Published online Sep 21, 2006. doi: 10.3748/wjg.v12.i35.5680
Revised: March 24, 2006
Accepted: March 27, 2006
Published online: September 21, 2006
AIM: To determine the prevalence of osteoporosis in a cohort of patients with Crohn’s disease (CD) and to identify the relative significance of risk factors for osteoporosis.
METHODS: Two hundred and fifty-eight unselected patients (92 M, 166 F) with CD were studied. Bone mineral density (BMD) was measured at the lumbar spine and hip by dual X-ray absorptiometry. Bone formation was assessed by measuring bone specific alkaline phosphatase (BSAP) and bone resorption by measuring urinary excretion of deoxypyridinoline (DPD) and N-telopeptide (NTX).
RESULTS: Between 11.6%-13.6% patients were osteoporotic (T score < -2.5) at the lumbar spine and/or hip. NTX levels were significantly higher in the patients with osteoporosis (P < 0.05) but BSAP and DPD levels were not significantly different. Independent risk factors for osteoporosis at either the lumbar spine or hip were a low body mass index (P < 0.001), increasing corticosteroid use (P < 0.005), and male sex (P < 0.01). These factors combined accounted for 23% and 37% of the reduction in BMD at the lumbar spine and hip respectively.
CONCLUSION: Our results confirm that osteoporosis is common in patients with CD and suggest that increased bone resorption is the mechanism responsible for the bone loss. However, less than half of the reduction in BMD can be attributed to risk factors such as corticosteroid use and low BMI and therefore remains unexplained.
- Citation: Bartram SA, Peaston RT, Rawlings DJ, Walshaw D, Francis RM, Thompson NP. Mutifactorial analysis of risk factors for reduced bone mineral density in patients with Crohn’s disease. World J Gastroenterol 2006; 12(35): 5680-5686
- URL: https://www.wjgnet.com/1007-9327/full/v12/i35/5680.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i35.5680
Osteoporosis is now recognised as a common complication of inflammatory bowel disease (IBD) and in particular Crohn’s disease (CD). Estimates of prevalence vary but those studies employing the World Health Organisation[1] diagnostic criteria (a bone density 2.5 or more standard deviation units below the mean value for young adults) report rates of 13%-42%[2-4]. The pathogenesis of osteoporosis in patients with CD is likely to be multifactorial. Most, but not all studies, have found an association with current or cumulative corticosteroid use[5-11] but other factors such as disease duration[3,4,12] low body weight or body mass index[4,10,13] calcium and vitamin D deficiency[12,14] small bowel involvement or resection[10] smoking[15] gender and increasing age[13] have been implicated. At the molecular level in vitro studies have demonstrated that serum from children with CD can inhibit bone formation suggesting that pro-inflammatory cytokines such as IL-6 may be involved[16].
The mechanism of bone loss is poorly understood. Normal bone in healthy adults is in a state of equilibrium, the rate of osteoblastic bone formation equaling the rate of bone resorption by osteoclasts. Biochemical markers of bone turnover such as deoxypyridinoline (DPD) and cross-linked N-telopeptides of type 1 collagen (Ntx), both markers of bone resorption, and osteocalcin and bone specific alkaline phosphatase (BSAP), markers of bone formation, are now available and have been used in several studies examining the possible mechanisms of bone loss in patients with IBD. Consistent findings are raised levels of either DPD or Ntx[2,4,12,17,18] suggesting increase in bone resorption, although there is also evidence that bone formation is reduced[7].
The aim of this cross sectional study was to determine the prevalence of osteoporosis in an unselected group of patients with CD and to identify the relative importance of possible risk factors and the mechanism of bone loss.
Patients were recruited from the IBD register at the Freeman Hospital and additionally from the Royal Victoria Infirmary, also in Newcastle-upon-Tyne, and the Queen Elizabeth Hospital, Gateshead. Patients were under the care of either surgical or medical gastroenterologists and all fulfilled at least two of four diagnostic criteria (histological, radiographic, endoscopic and surgical)[19]. Patients were contacted by letter inviting them to participate in the study and were then seen at the Freeman Hospital by either SB or NT. Those who did not attend were sent one further letter reminding them to do so. We included patients aged between 25 and 70 years only and excluded women who were pregnant or planning a pregnancy because of the potential risk from exposure to ionizing radiation during bone densitometry.
A questionnaire was completed with the patient detailing age, tobacco and alcohol consumption, fracture history, and in women, reproductive and menstrual history. Details of duration and site of disease, corticosteroid use (expressed as number of months on corticosteroids), relevant surgical history and drug history were obtained at interview and by careful scrutiny of the medical notes. Height and weight was measured immediately prior to bone densitometry and these figures were used to calculate body mass index [weight/height2, (kg/m2)]. The study was approved by the Newcastle-upon-Tyne Joint Ethics Committee.
Bone mineral density (BMD) was measured at the lumbar spine (L1-L4) and left hip (total hip) by dual X-ray absorptiometry (Hologic Inc. QDR 2000, Waltham, MA.). The coefficient of variation (CV) is 0.7% at the lumbar spine and 1.0% at the hip[20]. BMD results are expressed as an areal density in g/cm2, but have been compared with the manufacturer’s mean value for young adults and for normal people of the same age and sex to give T scores and Z scores respectively. The T score is the number of standard deviation units above or below the mean value for young adults of the same sex, whilst the Z score is the number of standard deviation units above or below the age related mean value. The WHO has defined osteopenia as a T score of < -1 but > -2.5, whilst osteoporosis is defined as a T score of -2.5 or lower. These diagnostic criteria are not necessarily thresholds for intervention, particularly for patients on oral corticosteroids, where a higher T score of -1.5 would be more appropriate[21].
Serum and urine samples were taken between 2 pm and 4 pm. The serum was immediately centrifuged and all samples were stored at -30°C. Serum calcium and phosphate levels were measured using standard methods on an Olympus 600 automated system, (interassay CVs for calcium and phosphate are < 1% and < 2% respectively).
Bone resorption was assessed by measurement of the urinary excretion of free DPD and Ntx with values expressed as a fraction of urinary creatinine excretion. Free DPD was measured by competitive immunoassay (Chiron Diagnostics Corporation, MA) (interassay CV < 8%). The reference range (manufacturer’s data), is 3.0-7.4 nmol DPD/mmol creatinine for females aged 25-44 years and 2.3-5.4 nmol DPD/mmol creatinine for males aged 25-55 years. Ntx was measured by enzyme-linked immunoabsorbant assay (“Osteomark”, Ostex International, Seattle, WA) with results expressed as bone collagen eqivalents (BCE). The reference ranges for men and women (manufacturer’s data) are 3-51 and 5-65 nm/mmol creatinine respectively (interassay CVs < 9%). Bone formation was assessed by measurement of serum BSAP using enzyme-linked immunoabsorbant assay (Metra BioSystems, Southampton, UK) (interassay CV < 6%).
Patients with a T score of -1.5 or less at either the lumbar spine were investigated further to look for other secondary causes of osteoporosis, such as vitamin D deficiency with secondary hyperparathyroidism, thyroid disease and, in men, hypogonadism.
Intact parathyroid hormone (PTH) levels, reference range 12-72 ng/L, were measured by immunometric assay (Immulite Intact PTH, Diagnostics Products Corporation, Los Angeles, CA.) (interassay CV < 5%). Vitamin D levels (25 OH Cholecalciferol and 25 OH Ergocalciferol), normal range 10-50 nmol/L, were assayed by high performance liquid chromatography performed at the Department of Clinical Biochemistry, Royal Victoria Infirmary, Newcastle-upon-Tyne (interassay CV < 12%). Thyroid stimulating hormone (TSH), reference range 0.3-4.1 Mu/L, was measured with a two site immunoassay using direct chemiluminometric detection and monoclonal/polyclonal antibodies (interassay CV < 4.0%). Testosterone, reference range 9-20 nmol/L, was measured with a competitive immunoassay using direct chemiluminometric detection and a polyclonal antibody (interassay CV < 5.2%) and Sex Hormone Binding Globulin (SHBG) was measured with a non-competitive immunoradiometric assay (interassay CV < 5%). The free androgen index (FAI), reference range > 3 nmol/nmol, was calculated after dividing the total serum testosterone by the SHBG level.
The results are expressed as means ± SD. Comparison between group means was anlaysed using Student’s unpaired t-test or, where data was not normally distributed, the Mann-Whitney U-test. Multiple regression analysis was performed to determine independent risk factors for osteoporosis and the adjusted χ2 value was calculated to determine the proportion of the variability in BMD attributable to these factors. The chi-squared test was used to compare incidences. A P value of < 0.05 was taken to be statistically significant. Analyses were made with GraphPad Prism (GraphPad Software Inc) and Minitab (Version 12.1, Minitab Inc).
Two hundred and sixty five patients responded to the correspondence inviting them to participate in the study, 7 of whom subsequently declined a DXA scan and were excluded from the study. The remaining 258 patients who completed the study comprised 92 men and 166 women. One patient was of Asian descent, the remainder were white. Table 1 summarizes the clinical characteristics of the group.
| n | 258 (166 F, 92 M) |
| Age (yr) | 44.5 (± 11.5) |
| Postmenopausal women | 67 (25.9%) |
| Fractures | 28 (10.8%) |
| BMI (kg/m2) | 24.7 (± 4.6) |
| Disease duration (yr) | 14.3 (± 9.5) |
| Small bowel involvement | 192 (74.4%) |
| Small bowel resection | 135 (52.3%) |
| Large bowel involvement | 173 (67.1%) |
| Current CS use | 77(29.8%) |
| Previous CS use | 152 (58.9%) |
| Duration CS use (mo) | 51.0 (± 68.4) |
| Current smokers | 83 (32.2%) |
Twenty-eight patients (10.9%) reported a low trauma fracture in adulthood, 12 of whom had osteoporosis. The fractures comprised wrist[9], forearm[5] and vertebral[3] fractures, and 2 each of metatarsal, rib and ankle fractures, 1 patella fracture and 1 femoral fracture. Two patients had more than 1 fracture and in 5 cases the fracture site was not documented.
One hundred and ninety two (74.4%) had small bowel disease, of which 70 (27.1%) had small bowel involvement alone and 88 (34.1%) had small bowel involvement with colonic disease. Forty-nine (18.9%) had isolated colonic disease. At least one partial small bowel resection had been performed in 135 (52.3%) of the study group, whilst 92 (35.7%) had undergone partial or total resection of their large bowel.
Seventy-seven patients (29.8%) were currently taking oral corticosteroids and 152 (58.9%) had previously taken corticosteroids to treat complications of their Crohn’s disease. Forty-five patients (17.4%) were currently taking bone active treatment (calcium supplementation with or without vitamin D, bisphosphonates or hormone replacement therapy).
Eighty-three patients (32.2%) were current cigarette smokers and 78 (30.2%) previous smokers. The mean alcohol intake was 8.8 units/week among the 160 patients who drank alcohol.
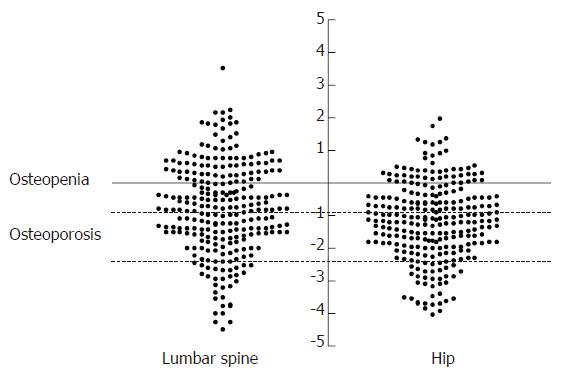
Measurements at the lumbar spine were available in 257 patients, one was excluded because of the presence of surgical metal rods. Measurements at the hip were available in all 258 patients. Details of the mean BMD measurements, mean Z and T scores and numbers with osteopenia or osteoporosis are presented in Table 2 and Figure 1. Employing the WHO definitions for osteoporosis 18 (6.9%) were osteoporotic at both the lumbar spine and hip. Considering each site separately, 30 (11.6%) patients were osteoporotic at the lumbar spine (16 men, 14 women) and 35 (13.6%) were osteoporotic at the hip (15 men, 20 women) whilst a further 77 (29.8%) and 67 (25.9%) fulfilled the criteria for osteopenia at the lumbar spine and hip respectively.
| Site | BMD(g/cm2) | Z score | T score | Osteopenia | Osteoporosis |
| Lumbar spine | 0.975 ± 0.15 | -0.308 ± 1.39 | -0.795 ± 1.39 | 77 (29.8%) | 30 (11.6%) |
| Total hip | 0.860 ± 0.15 | -0.675 ± 1.19 | -1.181 ± 1.21 | 67 (25.9%) | 35 (13.6%) |
Table 3 shows the characteristics of those patients with and without osteoporosis. At the lumbar spine patients with osteoporosis had a significantly lower BMI and had taken corticosteroids for longer, there was a trend towards longer disease duration although this did not achieve statistical significance. Patients with osteoporosis at the hip were also likely to have a lower BMI, a longer disease duration and have taken corticosteroids for longer. Gender, small bowel resection and current tobacco use were not significant factors at either site. One hundred and fifteen patients (44.6%) had T scores < -1.5 at the lumbar spine and/or hip respectively. The T score within individual patients was significantly lower at the hip compared with the lumbar spine (-0.80 ± 1.4 vs -1.19 ± 1.2, P < 0.0001).
| Risk factor | Osteoporosis atlumbar spineand/or hip( n = 47) | Osteopenia/normal bonemineral density(n = 211) | P |
| BMI (kg/m2) | 22.3 ± 0.6 | 25.2 ± 0.3 | < 0.0001 |
| Corticosterid use (mo) | 92.3 ± 16.7 | 34.9 ± 3.2 | < 0.0001 |
| Disease duration (yr) | 18.9 ± 1.5 | 13.2 ± 0.6 | < 0.005 |
| Age (yr) | 46.8 ± 11.4 | 44.1 ± 11.5 | NS |
| Small bowel resection (Y/N) | 10/37 | 105/106 | NS |
| Current smokers (Y/N) | 11/36 | 72/139 | NS |
| Gender (M/F) | 22/25 | 69/142 | NS |
Postmenopausal women had significantly lower T scores at the lumbar spine and hip compared to the rest of the study group (-1.30 ± 0.16 vs -0.63 ± 0.10 and -1.5 ± 0.14 vs -1.09 ± 0.09 respectively, P < 0.0001). This group accounted for 11/29 patients with osteoporosis at the spine and 12/34 at the hip.
Multivariate analysis was used to determine independent predictors for the presence of osteoporosis. Variables included in the analysis were age, sex, disease duration, BMI, length of corticosteroid use and small bowel resection. Of these, decreasing BMI (P < 0.001), length of corticosteroid use (P = 0.003) and male sex (P = 0.009) were the significant predictors at the lumbar spine whilst at the hip decreasing BMI (P < 0.001), number of months treatment with corticosteroids (P < 0.001), increasing age (P = 0.003) and male sex (P = 0.005) were significant. The adjusted r2 value was calculated using sex, age, sex-age interaction, corticosteroid use, BMI, disease duration and small bowel resection (Yes/No) as variables. These factors accounted for 22.9% and 37.2% in the variability in BMD at the lumbar spine and hip respectively.
The mean levels of total calcium and phosphate were normal (Table 4). There was no difference in the means between those with and those without osteoporosis. Patients with a T score < -1.5 at either site (n = 115) were investigated further and in these patients mean levels of TSH and 25 OHD were normal (Table 5). Five patients had abnormal thyroid function tests. Two patients had low TSH levels, one of these patients was on thyroid replacement therapy. Of the three with above normal TSH levels, none had abnormal tri-iodothyrine or thyroxine levels. Two patients had biochemically low 25 OHD levels (defined as < 10 mol/L) but neither was associated with a secondary hyperparathyroidism or with small bowel resection. Mean PTH levels were normal. PTH levels were raised (defined as twice the upper limit of normal) in 4 patients (range 153-613 ng/L) including one patient with severe nutritional deficiency (BMI < 19, anaemia, hypoalbuminaemia) and severe osteoporosis who nevertheless had normal 25 OHD levels because of treatment with vitamin D metabolites prior to inclusion into the study. Three men (6.5%) were hypogonadal (FAI < 3 nmol/nmol).
| Biochemistry(normal range) | Osteoporosis atlumbar spineand/or hip(n = 47) | Osteopenia/normal bonemineral density(n = 211) | P |
| Total calcium (2.12-2.55 mmol/L) | 2.30 ± 0.03 | 2.31 ± 0.01 | NS |
| Phosphate (0.65-1.3 mmol/L) | 1.11 ± 0.03 | 1.11 ± 0.03 | NS |
| BSAP (11.6-30.6 μg/L) | 19.7 ± 1.4 | 18.6 ± 0.6 | NS |
| DPD/creat (2.3-7.4 nmol/mmol) | 5.8 ± 1.0 | 5.6 ± 0.4 | NS |
| Ntx/creat (3.0-65.0 BCE) | 63.7 ± 9.5 | 40.6 ± 3.6 | P < 0.05 |
Mean levels of BSAP and DPD were normal, there was no significant difference between those with and without osteoporosis (Table 4). Ntx levels, although also within the normal range, were significantly higher in patients with osteoporosis.
We have shown that between 11%-13% of a large group of unselected patients with CD are osteoporotic. A low BMI, increasing exposure to corticosteroids and male sex appear to be the main predictors of osteoporosis which, however, only explain between 23%-37% of the variability in BMD described. Biochemical abnormalities such as 25 OHD deficiency and secondary hyperparathyroidism, thyroid disease and male hypogonadism are unusual but our results suggest that increased bone resorption, assessed by measuring urinary excretion of DPD and Ntx, may be the mechanism responsible for the accelerated bone loss.
As far as we are aware this is the largest and most complete study to date of the prevalence of osteoporosis in patients with CD. Our group of patients are unselected and we consider a reasonable representation of the larger population with CD in terms of age and sex composition, duration and site of disease and disease management. Between 11%-13% of our patients are osteoporotic. This is perhaps lower than we expected given the unselected nature of our patient group, although broadly in keeping with other studies using the same diagnostic criteria. However, there is considerable discrepancy among prevalence rates reported. This may be accounted for by differences in the site and the methods used to measure BMD and in the patient groups studied, some for example including those not only with CD but also ulcerative colitis (UC), where there is evidence that osteoporosis is less common[9,22]. Other groups have studied patients with CD only and have selected further to exclude for example those currently taking corticosteroids or at risk of metabolic bone disease[2].
We found that the BMD at the hip was significantly lower compared with the lumbar spine. This has previously been reported in other studies of patients with IBD[2-4,8,23] and is also the pattern of bone loss seen in other chronic inflammatory conditions such as rheumatoid arthritis. It may reflect a degree of degenerative change at the lumbar spine in some of the older patients which would spuriously elevate the density at that site, however we attempted to minimise this effect by excluding patients over the age of 70.
The clinical significance of osteoporosis is the increased susceptibility to fracture and the resulting morbidity and mortality. Eleven percent of our patients reported low trauma fractures, mostly of the upper limb. We did not perform radiography in our patients and, as many vertebral fractures are asymptomatic, the true prevalence is likely to be higher. Relatively few studies have examined fracture incidence and prevalence in adult patients with CD. In retrospective studies, fracture prevalence, calculated from plain radiography of symptomatic areas or self reported fracture, varies between 7% and 27%[7,9,12]. Vestergaard et al, in a study of over 800 patients with IBD[24] conducted via a postal questionnaire, reported an increased relative risk of low trauma fractures in women with CD of 2.5. The increase in fracture risk was seen mostly in premenopausal women but men with CD or patients of either sex with UC were not at increased risk of fracture. The authors speculated that their findings were due to a number of factors including the increased use of continuous corticosteroids and more severe systemic inflammation in CD compared to UC, and possible hypogonadism in the female patients.
A low BMI, increasing corticosteroid use, male sex and increasing age were independent risk factors in predicting those with osteoporosis although these factors together only accounted for between 20%-40% of the variability in BMD. Low body weight or a low BMI have been reported in other studies of patients with IBD to be significant risk factors for osteoporosis, particularly at the peripheral sites[4-6,9-11]. Within the context of IBD a low BMI may merely reflect severe inflammatory disease and/or malabsorption although there is also good evidence that low body mass per se is a significant factor in determining BMD[25,26].
In our study male sex was a significant independent risk factor for osteoporosis at both sites. Most groups have reported no significant differences between the sexes and only one study has looked specifically at men with IBD. Robinson et al studied the hormone profile of 48 men with CD[27] and found biochemical evidence of hypogonadism in only 3 patients, 2 of whom had osteopenia. Three of our male patients (6.5%) with reduced BMD were hypogonadal. This is lower than we might expect given the number of our patients taking corticosteroids which might lead to suppression of gonadotrophin, testosterone and SHBG levels.
The role of corticosteroids in the pathogenesis of osteoporosis in patients with IBD is complex. Whilst some studies have shown a clear relationship between lifetime corticosteroid dose and vertebral fracture rate[5] or low BMD other studies have suggested that BMD is unrelated to corticosteroid use[2,3,6,10]. There have been several prospective studies examining the role of corticosteroids in the rate of bone loss[22,28,29]. The largest of these[28], a longitudinal study of over 100 patients with IBD and bowel resection who were followed up for a mean period of over 5 years found no relationship between bone loss and corticosteroid use. The conflicting results are probably a reflection of the heterogeneity of patient groups studied and the complex relationship between disease severity, systemic inflammation and treatment with corticosteroids.
We found a very low prevalence of biochemical abnormalities in our patients and in particular no evidence of 25 OHD deficiency and secondary hyperparathyroidism or of a correlation with small bowel resection. The relationship between 25 OHD levels and BMD in patients with IBD is not clear cut. Andreassen[14] reporting one of the higher prevalences of 25 OHD deficiency (44%) in a study of 115 patients with CD nevertheless found that this, in combination with PTH levels, only accounted for 4% of the variation in BMD. Croucher[30] described the histomorphometric findings in a study of 19 patients with IBD, all of whom had osteoporosis and 16 of whom had undergone bowel resection. Despite only one patient having a low 25 OHD level, there was evidence of a mild mineralisation defect in the patient group compared with the control group, although none had the classical changes associated with osteomalacia.
Biochemical markers of bone formation and resorption are now widely available and have been used to predict bone loss[31,32] and response to treatment of osteoporosis with bisphosphonates and hormone replacement therapy (HRT) in postmenopausal women[33,34]. Several studies have now used these markers in exploring the mechanisms of bone loss in patients with IBD. Most studies have demonstrated an increase in bone resorption without a corresponding increase in bone formation[2,4,12,17,18]. Our results, which show that patients with osteoporosis have significantly higher levels of Ntx (but not DPD), would support the results of these studies. It should be noted however that the mean value, even in patients with osteoporosis, was within the normal range and their role in predicting those patients with IBD at risk of losing bone is not clear. Pollak et al has reported that increased levels of Ntx predicted rates of bone loss in a prospective study of 36 patients with IBD over a period of 2 years[35] whilst Schulte[29] found that they did not discriminate between those with accelerated bone loss and those without. Despite this they may be useful in targeting those patients to treat more aggressively with anti-resorptive agents such as HRT and the bisphosphonates.
Management of patients with IBD and osteoporosis remains problematic although recent guidelines for the management and prevention of osteoporosis in IBD have been published in the United Kingdom[36]. The guidelines recommend, amongst other measures, that all patients currently taking corticosteroids and with a T score of < -1.5 should be prescribed a bisphosphonate in addition to vitamin D supplementation. This study highlights the potential clinical implications of this strategy, as approximately 20% of our patients would require bisphosphonates if these guidelines were implemented.
We have also established that although there are several significant risk factors for osteoporosis, these combined explain less than 40% of the variability in BMD. A genetic predisposition to osteoporosis complicating other chronic conditions such as rheumatoid arthritis has been described[37]. This may be of significance in our population and merits further investigation.
We would also like to acknowledge the following consultant staff who gave permission for their patients to be involved in the study: Dr. R Lendrum, Dr. M Hudson, Dr. J Mansfield, Dr. K Oppong, Dr. C Record, Mr. P Wright and Mr. P Hainsworth as well as the patients who participated in the study.
S- Editor Liu Y L- Editor Lutze M E- Editor Bi L
| 1. | Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4:368-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1393] [Cited by in F6Publishing: 1380] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 2. | Bjarnason I, Macpherson A, Mackintosh C, Buxton-Thomas M, Forgacs I, Moniz C. Reduced bone density in patients with inflammatory bowel disease. Gut. 1997;40:228-233. [PubMed] [Cited in This Article: ] |
| 3. | Pollak RD, Karmeli F, Eliakim R, Ackerman Z, Tabb K, Rachmilewitz D. Femoral neck osteopenia in patients with inflammatory bowel disease. Am J Gastroenterol. 1998;93:1483-1490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 162] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Schulte C, Dignass AU, Mann K, Goebell H. Reduced bone mineral density and unbalanced bone metabolism in patients with inflammatory bowel disease. Inflamm Bowel Dis. 1998;4:268-275. [PubMed] [Cited in This Article: ] |
| 5. | Compston JE, Judd D, Crawley EO, Evans WD, Evans C, Church HA, Reid EM, Rhodes J. Osteoporosis in patients with inflammatory bowel disease. Gut. 1987;28:410-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 209] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Pigot F, Roux C, Chaussade S, Hardelin D, Pelleter O, Du Puy Montbrun T, Listrat V, Dougados M, Couturier D, Amor B. Low bone mineral density in patients with inflammatory bowel disease. Dig Dis Sci. 1992;37:1396-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 98] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Abitbol V, Roux C, Chaussade S, Guillemant S, Kolta S, Dougados M, Couturier D, Amor B. Metabolic bone assessment in patients with inflammatory bowel disease. Gastroenterology. 1995;108:417-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 174] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Silvennoinen JA, Karttunen TJ, Niemelä SE, Manelius JJ, Lehtola JK. A controlled study of bone mineral density in patients with inflammatory bowel disease. Gut. 1995;37:71-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 192] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Jahnsen J, Falch JA, Aadland E, Mowinckel P. Bone mineral density is reduced in patients with Crohn's disease but not in patients with ulcerative colitis: a population based study. Gut. 1997;40:313-319. [PubMed] [Cited in This Article: ] |
| 10. | Robinson RJ, al-Azzawi F, Iqbal SJ, Kryswcki T, Almond L, Abrams K, Mayberry JF. Osteoporosis and determinants of bone density in patients with Crohn's disease. Dig Dis Sci. 1998;43:2500-2506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Martin JP, Tonge KA, Bhonsle U, Jacyna MR, Levi J. Bone mineral density in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1999;11:537-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Bischoff SC, Herrmann A, Göke M, Manns MP, von zur Mühlen A, Brabant G. Altered bone metabolism in inflammatory bowel disease. Am J Gastroenterol. 1997;92:1157-1163. [PubMed] [Cited in This Article: ] |
| 13. | Andreassen H, Hylander E, Rix M. Gender, age, and body weight are the major predictive factors for bone mineral density in Crohn's disease: a case-control cross-sectional study of 113 patients. Am J Gastroenterol. 1999;94:824-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Andreassen H, Rix M, Brot C, Eskildsen P. Regulators of calcium homeostasis and bone mineral density in patients with Crohn's disease. Scand J Gastroenterol. 1998;33:1087-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Silvennoinen JA, Lehtola JK, Niemelä SE. Smoking is a risk factor for osteoporosis in women with inflammatory bowel disease. Scand J Gastroenterol. 1996;31:367-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Hyams JS, Wyzga N, Kreutzer DL, Justinich CJ, Gronowicz GA. Alterations in bone metabolism in children with inflammatory bowel disease: an in vitro study. J Pediatr Gastroenterol Nutr. 1997;24:289-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 102] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Silvennoinen J, Risteli L, Karttunen T, Risteli J. Increased degradation of type I collagen in patients with inflammatory bowel disease. Gut. 1996;38:223-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Robinson RJ, Iqbal SJ, Abrams K, Al-Azzawi F, Mayberry JF. Increased bone resorption in patients with Crohn's disease. Aliment Pharmacol Ther. 1998;12:699-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Kyle J. Crohn's disease in the northeastern and northern Isles of Scotland: an epidemiological review. Gastroenterology. 1992;103:392-399. [PubMed] [Cited in This Article: ] |
| 20. | Kelly TL Sp, von Stetton E. Performance evaluation of a multi-detector DXA device. J Bone Miner Res. 1991;6 Suppl 1:S168. [Cited in This Article: ] |
| 21. | Eastell R, Reid DM, Compston J, Cooper C, Fogelman I, Francis RM, Hosking DJ, Purdie DW, Ralston SH, Reeve J. A UK Consensus Group on management of glucocorticoid-induced osteoporosis: an update. J Intern Med. 1998;244:271-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 210] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Ghosh S, Cowen S, Hannan WJ, Ferguson A. Low bone mineral density in Crohn's disease, but not in ulcerative colitis, at diagnosis. Gastroenterology. 1994;107:1031-1039. [PubMed] [Cited in This Article: ] |
| 23. | Scharla SH, Minne HW, Lempert UG, Leidig G, Hauber M, Raedsch R, Ziegler R. Bone mineral density and calcium regulating hormones in patients with inflammatory bowel disease (Crohn's disease and ulcerative colitis). Exp Clin Endocrinol. 1994;102:44-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Vestergaard P, Krogh K, Rejnmark L, Laurberg S, Mosekilde L. Fracture risk is increased in Crohn's disease, but not in ulcerative colitis. Gut. 2000;46:176-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 141] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Edelstein SL, Barrett-Connor E. Relation between body size and bone mineral density in elderly men and women. Am J Epidemiol. 1993;138:160-169. [PubMed] [Cited in This Article: ] |
| 26. | Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res. 1993;8:567-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 644] [Cited by in F6Publishing: 631] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 27. | Robinson RJ, Iqbal SJ, Al-Azzawi F, Abrams K, Mayberry JF. Sex hormone status and bone metabolism in men with Crohn's disease. Aliment Pharmacol Ther. 1998;12:21-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Staun M, Tjellesen L, Thale M, Schaadt O, Jarnum S. Bone mineral content in patients with Crohn's disease. A longitudinal study in patients with bowel resections. Scand J Gastroenterol. 1997;32:226-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Schulte C, Dignass AU, Mann K, Goebell H. Bone loss in patients with inflammatory bowel disease is less than expected: a follow-up study. Scand J Gastroenterol. 1999;34:696-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Croucher PI, Vedi S, Motley RJ, Garrahan NJ, Stanton MR, Compston JE. Reduced bone formation in patients with osteoporosis associated with inflammatory bowel disease. Osteoporos Int. 1993;3:236-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Hansen MA, Overgaard K, Riis BJ, Christiansen C. Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year study. BMJ. 1991;303:961-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 346] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 32. | Uebelhart D, Schlemmer A, Johansen JS, Gineyts E, Christiansen C, Delmas PD. Effect of menopause and hormone replacement therapy on the urinary excretion of pyridinium cross-links. J Clin Endocrinol Metab. 1991;72:367-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 251] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 33. | Garnero P, Shih WJ, Gineyts E, Karpf DB, Delmas PD. Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab. 1994;79:1693-1700. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Chesnut CH 3rd, Bell NH, Clark GS, Drinkwater BL, English SC Jr, Johnson CC, Notelovitz M, Rosen C, Cain DF, Flessland KA. Hormone replacement therapy in postmenopausal women: urinary N-telopeptide of type I collagen monitors therapeutic effect and predicts response of bone mineral density. Am J Med. 1997;102:29-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 196] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 35. | Dresner-Pollak R, Karmeli F, Eliakim R, Ackerman Z, Rachmilewitz D. Increased urinary N-telopeptide cross-linked type 1 collagen predicts bone loss in patients with inflammatory bowel disease. Am J Gastroenterol. 2000;95:699-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Scott EM, Gaywood I, Scott BB. Guidelines for osteoporosis in coeliac disease and inflammatory bowel disease. British Society of Gastroenterology. Gut. 2000;46 Suppl 1:i1-i8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 175] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Gough A, Sambrook P, Devlin J, Lilley J, Huisoon A, Betteridge J, Franklyn J, Nguyen T, Morrison N, Eisman J. Effect of vitamin D receptor gene alleles on bone loss in early rheumatoid arthritis. J Rheumatol. 1998;25:864-868. [PubMed] [Cited in This Article: ] |