Published online Oct 7, 2006. doi: 10.3748/wjg.v12.i37.5959
Revised: August 2, 2006
Accepted: August 15, 2006
Published online: October 7, 2006
AIM: To study the mechanism of cytotoxicity of a new active 5-alkyl resorcinol [1, 3-dihydroxy-5- (tridec-4’, 7’-dienyl) benzene] isolated from Lithraea molleoides leaves on liver tumor cells.
METHODS: Human hepatocarcinoma cell lines (HepG2 and Hep3B) in culture were treated with inhibitory concentrations, 50% of the compound, for 24 h. The induction of apoptosis was detected in treated cells by analysis of DNA fragmentation, DNA content, and acridine orange and propidium iodide staining.
RESULTS: After 24 h of 5-alkyl resorcinol treatment, both cell lines showed: (1) the typical morphological alterations of apoptosis; (2) DNA fragmentation, detected by laddering and appearance of a subG0 population by flow cytometry; and (3) condensed and fragmented nuclei by acridine orange-propidium iodide staining.
CONCLUSION: Based on the results, this compound exerts its cytotoxic effect in both hepatocellular cell lines through apoptotic cell death. For Hep3B, cells with mutated p53 and Fas, apoptosis would proceed by p53- or Fas-independent pathways.
- Citation: Barbini L, Lopez P, Ruffa J, Martino V, Ferraro G, Campos R, Cavallaro L. Induction of apoptosis on human hepatocarcinoma cell lines by an alkyl resorcinol isolated from Lithraea molleoides. World J Gastroenterol 2006; 12(37): 5959-5963
- URL: https://www.wjgnet.com/1007-9327/full/v12/i37/5959.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i37.5959
Hepatocellular carcinoma is the 5th most common cancer in the world and the 4th most common cause of cancer-associated mortality[1]. Surgical resection and local treatment are frequently limited, as a result of metastasis, cirrhosis, and other pathological changes in the liver parenchyma. The development of chemotherapeutic or chemopreventive agents for hepatocellular carcinoma is important to reduce the mortality caused by this disease. Since cell homeostasis depends on the balance between proliferation and apoptosis, effective compounds inducing apoptosis appear to be a relevant strategy to suppress tumor growth[2]. Cytotoxic drugs cause cell death in sensitive cells, at least partly, by induction of apoptosis.
The Anacardiaceae family comprises many medicinal species from which a number of biologically active substances, such as various phenolic lipids (alkylresorcinols, alkylphenols, alkylcatechols), have been isolated. These compounds present antibacterial, fungicidal and cytotoxic properties[3,4]. In addition, cytotoxic activity on tumor cells (B-16, PC-13, L-5178Y, P-388 and Hep-2) and antitumor activity against S-180 tumors in mice have been reported[5,6]. Lithraea molleoides (Vell.) Engl, a member of the Anacardiaceae family, is a Southamerican tree that grows in Argentina, Brazil and Uruguay[7,8]. We have previously reported cytotoxic activity for the methanol extract of L. molleoides on HepG2 cells[9]. Further activity-guided fractionation of the dichloromethane extract has led to the isolation of a pure bioactive compound, a new cytotoxic 5-alkyl resorcinol (5-AR) derivative: 1,3-dihydroxy-5-(tridec-4’,7’-dienyl) benzene[10].
The aim of the present study was to analyze the mechanism of cytotoxicity of this compound, by studying apoptosis induction on treated HepG2 and Hep3B hepatoma cell lines.
The isolation and characterization of the L. molleoides compound 1, 3-dyhydroxy-5- (tridec-4´, 7´-dienyl) benzene has been previously described[10].
HepG2 and Hep3B cells, derived from human hepatoma, were obtained from the American Type Tissue Collection (ATCC, HB 8065 and HB 8064, respectively). They were cultured in minimal Eagle’s medium (MEM) supplemented with 100 ml/L fetal bovine serum (FBS), 2 mmol/L glutamine, 1.5 g/L sodium bicarbonate, 1.0 mmol/L non-essential aminoacids, 1.0 mmol/L sodium pyruvate, and incubated at 37°C in a humidified atmosphere containing 5% CO2.
The cytotoxicity assay was carried out as previously described[9]. Briefly, 5 × 103 of HepG2 or Hep3B cells in growth medium were seeded in each well of a 96 well-microtiter plate, and incubated for 24 h at 37°C. Different concentrations of the 5-AR (in quadruplicates) were added to the exponentially growing cells. Cell controls in absence of the compound were included. After an incubation period of 24 h at 37°C, the MTT assay was performed following the manufacturer’s instructions. The absorbance values at 546 nm and 650 nm were recorded in an ELISA plate reader (Meterech 960). The 50% inhibitory concentrations (IC50) for both cell lines were determined by linear regression from dose-response curves.
Approximately 1-2 × 106 of control or treated cells were harvested and washed twice with PBS. DNA was extracted by the Wizard Genomic DNA Purification Kit (Promega), according to the manufacturer´s instructions. The DNA pellet was washed, air-dried, resuspended and electrophoresed on 1% agarose gel at 50 volts for 3 h. The gel was visualized under UV transilluminator and photographed.
After a 24-h 5-AR treatment, control or treated cells were harvested and washed twice with 1 g/L bovine serum albumin (BSA) in PBS. They were fixed in 700 mL/L ethanol for 1 h at 4°C, washed twice with 1 g/L BSA in PBS and resuspended in the same buffer. After the addition of a DNA extraction buffer (192 mmol/L PO4HNa2; 4 mmol/L citric acid; pH 7.8), incubation at room temperature (RT) for 5 min and centrifugation, the cells were stained with propidium iodide (Sigma, 50 µg/mL) and treated with RNAse A (Sigma, 0.5 mg/mL) for 30 min at RT. Cells were analyzed on a flow cytometer (FACSCalibur, Becton Dickinson) containing an argon laser (488 nm). The red fluorescence of propidium iodide, proportional to the DNA content, was collected through a 620 ± 15 nm band pass filter. A minimum of 10 000 cells per sample was collected and DNA hystograms were further analyzed by the WinMDI 2.8 program.
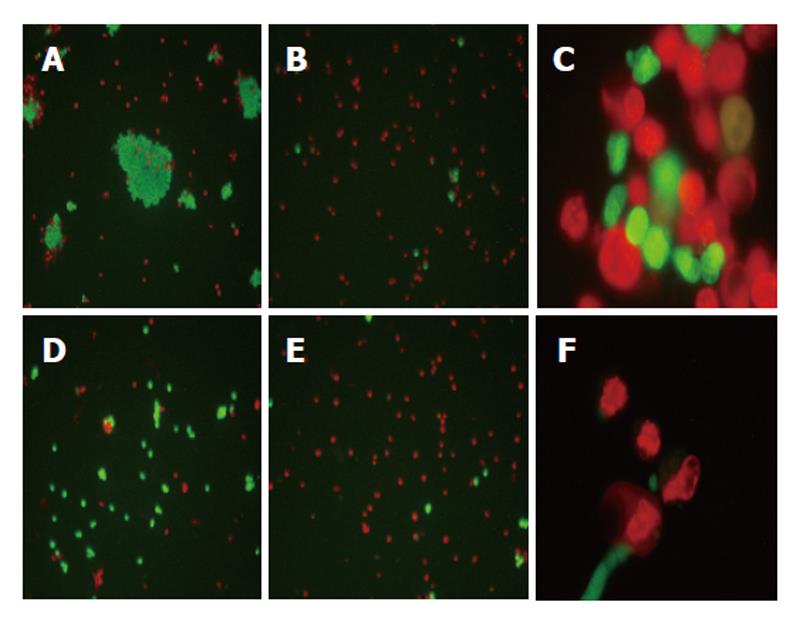
Approximately 1 × 106 of control or treated cells were resuspended in 5 mL of PBS containing 5 µg/mL of acridine orange (Sigma) and 5 µg/mL of propidium iodide (Sigma). The cell suspension was immediately dispensed onto slides, viewed under fluorescent microscopy (Nikon Eclipse 400) and photographed (Nikon Coolpix 4500).
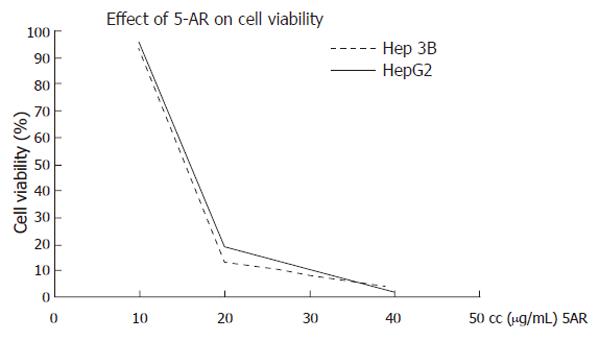
The cytotoxicity of 5-AR on HepG2 and Hep3B human hepatoma cell lines was assessed by a cell viability assay, in the presence of different concentrations of the compound for 24 h. Under these experimental conditions, 5-AR exhibited a significant cytotoxic effect on both HepG2 and Hep3B cells. The IC50 values for HepG2 and Hep3B were interpolated from linear regression curves: 13.12 µg/mL (45.49 µmol/L) and 12.45 µg/mL (43.17 µmol/L), respectively (Figure 1). Both viability curves for 5-AR were practically overlapped, suggesting a parallel effect for cell death in both cell lines. This effect is independent of the p53 status of the cells, as cytotoxicity by 5-AR was similar for HepG2, which posses wild-type p53, and Hep3B, which are p53-deficient.
Based on the detected cytotoxic activity, the effect of the compound on cell apoptosis induction was examined in both cell lines by different methods.
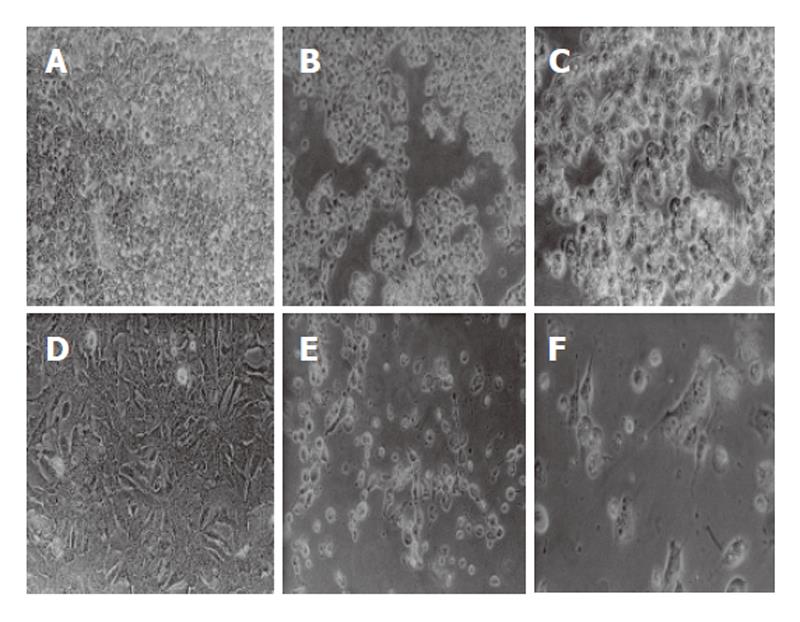
Apoptosis, characterized by cell shrinkage, membrane blebbing, nuclear pyknosis, chromatin condensation, and genomic fragmentation, represents a universal and exquisitely efficient cellular suicide pathway. The cytoplasm condenses and cells shrink to finally form apoptotic bodies. HepG2 or Hep3B cells were treated for 24 h with the previously determined 24 h IC50 of the compound. After this period, the cells were observed under contrast phase microscopy (Nikon TMS) and photographed (Nikon FDX-35). The observation of cell morphology revealed that 5-AR-treated cells showed significant morphological changes compatible with programmed cell death (Figure 2). The effects on cell morphology were dose-dependent (data not shown).
DNA fragmentation, the typical hallmark of apoptosis, was analyzed by DNA laddering on agarose gels and by the appearance of a subdyploid cell population with lower DNA content by flow cytometry.
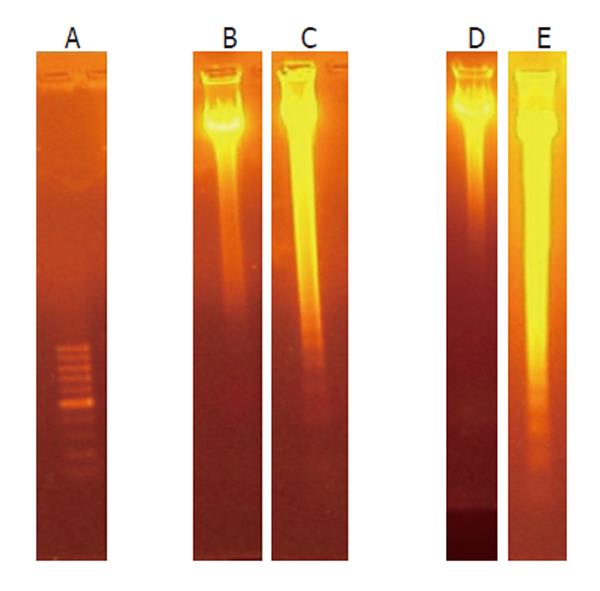
The apoptotic process leads to fragmentation of cellular DNA in characteristic oligonucleosomal fragments, multiples of 200 base pairs. HepG2 and Hep3B cells, treated for 24 h with 5-AR, showed the typical DNA ladder on agarose gels (Figure 3). The intensity of this typical DNA ladder of multiples of 200 base pairs fragments was dose- and incubation time-dependent.
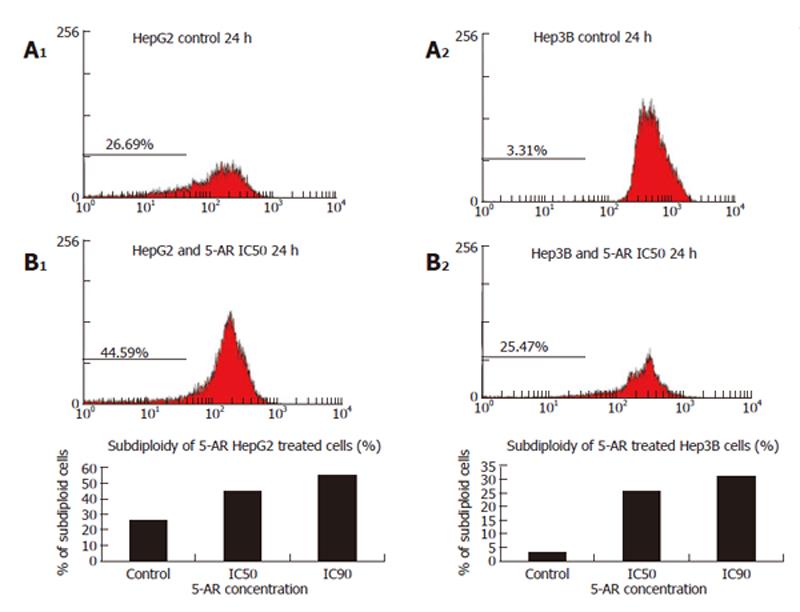
These results were further confirmed by flow cytometer hystograms obtained from treated cells, stained with propidium iodide. For HepG2 cells, the 5-AR treatment produced a significant increase in the percentage of the subG0 cell population (control cells: 26.09%; IC50: 44.59%). For Hep3B cells, the 5-AR treatment also produced a significant elevation of the subG0 cell population (control cells: 3.39%; IC50: 25.47%). The typical hypoploid population or subG0 peak, corresponding to cells with low DNA content, is shown in cytometry hystograms (Figure 4).
Control or 5-AR-treated HepG2 and Hep3B cells were stained with acridine orange and propidium iodide. A significant increase in the percentage of red cells and in condensed and fragmented nuclei were observed in treated cells, all these findings compatible with apoptotic cell death (Figure 5).
The search for novel anticancer drugs from natural sources has continued through the collaboration of scientists worldwide in looking for new bioactive compounds[12]. Experimental agents derived from natural products offer opportunities to evaluate not only totally new chemical classes of anticancer agents, but also novel and potentially relevant mechanisms of action[13].
With the aim of searching for new cytotoxic compounds from Argentine medicinal plants, a 5-AR was isolated from L. molleoides. The dose-dependent cytotoxic activity detected on many human tumoral cell lines suggested that it may contain some kind of antitumoral activity[10]. Due to the relevance of human hepatocarcinoma and the lack of available successful treatments, human hepatocarcinoma cells were selected to study the 5-AR cytotoxicity and to deepen into the mechanism of cell death induced by this compound.
Apoptotic pathways are involved in the cytotoxic mechanism of antitumoral drugs. Some anticancer drugs are known to upregulate Fas ligand, leading to its interaction with Fas and triggering the apoptotic pathway. On the other hand, p53 exerts its effects on cells as a transcription factor. An increase in p53 leads to the expression of pro-apoptotic proteins, which prompt cells to undergo apoptosis. It has been reported that p53-dependent apoptosis modulates the cytotoxicity of anticancer agents[14].
Considering the importance of Fas and p53 in hepatocyte cell death, two cell lines were selected based on their differential p53 and Fas phenotype. While HepG2 cells exhibit normal expression of both proteins, Hep3B cells present mutated p53 and Fas[15]. In this work, the treatment of HepG2 and Hep3B with IC50 doses of the 5-AR induced apoptosis in both cell lines, as evidenced by all the methodologies used in this work. Experiments with lower concentrations of the compounds also showed apoptosis induction in both cell lines (data not shown).
The fact that 5-AR can induce programmed cell death in Hep3B with non-functional p53[15] and Fas[16] evidences that the cytotoxic effect of 5-AR in this cell line is independent of their p53 or Fas phenotypic profile. As p53 is the most common mutated gene in hepatocellular carcinomas, it is important to have cytotoxic compounds that exert their apoptotic activity in a p53-independent pathway to treat this kind of tumors.
The induction of apoptosis is known to be an efficient strategy for cancer therapy[17]. Recently, many plant extracts have demonstrated to posses the ability of triggering the apoptotic pathway[18,19]. The present study demonstrates that a pure compound isolated from L. molleoides is highly cytotoxic and presents apoptogenic activity on human hepatocarcinoma cell lines. The molecular mechanistic pathway involved in this process will be studied in our future experiments. Our results and the previously reported cytotoxic and antitumoral activities of other alkyl resorcinols justify further in vitro and in vivo studies to evaluate the potential use of this 5-AR as an antitumoral agent for hepatocellular carcinoma.
The authors are grateful to Lic. Alejandra Hidalgo (Centro de Biologia y Medicina Experimental, Hospital Italiano de Buenos Aires) for assistance in phase contrast and fluorescence microscopy images.
S- Editor Liu Y L- Editor Kumar M E- Editor Liu WF
| 1. | McGlynn KA, Tsao L, Hsing AW, Devesa SS, Fraumeni JF. International trends and patterns of primary liver cancer. Int J Cancer. 2001;94:290-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 234] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000;256:42-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 845] [Cited by in F6Publishing: 860] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 3. | Kozubek A, Zarnowski R, Stasiuk M, Gubernator J. Natural amphiphilic phenols as bioactive compounds. Cell Mol Biol Lett. 2001;6:351-355. [Cited in This Article: ] |
| 4. | Chaturvedula VS, Schilling JK, Miller JS, Andriantsiferana R, Rasamison VE, Kingston DG. New cytotoxic bis 5-alkylresorcinol derivatives from the leaves of Oncostemon bojerianum from the Madagascar rainforest. J Nat Prod. 2002;65:1627-1632. [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Kozubek A, Tyman JH. Resorcinolic Lipids, the Natural Non-isoprenoid Phenolic Amphiphiles and Their Biological Activity. Chem Rev. 1999;99:1-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 415] [Cited by in F6Publishing: 355] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 6. | Arisawa M, Ohmura K, Kobayashi A, Morita N. A cytotoxic constituent of Lysimachia japonica THUNB. (Primulaceae) and the structure-activity relationships of related compounds. Chem Pharm Bull (Tokyo). 1989;37:2431-2434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Toursarkissian M. Plantas Medicinales de la Argentina. 1980;. [Cited in This Article: ] |
| 8. | Muñoz J. Usos principales de las especies de Anacardiaceae, particularmente del Paraguay. Candollea. 1990;45:671-680. [Cited in This Article: ] |
| 9. | Ruffa MJ, Ferraro G, Wagner ML, Calcagno ML, Campos RH, Cavallaro L. Cytotoxic effect of Argentine medicinal plant extracts on human hepatocellular carcinoma cell line. J Ethnopharmacol. 2002;79:335-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | López P, Ruffa MJ, Cavallaro L, Campos R, Martino V, Ferraro G. 1,3-dihydroxy-5-(tridec-4',7'-dienyl)benzene: a new cytotoxic compound from Lithraea molleoides. Phytomedicine. 2005;12:108-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Valcic S, Wachter GA, Eppler CM, Timmermann BN. Nematicidal alkylene resorcinols from Lithraea molleoides. J Nat Prod. 2002;65:1270-1273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Cragg GM, Newman DJ. Discovery and development of antineoplastic agents from natural sources. Cancer Invest. 1999;17:153-163. [PubMed] [Cited in This Article: ] |
| 13. | Mukherjee AK, Basu S, Sarkar N, Ghosh AC. Advances in cancer therapy with plant based natural products. Curr Med Chem. 2001;8:1467-1486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 229] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 14. | Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2022] [Cited by in F6Publishing: 2048] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 15. | Bressac B, Galvin KM, Liang TJ, Isselbacher KJ, Wands JR, Ozturk M. Abnormal structure and expression of p53 gene in human hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:1973-1977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 321] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Jiang S, Song MJ, Shin EC, Lee MO, Kim SJ, Park JH. Apoptosis in human hepatoma cell lines by chemotherapeutic drugs via Fas-dependent and Fas-independent pathways. Hepatology. 1999;29:101-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78:539-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 906] [Cited by in F6Publishing: 966] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 18. | Liu J, Shen HM, Ong CN. Salvia miltiorrhiza inhibits cell growth and induces apoptosis in human hepatoma HepG(2) cells. Cancer Lett. 2000;153:85-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Liu J, Shen HM, Ong CN. Role of intracellular thiol depletion, mitochondrial dysfunction and reactive oxygen species in Salvia miltiorrhiza-induced apoptosis in human hepatoma HepG2 cells. Life Sci. 2001;69:1833-1850. [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |