CASE REPORTS
Case 1
A 55-year-old normotensive, nondiabetic, non-alcoholic man with no history of chronic liver disease, presented with the complaints of low backache and generalized weakness for 20 d. Clinically, he had tenderness in the presacral region. Further investigations revealed normal hematology and biochemical parameters. Magnetic resonance imaging (MRI) of the whole spine showed a tumor deposit in the presacral area. Whole body 99 mTc bone scan revealed increased radiotracer uptake in right ilium, sacrum including both sacroiliac joints and D3 vertebra. Computerised Tomography (CT) guided biopsy and fine needle aspiration cytology (FNAC) from the presacral tumor were consistent with the metastatic poorly differentiated neoplasm. Serum alpha feto protein (AFP) level was more than 500 IU/mL (normal AFP level < 10 IU/mL). Ultrasound if testis, upper GI endoscopy, CT chest scan were unremarkable but CT scan of the abdomen revealed a large well defined hypodense lesion, 7.5 cm × 5.8 cm in dimensions with areas of necrosis, in the right liver lobe, in segment V, with no involvement of the portal vein. Additionally there was a destructive lesion in the right ala of the sacrum associated with a bulging soft tissue component, 5.3 cm × 3.9 cm in dimensions, which corroborated with MRI spine and bone scan findings. The patient underwent CT guided FNAC and Tru-cut biopsy of the right liver lobe lesion. Histopathological examination (HPE) was suggestive of poorly differentiated primary HCC, showing increased trabecular thickness and cellular pleomorphism with vesicular nuclei (Figure 1). Viral marker profile was positive for Hepatitis B surface antigen (HbsAg) and was non-reactive to hepatitis C virus. He was diagnosed as having primary HCC with bone metastases. The option of palliative chemotherapy was declined by the patient and he was started on palliative external beam radiotherapy (EBRT) to the right pelvis in view of pain. He developed acute onset paraplegia along with urinary retention shortly after initiation of EBRT. Immediate intravenous Solumedrol and other supportive measures were taken. Whole spine MRI was done and revealed multiple vertebral metastases at D2-D3, L1, L5, S1,S3 vertebral levels (Figure 2A and 2B) and epidural deposits with extradural cord compression at D2 and D3 vertebrae (Figure 2C). FNAC from vertebral metastases (Figure 3) revealed metastatic carcinoma consistent with primary HCC. Decompression and fixation surgery was suggested, but the family did not opt for any surgical intervention. He continued to receive EBRT for SCC. Although, there was symptomatic improvement in the pain intensity, there was no significant improvement in the neurological status. After further discussion with the family, oral chemotherapy was performed with Capecitabine and Thalidomide with no response after two cycles. Despite prompt treatment of SCC with steroids and EBRT, the patient succumbed to the disease five months after primary diagnosis.
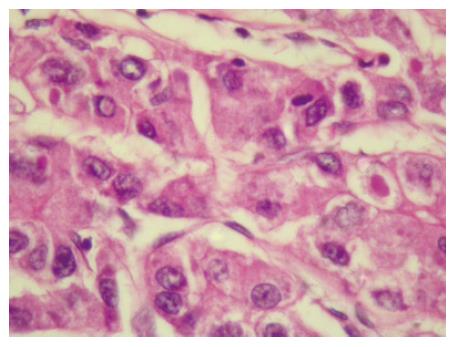
Figure 1 Tru cut biopsy of liver lesion showing increased trabecular thickness, cellular pleomorphism with vesicular nuclei.
Sinusoids are lined by flat endothelial cells, Kupffer cells are absent.
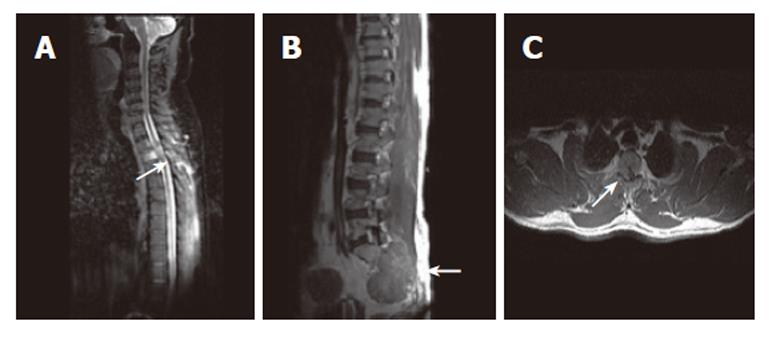
Figure 2 A: Sagittal MR image of cervicodorsal spine showing vertebral lesions with most prominent D3 vertebral lesion (arrow); B: Post contrast sagittal T1W SE MR image of the same patient showing lesion involving S2, S3 vertebrae (arrow) with associated large soft tissue component; C: T1W SE axial post contrast MR image showing right lateral epidural soft issue enhancing component (arrow) causing cord compression.
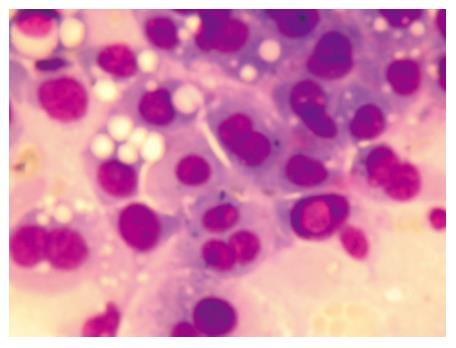
Figure 3 FNAC of vertebral metastasis showing cellular monomorphism, moderate pleomorphism with occasional intranuclear inclusion.
Case 2
A 52-year-old non-alcoholic man presented with complaints of heaviness and discomfort in the right upper abdomen of two months duration. Whole abdomen ultrasound done elsewhere was suggestive of liver mass. Clinical examination revealed presence of mild hepatomegaly. Laboratory investigations showed a normal hematological profile and mild derangement of liver function tests (LFTs). Viral marker profile was positive for HbsAg and non-reactive to HCV. Whole abdomen CT scan revealed mild hepatic enlargement with an ill-defined hypodense lesion of 3.8 cm in diameter, in segment V and VI of the liver with no involvement of portal vein. His AFP levels were 3540 IU/mL. Magnetic resonance cholangio pancreatography (MRCP) revealed a nodular outline and texture of liver consistent with cirrhosis, with a focal lesion in segment V and VI, measuring 3.5 cm × 4.0 cm × 4.1 cm. He underwent CT guided FNAC from right liver lobe mass which was suggestive of primary HCC. Whole body 99 m Tc bone scan revealed no evidence of bone metastases. He was diagnosed as having primary HCC with no evidence of extra-hepatic spread. He underwent hepatic segmentectomy. HPE of the resected specimen of liver segment revealed poorly differentiated HCC with cirrhosis and regenerative nodules. CT scan of the abdomen after surgery as a part of revaluation revealed a new focal hypodense lesion measuring 2.4 cm × 2.5 cm, in the caudate lobe of the liver and another ill defined hypodense lesion in the right lobe of the liver with an expansile lesion with soft tissue component in the right seventh rib. Whole body 99 mTc bone scan showed irregular radiotracer uptake in the right seventh rib anterolaterally. He underwent FNAC from right seventh rib, which revealed metastatic deposit of HCC. There was a rise in serum AFP level from 3540 IU/mL at the time of diagnosis to 7920 IU/mL, suggestive of relapse. He received palliative chemotherapy with Gemcitabine and Cisplatin. CT scans after three cycles of chemotherapy revealed complete regression of caudate lobe, right liver lobe and right seventh rib lesion with a new development of a well defined lytic lesion in L2 vertebra. He also had pain in the right hip joint. Chemotherapy was continued with the same protocol for three more cycles. Subsequent CT chest scan revealed development of a new lesion in the left third rib with destruction of the left transverse process of D3 vertebra along with erosion of D5 vertebra. CT guided FNAC from the third left rib lesion was positive for malignant cells, consistent with primary in liver. He received palliative EBRT to the symptomatic areas like right hip joint, C7 to D7 vertebrae and L1 to L4 Vertebrae. One month later, he developed paraplegia with complete loss of bladder and bowel control. MRI of Dorso-lumber spine was suggestive of metastatic lesion at dorsal and lumbo-sacral spine with cord compression at the D6 vertebral level. The patient underwent D5 to D6 laminectomy with posterior fixation. HPE of laminectomied specimen was reported as metastatic carcinoma consistent with primary HCC. Postoperatively there was no significant improvement in the paraplegia and he did not regain bladder and bowel control. He was supported with intravenous steroids, bisphosphonates and physiotherapy. After laminectomy, although palliative EBRT was planned to the dorsal spine, (D4 to D7) vertebrae and to the lower dorsal spine, his general condition deteriorated and he succumbed to his illness 11 mo after the primary diagnosis.
Case 3
A 70-year-old man who was alcoholic, diabetic and a smoker, presented with complaints of pain in the right side of the chest. Clinical examination revealed mild hepatomegaly and pallor. Whole abdomen CT scan revealed left liver lobe mass suggestive of primary HCC with portal vein thrombosis and abdominal lymphadenopathy. He underwent MRCP and MR angiography, which revealed a soft tissue lesion in the left lobe of the liver with portal vein thrombosis and abdominal lymphadenopathy, with no signs of cirrhosis of liver. His hematological and biochemical profile were within normal range. HbsAg and HCV were not reactive in viral marker profile. His serum AFP level was 250 IU/mL. Tru-cut biopsy of left liver lobe lesion was consistent with the diagnosis of HCC. Whole body 99 mTc bone scan revealed no evidence of bone metastases at the time of diagnosis. Chemotherapy was given with Gemcitabine and Capecitabine. After the second cycle, he had sudden onset of weakness of bilateral lower limbs. Neurological examination revealed paraplegia with sensory loss below D5 level with moderate sphincteric involvement. Whole spine MRI showed extradural cord compression at D4 and D5. Whole body 99 mTc bone scan revealed abnormal areas of radiotracer uptake in D4 and D5 vertebrae, consistent with bone metastases. Emergency D1 to D4 vertebral laminectomy was performed with posterior fixation from C7 to D5 vertebrae. HPE of laminectomied specimen showed metastasis from HCC. He received EBRT to the spine along with aggressive physiotherapy and with other supportive measures after surgery. He achieved good neurological and symptomatic recovery, and was discharged in the hemodynamically stable condition. As the patient was not willing to receive further treatment, he was followed up on palliative supportive care. However, he succumbed to the disease four months after the diagnosis.
Case 4
A 62-year-old normotensive, non diabetic, nonalcoholic man presented with complaints of pain in neck and lower back of one month duration. Pain radiated from neck to fingers bilaterally. He also had difficulty in walking due to radicular pain. Clinical examination revealed painful neck movement and mild hepatomegaly. Hematological profile was normal mild derangement of liver enzymes. Both HbsAg and HCV were non-reactive. Whole abdomen ultrasound revealed a well defined solid mass, measuring 8.4 cm × 4.9 cm, in the right lobe of the liver, with heterogeneous echotexture. Upper abdomen CT scan also revealed enlarged liver with a large hypodense lesion, measuring 10.5 cm × 10.1 cm, in the right lobe of the liver. Whole spine MRI was suggestive of multiple bone metastases in C5-C6, D9, D11-D12 and L4 vertebrae and the bodies of D9 and L4 vertebrae revealed wedge compression fracture. A small anterior epidural space soft tissue collection was also present, causing compression of the thecal sac at L4 vertebral level. Whole body 99 mTc bone scan revealed increased radiotracer uptake in D7, D9, D11-D12 and the L4 vertebrae, sacrum and bilateral sacroiliac joints, suggestive of bone metastases. He underwent CT guided FNAC from right liver lobe mass which revealed primary HCC. FNAC from vertebra was consistent with metastasis from primary HCC. Serum AFP level was elevated to 121 IU/mL at the time of diagnosis. He was diagnosed as having primary HCC with multiple bone metastases with SCC, manifesting primarily as radiculopathy. The patient and the family were given the options of surgical decompression as well as chemotherapeutic management, which they were not inclined for in view of disseminated disease. Hence, he was started on palliative EBRT to L2-L5 vertebrae and to C4-C7 vertebrae. EBRT to the thoracic spine was performed later. After completion of radiotherapy (RT) there was symptomatic improvement in the pain intensity. However, after EBRT, there was progression of SCC leading to development of paraparesis with urine incontinence. He was managed conservatively in view of deteriorating general condition. He died from his illness three months after the primary diagnosis.
DISCUSSION
HCC is the most common solid organ tumor with a high mortality[2]. Most patients with HCC present with right upper quadrant pain or an abdominal mass due to the presence of hepatomegaly[7,8,9]. Rarely though, the patients may have initial symptoms related exclusively to the extra-hepatic metastases[6]. Serum AFP level is the most useful serum tumor marker for primary HCC. Serum AFP level is elevated in most of the patients, which is highly specific for a tumor larger than three cm in diameter, and also is of prognostic value as rise after initial effective chemotherapy or surgery suggests a relapse[3]. This was evident from the fact that AFP level was elevated in all our cases and relapse occurred after hepatic segmentectomy in the second patient corresponded to the elevation of AFP levels.
Extra-hepatic spread from HCC is not uncommon and reported to be 30%-78% at autopsy examination. Bone metastases are rare with an incidence of only about 2%-20%[2-5,10,11]. Although incidence of metastasis of HCC to the bones is low, recent reports have shown an increasing incidence and is estimated to be about 28%[1].
The most frequent sites of the bone metastases are ribs, spine, femur, pelvis and humerus according to Khulman et al[2]. Patients with bone metastases most often present with pain as the principal symptom[3,7]. Two of our patients presented with the chief complaints of backache. Very rarely patient may present with bone pains without any symptom of underlying hepatic pathology, as seen in our first case.
In the majority of cases, vertebral body metastases result from hematogenous dissemination of tumor which is evident by the vertebral column bone marrow involvement[6,10]. Radiologically, bone metastases from HCC appear osteolytic on plain films. All the four patients in our series had osteolytic lesions. They are destructive, expansive and often associated with large soft tissue masses[2-6,10]. Conventional radiography is however, not a very sensitive modality for the diagnosis of early bone metastases as the cancellous part of the bone is usually the first site of bone metastases and cortical part of the bone is responsible for most of the bone density depicted on plain X-Ray films[6]. For the same reason the bone scan is also less sensitive. MRI is most helpful for early diagnosis as well as delineation of the extent of metastases[2,6].
The histological appearance of the bone metastases from primary HCC is similar to that of the primary tumor, with positive bile staining[2,4]. Recent insight into the causation of bone metastases of HCC has been correlated with angiogenesis[8]. Significant hemorrhage from metastatic lesions is reported to occur either spontaneously or after biopsy of the lesion[2].
The level of vascular endothelial growth factor (VEGF) has been reported to be elevated in HCC with bone metastases[8]. VEGF, the most important angiogenic factor, has been shown to stimulate bone resorption through its effects on osteoclasts[8,12]. Thus in the era of targeted therapy, VEGF could be an important target for the treatment of these tumors. In their study on metastatic HCC, S. Kummar et al have evaluated TNP-470, a derivative of fumagillin and a potent angiogenetic inhibitor, as a treatment for experimentally induced HCC in animal models[8]. Similarly, the serum levels of C- terminal telopeptide of type 1 collagen are also significantly elevated in patients with bone metastases[13].
The overall frequency of the malignant SCC has been reported to be approximately 5% in cancer patients. In approximately 95% of cases, SCC is caused by extradural metastases from tumors involving the vertebral column. Thoracic spine (70%) is most commonly involved as compared to the lumbosacral (20%) and the cervical spine (10%)[12,14]. Three cases reported by us had SCC at thoracic vertebrae level and all of them had extradural compression. Although SCC is rare with HCC, the symptoms and involvement are similar to other primary tumors, commonly leading to SCC, such as lung, breast and prostate cancer.
Omura et al in 1989 described a case of a 57-year-old male with primary HCC diagnosed when he developed paraplegia secondary to a SCC due to vertebral tumor. After laminectomy, the tumor histology was reported to be metastatic HCC[15]. Kantharia et al in 1993, also described a case of radiculopathy and rapidly developing SCC, due to bone metastases and diagnosed as HCC at autopsy[6]. Pinazo Seron et al in 1999 reported a case of a 55-year-old man, who had alcoholic cirrhosis and HCC. This patient developed SCC due to soft tissue epidural metastases, seated at the paravertebral zone. Plain radiography and bone scan were normal and diagnosis was achieved by MRI and FNAC[16]. Cho et al in 2002 reported a case of pathologically confirmed HCC who developed lower leg weakness, which was found to be due to spinal metastases as evidenced by MRI spine. This patient received emergency radiotherapy (RT) and recovered from SCC[17]. Melicher et al in 2002 also described a case of asymptomatic liver mass of uncertain histology of one year duration. He presented with back pain and developed signs of SCC, and he underwent laminectomy, which established the diagnosis of metastatic HCC[18].
Cord compression occurs due to invasion of epidural space, most often as a direct extension of vertebral body metastases. There are various routes of the epidural invasion by tumor cells, hematogenous being the most common mode of spread. Hematogenous spread occurs directly or via the involvement of Batsons venous plexuses[19].
In metastatic SCC, back pain is not only the most common symptom but also the earliest manifestation of SCC, as seen in two of our patients. First sign after development of pain is weakness due to myelopathy. Once lost, neurological functions cannot be regained in most of the patients[12,20,21].
Though various modalities such as plain radiography, CT scan, CT-myelography and MRI can be used to evaluate SCC, whole spine MRI is the best method of evaluating epidural SCC. Apart from being most sensitive, cost-effective and noninvasive, MRI is also helpful in distinguishing between benign and malignant causes of SCC[20,21]. In all our patients, SCC was diagnosed with the help of MRI spine, which revealed the site of extradural cord compression with precision. Presently CT-myelography is used only for patients in whom MRI is contraindicated[21].
The pre-treatment degree of neurologic dysfunction is the strongest predictor of therapeutic outcome. However, the most important weapon against the prevention of devastating complications of SCC is the high index of suspicion and the awareness that SCC is a potential oncologic emergency[20]. Thus the development of any new pain or any change in the character of the pain mandates complete neurological examination along with MRI whole spine as a screening modality to rule out early signs of SCC[21].
The treatment modalities available for SCC are individualized with a definite role of corticosteriods, RT, chemotherapy and surgery. Corticosteriods act by relieving edema and help preserve neurological function. They may also improve overall outcome after specific therapy[19,22,23].
RT is an important part of the management of SCC and it helps in pain relief, cytoreduction of tumor, prevention of progressive neurologic dysfunction and structural damage to the cord. RT reduces pain in approximately 70%, improves motor function in 45% to 60%, and reverses paraplegia in 11% to 21% of the patients. The outcome of RT is related to the neurological status prior to the treatment and the radiosensitivity of the tumor. RT should also be given following surgery in patients who have not previously received radiation[24-27]. Our third patient was given EBRT to the affected region after emergency posterior laminectomy was performed.
Surgical interventions are usually indicated in situations where the diagnosis is unknown, in cases of spinal instability or compression by the bone requiring prompt relief of pressure related symptoms[12,20,27]. These four cases suggest that surgery should be the integral part of SCC management in HCC. Our first patient immediately received RT after developing SCC, with no improvement in neurological status, and in the second patient, SCC developed at the vertebra which was earlier irradiated. RT did not help prevent paraparesis in the fourth patient. Whereas, the third patient who underwent emergency laminectomy followed by EBRT had best palliation. Surgery should also be considered in the patients who fail to respond to RT or deteriorate further while on RT and the patients who have received maximal allowable radiation dose to the spinal cord[12,20]. The surgical approach should be determined based on the location of vertebral involvement and the direction of compression[20]. The two main surgical approaches used for decompression are laminectomy with posterior fixation and anterior decompression of the spine with reconstruction. Anterior decompression of the spine with mechanical stabilization has been accepted as the surgical intervention of choice for anterior vertebral body involvement[21,28]. Our second and third patient underwent posterior laminectomy for emergency decompression without any immediate morbidities. But the aim is to diagnose SCC in the early stage so that emergency decompressive surgery will not be needed.