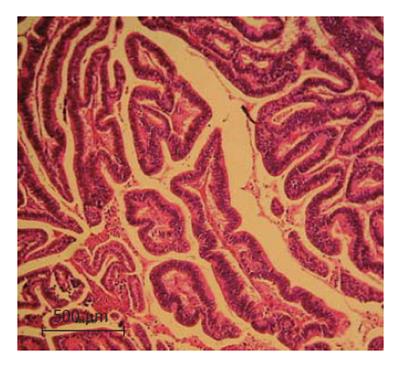
The lesion was classified as a well-differentiated peripheral cholangiocarcinoma at stage T2N0M0 according to the UICC standardization (Figure 1).
After the tumor tissue was surgically removed from the patient, it was immediately suspended in transfer medium [HAM’s F12 (GIBCO, Grand Island, NY, USA) containing antibiotics (GIBCO, Grand Island, NY, USA)] at 4°C. The tissue was quickly washed in PBS pH 7.4 several times before being minced. Later the cell suspension was placed in a 100 mm × 20 mm plastic tissue culture dish (CORNING, New York, USA) containing 10 mL of growth medium (HAM’s F12, 20% fetal bovine serum (FBS) (GIBCO, Grand Island, NY, USA), 1 × 10-5 g/L epidermal growth factor (EGF) (Pacific Science, Peprotech, New Jersey, USA), 0.1 U/L penicillin G sodium, 0.1 g/L streptomycin sulfate and 2.5 × 104 g/L amphotericin B). The cell cultures were then incubated at 37°C in a humidified 50 mL/L CO2 atmosphere and observed daily. Tumor cells were separated from the contaminating fibroblast cells by manually dropping 2.5 mL/L trypsin containing 0.2 mL/L EDTA (GIBCO, Grand Island, NY, USA) in PBS onto an isolated tumor colony. Subsequently the detached tumor cells were transferred by pipettes to a new culture dish under a phase contrast microscope. After about 1 mo, a homogeneous layer of epithelial tumor cells with sustained growth pattern was established.
A suspension of 2 × 103 cells was cultured in triplicates in 100 μL of Ham’s F12 medium supplemented with 100 mL/L FBS in a 96-well plate (CORNING, New York, USA). At time intervals, 10 μL of 5 mg/mL MTT (USB, Cleveland, OH, USA) solution was added to the individual wells, followed by incubation for 4 h at 37°C in a humidified atmosphere containing 50 mL/L CO2. MTT converted to insoluble formazan dye in live cells was then dissolved by addition of 200 μL DMSO (Sigma, St. Louis, MO, USA) before the absorbency was read at 540 nm. The doubling time of the cell population was determined from the exponential phase of the growth curve.
The monoclonal antibody mixture AE-1/AE-3 (DAKO, Denmark) recognized all known basic and most acidic keratin, thus it was used as a general marker of epithelial cells. Cytokeratin 7 (monoclonal mouse Anti-Human Cytokeratin 7, DAKO, Denmark) and cytokeratin 19 (monoclonal mouse Anti-Human Cytokeratin 19, DAKO, Denmark) were used to specifically distinguish biliary epithelial cells from hepatocytes[7,8]. RMCCA-1 cells were grown on sterile coverslips until confluent before being fixed in methanol for 15 min, blocked in 10 mg/L bovine serum albumin (BSA), and incubated with primary antibody for 60-min. After that, a secondary antibody conjugated with fluorescein isothiocyanate (DAKO, Denmark) was added and the incubation was allowed to proceed for 60 min at 37°C. After washing, the coverslips were examined under a fluorescence microscope (Nikon Eclipse TE 2000-U, Kanagawa, Japan).
The established tumor cells at 12th passage were subjected to chromosomal analysis. The cells were treated with 10 μmol/L Colchicine (Sigma, St. Louis, MO, USA) for 30 min and suspended in hypotonic solution, 0.075 mol/L KCl (Sigma, St. Louis, MO, USA), for 7 min, fixed in Carnoy’s fixative and spreaded onto cold glass slides. The cells were stained with Giemsa and the representative chromosome sets were photographed for karyotype analysis. Interpretation of the karyotype was based on ISCN (1995). The modal chromosome number was determined from 20 cells.
A duplicate slide, prepared from the same culture used for G-banding, was subjected to mFISH analysis according to the manufacturer’s protocol (Metasystem, Germany). The 24 XCyte mFISH kit (Metasystem, Germany) with five fluorophors were used for hybridization, including FITC, Spectrum Orange, Texas Red, Cy5 and DEAC. Each chromosome (1-22, X and Y) was painted with different colors using various combinations of the fluorophores. After hybridization, the slides were evaluated under a fluorescence microscope (Axioimage, Zeiss, Germany), and the images were captured and analyzed using the Program Isis (Metasystem, Germany).
A suspension of 105 tumor cells was cultured in serum-free HAM’s F12 medium for 24 h before the conditioned medium was collected and centrifuged at 1800 r/min for 10 min. The supernatant was then collected for detection of CA 19-9, CEA and AFP by using chemiluminescence detection system on an ELECHYS 2012 instrument (Roche, USA).
Cancer cell invasiveness was determined using transwell chamber (Costar, Cambridge, MA, USA) coated with 0.3 g/L matrigel (Collaborative Research Inc., Bedford, MA, USA). Approximately, 1 × 105 cells (RMCCA-1, KKU-100, KKU-213 and HuCCA-1) in culture medium containing FBS were added into the upper compartment of the transwell, and incubated at 37°C in a humidified atmosphere containing 50 mL/L CO2 for 6 h. The lower compartment contained culture medium plus FBS. The filters were fixed with methanol and stained with 0.5% crystal violet in 25% methanol for 1 h, before rinsing with tap water several times. The cells on the upper surface of the filters were gently removed using cotton swabs and the cells that have invaded into the lower surface were counted under a microscope. The numbers of invaded cells in five random × 10 microscopic fields were counted and expressed as number of cells per well. The results shown represented mean ± SE of the number of invaded cells from three independent experiments, each carried out in triplicates.
Motility assay was performed in a similar fashion to the invasion assay, except no matrigel coating was applied to the upper surface of the transwell filters. The numbers of migrating cells in five random × 10 microscopic fields were counted and expressed as number of cells per well. The results shown represented mean ± SE of the number of migrating cells from three independent experiments, each carried out in triplicates.
Cells were starved by culturing in the medium without FBS for 24 h before collection of the conditioned medium. The conditioned medium was mixed with 5 × SDS-sample buffer before separation in a 120 g/L SDS-PAGE containing 1 mg/mL gelatin (Sigma, St. Louis, MO, USA). After electrophoresis at 200 V for 1 h, the gel was washed in a 25 mL/L Triton X-100 (Amersham, Piscataway, NJ, USA) solution twice. The gel was then incubated in buffer containing 50 mmol/L Tris-HCl (Amersham, Piscataway, NJ, USA), pH 7.5, 10 mmol/L CaCl2 (Merck, Denmark), 1 mmol/L ZnCl2 (Merck, Denmark), 10 mL/L Triton X-100 for 16-18 h, after which the gel was stained with 5 g/L Coomassie blue in 300 mL/L methanol and 100 mL/L acetic acid. After destaining, the clear band of gelatinolytic activity was documented for size determination using a Biorad GS700 gel scanner (Biorad, Hercules, CA, USA)[9].
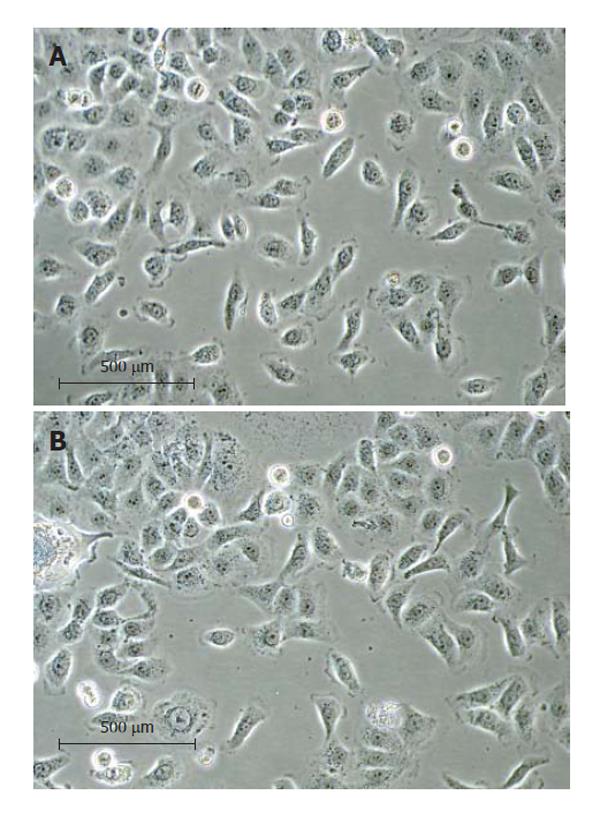
The human CCA tissue fragments adhered to the dish after plating for 6 h. After three weeks, a layer of epithelial cells appeared, from which contaminating spindle-shaped fibroblasts could be readily distinguished and separated from the epithelial tumor cells by differential trypsinization. After successive 16 passages, a homogeneous immortalized culture of tumor cells was established and was named RMCCA-1. The cells from passages 16th and 30th were then used for morphological analysis. These cells exhibited circular to spindle shape with many processes and ornamental fringes. The nucleus and cytoplasm appeared granulated (Figure 2).
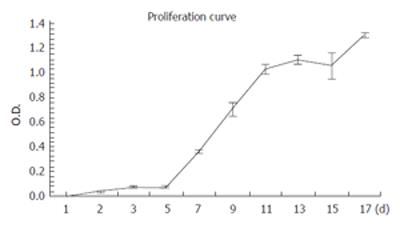
RMCCA-1 cells were in lag phase until d 5, after which they entered a logarithmic growth phase. The doubling time determined from the slope of the growth curve was 48 h (Figure 3).
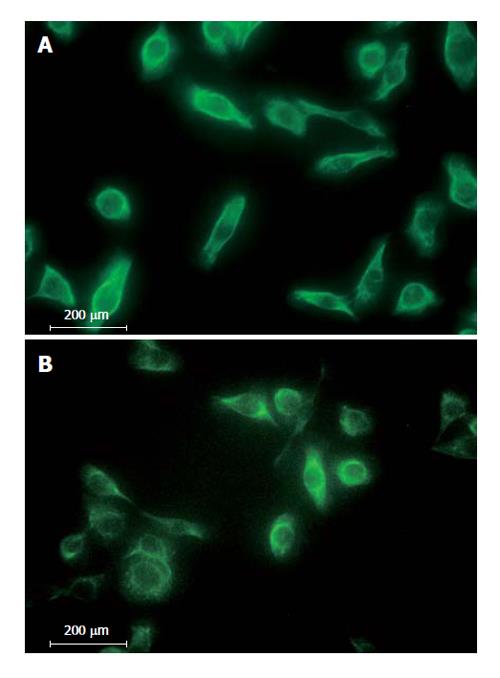
All RMCCA-1 cells showed positive staining with the AE-1/AE-3 monoclonal antibody mixture (Figure 4B), which recognizes the human epidermal cytokeratins, the signature of epithelial cells that distinguishes them from fibroblasts. Furthermore, specific markers for the adenocarcinoma and transitional cell carcinomas, cytokeratin 7 (Figure 4A) and 19 (data not shown), were also positive.
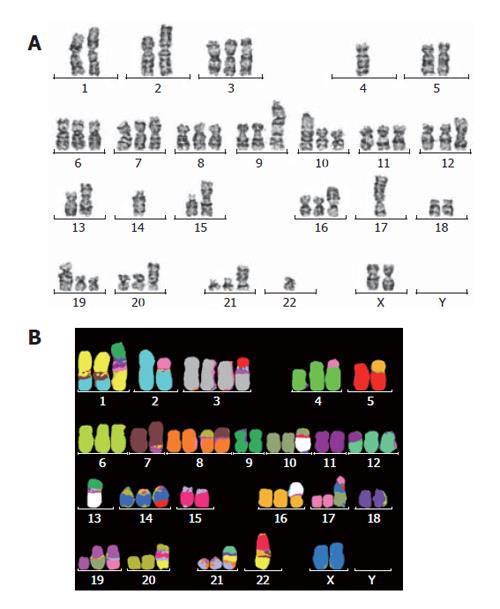
The G-banding analysis demonstrated aneuploidy karyotype with marked structural abnormalities of the chromosomes (Figure 5A and B). Although this cell line was established from a male patient, it lacks a Y chromosome and, instead, possesses two X chromosomes. The number of chromosomes ranges between 54 and 61, with a modal chromosome number of 59. The final karyotype was determined by consolidating the G-banding and mFISH results. Sixteen structural rearrangements were found, including 9 unbalanced translocations and 2 balanced translocations. In addition, the absence of Y chromosome in this cell line was also confirmed by both G-banding and mFISH techniques.
The level of CA19-9 in the spent medium of RMCCA-1 cells (72.92 ± 4.35 U/mL) was similar to that of the patient’s serum at admission (85.05 U/mL); both of which were about twice the maximum value of the normal range (39 U/mL, Table 1). The levels of CEA and AFP were normal (Table 1).
| CEA (ng/mL) | AFP (U/mL) | CA 19-9 (U/mL) | |
| 1RMCCA-1 | < 0.200 | < 0.500 | 72.92 ± 4.35 |
| Patient’s serum | 7.56 | 2.24 | 85.05 |
| Normal serum | 0.0-3.4 | 0.0-5.8 | 0.0-39 |
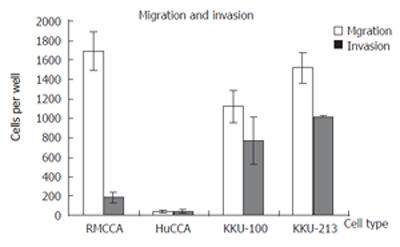
An important characteristic of metastatic cancer is the ability to migrate and invade the underlining basement membrane, the surrounding tissues and the blood vessels. We thus examine the invasiveness and motility of the RMCCA-1 cells, compared with those of three other established Cholangiocarcinoma cell lines from Thai patients (KKU-100, KKU-213 and HuCCA-1), using in vitro invasion and motility assay, respectively[10]. RMCCA-1 exhibited relatively higher migration rate (1,688 ± 207 cells/well) compared with the other 3 cell lines (1,514 ± 152 cells/well, 1,123 ± 163 cells/well, 40 ± 17 cells/well for KKU-213, KKU-100 and HuCCA-1, respectively). Surprisingly, the invasiveness of RMCCA-1 was significantly lower than those of KKU-213 and KKU-100 (181 ± 54 cells/well for RMCCA-1 vs 1021 ± 5 cells/well for KKU-213 and 765 ± 244 cells/well for KKU-100), while slightly higher than that of HuCCA-1 (38 ± 21 cells/well) (Figure 6).
Most metastatic cells secrete proteinases to facilitate its invasion through tissue barriers. One of the most characterized families of tissue-degrading enzymes from cancer is the matrix-metalloproteinases (MMPs). Here we examined the ability to secrete the MMPs from RMCCA-1 cells by gelatin zymography. KKU-100 secretes a high level of Mr 72 000 band previously shown to correspond to MMP-2 (Figure 7)[11,12]. A Mr 72 000 band is also present in FBS. Presumably, this band corresponds to the endogenous gelatinase activity present in the FBS (our unpublished observation). In contrary, the conditioned medium of RMCCA-1 lacks the activity (Figure 7).
Cholangiocarcinoma (CCA) is a cancer arising from the bile duct epithelium. It is a lethal disease of which little is known about its biology, pathogenesis and behavior. Up to presence, there has been no effective early detection protocol or curative strategy for the disease; most patients seek curative treatment at advanced stage with poor prognosis. Therapeutic options for cholangiocarcinoma have been limited due to poor response to chemotherapy and radiation therapy. Surgery is perhaps the only effective cure although the 5-year survival after surgical treatment is less than 40%[2,5]. Therefore, it is urgently required that the molecular markers for early detection are mapped and molecular targets for effective treatment are deciphered. One way to understand the nature of such disease is by studying the behavior of cell lines derived from the tumor in vitro. Here we describe the establishment and preliminary characterization of a cell line derived from a peripheral cholangiocarcinoma of a Thai patient which we have named RMCCA-1.
The population doubling time of this cell line was about 48 h, where those of other cholangiocarcinoma cell lines originated from Thai patients were 55 h for HuCCA1[7] and 72 h for KKU100[8]. Although we have not yet deciphered the mechanism of growth regulation for RMCCA-1, growth regulation mediated by COX-2, PGE2, EGFR and Akt had been demonstrated in a number of cholangiocarcinoma cell lines, including CCLP1, HuCCT1, SG231[13].
Analysis of the spent medium from RMCCA-1 cells showed that these cells have retained some functional characteristics of the original tumor, including over expression of the tumor marker CA19-9. The levels of CA19-9 in the spent medium of RMCCA-1 cells (72.92 ± 4.35 U/mL) was similar to that of the patient's serum at admission (85.05 U/mL); both of which were approximately twice the maximum value of the normal range (39 U/mL, Table 1). CA19-9 was often detected in patients with malignant cholangiocarcinoma and pancreatic cancer[14]. Many kinds of cancer cell lines also secrete CA19-9, including those derived from cholangiocarcinoma (TK)[15], pancreatic carcinoma (SUIT-2)[16] and colon cancer (SW1116)[17].
Analysis by immunofluorescence staining using monoclonal antibodies to AE1 and AE3 showed positive staining for human cytokeratins, verifying the epithelial origin of the tumor. RMCCA-1 also stained positively with antibodies to cytokeratins 7 and 19, distinguishing the bile duct epithelial cells from the hepatocytes. Together, these data confirm that RMCCA-1 indeed derived from the epithelium of the bile duct.
Tumor metastasis involves a series of complex processes including dysregulation of cell adhesion, cell motility, and enzymatic proteolysis of basement membrane and extracellular matrix. The RMCCA-1 cells exhibit a high level of migration rate, contradicting the relatively low level of invasiveness and the absence of MMP activity (Figures 6 and 7). The low level of invasiveness in RMCCA-1 can, at least in part, be explained by the lack of MMP activity in this cell line. Unlike the RMCCA-1 cells, KKU-100 exhibits a high degree of invasiveness, correlating with a high level of MMPs activity (Figures 6 and 7). Karyotype analysis by G-banding revealed a complex pattern of chromosomal abnormalities. Most of the chromosomes were triplicates, suggesting that the cell line was originated from a tripoid cell. The lost of Y chromosome was not unusual as similar finding has been shown in human sarcomatous cholangiocarcinoma (SCK) cells[18]. Other aberrations involving Y chromosome have also been reported, including the translocation between Y chromosome and chromosome 1 as shown in a human cholangiocarcinoma cell line, PCI-SG231[19].
With recent development of mFISH technique, it has become possible to identify and characterize complex chromosomal aberrations, previously unrevealed by G-banding analysis. This technique has allowed us to characterize the karyotype of RMCCA-1 in much greater detail with a high level of accuracy.
We have detected aberrations of chromosomes 1, 5, 7, and 12 in the RMCCA-1 cells, which were consistent with those of the other human cholangiocarcinoma cell lines including SCK, JCK, Cho-CK, Choi-CK, CC-SW-I, CC-LP-I, PCI: SG231, and RPMI-7451, and a rat cholangiocarcinoma cell line, CC-62[18-21]. In contrast, we did not detect structural rearrangement of X chromosome and chromosome 6 in the RMCCA-1 cells, nor did we detect the lost of chromosome 18, as found in SCK, JCK, Cho-CK, and Choi-CK[18].
In conclusion, we successfully established a new cholangiocarcinoma cell line which we named RMCCA-1. We have also performed preliminary characterization of its growth characteristics, karyotype, secreted tumor markers and invasive properties. This cell line will be further used in our research towards understanding and combating against cholangiocarcinoma.
We are grateful to Associate Professor Banchop Sripa who kindly provided KKU-100 and KKU-213 cell lines, and Professor Stitaya Sirisinha who kindly provided HuCCA-1 cell line.
S- Editor Liu Y L- Editor Rippe RA E- Editor Liu WF
| 1. | Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Thursz MR, Wasan H. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2005;51:vi1-vi9. [PubMed] [Cited in This Article: ] |
| 2. | Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 253] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 3. | de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368-1378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 708] [Cited by in F6Publishing: 668] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 4. | Vatanasapt V, Sriamporn S, Vatanasapt P. Cancer control in Thailand. Jpn J Clin Oncol. 2002;32 Suppl:S82-S91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Thongprasert S. The role of chemotherapy in cholangiocarcinoma. Ann Oncol. 2005;16 Suppl 2:ii93-ii96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Sripa B, Pairojkul C. Pathology of Cholangiocarcinoma. In Liver Cancer in Thailand. 2000;84-88. [Cited in This Article: ] |
| 7. | Sirisinha S, Tengchaisri T, Boonpucknavig S, Prempracha N, Ratanarapee S, Pausawasdi A. Establishment and characterization of a cholangiocarcinoma cell line from a Thai patient with intrahepatic bile duct cancer. Asian Pac J Allergy Immunol. 1991;9:153-157. [PubMed] [Cited in This Article: ] |
| 8. | Sripa B, Leungwattanawanit S, Nitta T, Wongkham C, Bhudhisawasdi V, Puapairoj A, Sripa C, Miwa M. Establishment and characterization of an opisthorchiasis-associated cholangiocarcinoma cell line (KKU-100). World J Gastroenterol. 2005;11:3392-3397. [PubMed] [Cited in This Article: ] |
| 9. | MacDougall JR, Bani MR, Lin Y, Rak J, Kerbel RS. The 92-kDa gelatinase B is expressed by advanced stage melanoma cells: suppression by somatic cell hybridization with early stage melanoma cells. Cancer Res. 1995;55:4174-4181. [PubMed] [Cited in This Article: ] |
| 10. | Frandsen TL. Assays for the study of human cancer cell invasion and metastasis. Fibrinolysis. 1992;6:71-76. [Cited in This Article: ] |
| 11. | Quax PH, de Bart AC, Schalken JA, Verheijen JH. Plasminogen activator and matrix metalloproteinase production and extracellular matrix degradation by rat prostate cancer cells in vitro: correlation with metastatic behavior in vivo. Prostate. 1997;32:196-204. [DOI] [Cited in This Article: ] |
| 12. | Muangman S, Thippornwong M, Tohtong R. Anti-metastatic effects of curcusone B, a diterpene from Jatropha curcas. In Vivo. 2005;19:265-268. [PubMed] [Cited in This Article: ] |
| 13. | Xu L, Han C, Wu T. A novel positive feedback loop between PPARdelta and PGE2 signaling pathways for human cholangiocarcinoma cell growth. J Biol Chem. 2006;11:1-44. [Cited in This Article: ] |
| 14. | Ozkan H, Kaya M, Cengiz A. Comparison of tumor marker CA 242 with CA 19-9 and carcinoembryonic antigen (CEA) in pancreatic cancer. Hepatogastroenterology. 2003;50:1669-1674. [PubMed] [Cited in This Article: ] |
| 15. | Watanabe M, Chigusa M, Takahashi H, Nakamura J, Tanaka H, Ohno T. High level of CA19-9, CA50, and CEA-producible human cholangiocarcinoma cell line changes in the secretion ratios in vitro or in vivo. In Vitro Cell Dev Biol Anim. 2000;36:104-109. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 16. | Taniguchi S, Iwamura T, Kitamura N, Yamanari H, Kojima A, Hidaka K, Seguchi K, Setoguchi T. Protein-free culture of the human pancreatic cancer cell line, SUIT-2. Hum Cell. 1994;7:207-214. [PubMed] [Cited in This Article: ] |
| 17. | Adachi M, Sekine T, Umemoto A, Tsukikawa S, Imai K, Yachi A. Mechanism of clearance of circulating CA19-9 in rats. Tumour Biol. 1990;11:51-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Kim DG, Park SY, You KR, Lee GB, Kim H, Moon WS, Chun YH, Park SH. Establishment and characterization of chromosomal aberrations in human cholangiocarcinoma cell lines by cross-species color banding. Genes Chromosomes Cancer. 2001;30:48-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 19. | Storto PD, Saidman SL, Demetris AJ, Letessier E, Whiteside TL, Gollin SM. Chromosomal breakpoints in cholangio-carcinoma cell lines. Genes Chromosomes Cancer. 1990;2:300-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Shimizu Y, Demetris AJ, Gollin SM, Storto PD, Bedford HM, Altarac S, Iwatsuki S, Herberman RB, Whiteside TL. Two new human cholangiocarcinoma cell lines and their cytogenetics and responses to growth factors, hormones, cytokines or immunologic effector cells. Int J Cancer. 1992;52:252-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 76] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Gil-Benso R, Martinez-Lorente A, Pellin-Perez A, Navarro-Fos S, Gregori-Romero MA, Carda C, Callaghan R, Peydro-Olaya A, Llombart-Bosch A. Characterization of a new rat cell line established from 2’AAF-induced combined hepatocellular cholangiocellular carcinoma. In Vitro Cell Dev Biol Anim. 2001;37:17-25. [DOI] [Cited in This Article: ] |