Published online Aug 28, 2006. doi: 10.3748/wjg.v12.i32.5168
Revised: July 20, 2005
Accepted: July 28, 2005
Published online: August 28, 2006
AIM: To evaluate the mechanism underlying the effects of 5-Fluorouracil (5-FU) on adenoviral infection.
METHODS: Low and high Coxsackievirus-Adenovirus Receptor (CAR) expressing human colon carcinoma cell lines were treated with 5-FU and two E1-deleted adenoviral constructs, one transferring GFP (Ad/CMV-GFP) the other bax (Ad/CEA-bax). The number of infected cells were monitored by GFP expression. To evaluate the effects of 5-FU in a receptor free system, Ad/GFP were encapsulated in liposomes and treated with 5-FU. Ad/GFP release was estimated with PCR and infection of 293 cells with the supernatant. Electron microscopy of the Ad5-GFP-liposome complex was made to investigate morphological changes of the liposomes after 5-FU.
RESULTS: Infection rates of all cell lines increased from 50% to 98% with emerging 5-FU concentrations. The enhanced viral uptake was independent of the CAR expression. Additionally, 5-FU treated liposomes released 2-2.5 times more adenoviruses. Furthermore, 5-FU-treated liposomes appeared irregular and porous-like.
CONCLUSION: adenoviral uptake is enhanced in the presence of 5-FU irrespective of CAR and is associated with morphological changes in membranes making the combination of both a promising option in gene therapy.
- Citation: Cabrele C, Vogel M, Piso P, Rentsch M, Schröder J, Jauch KW, Schlitt HJ, Beham A. 5-Fluorouracil-related enhancement of adenoviral infection is Coxsackievirus-adenovirus receptor independent and associated with morphological changes in lipid membranes. World J Gastroenterol 2006; 12(32): 5168-5174
- URL: https://www.wjgnet.com/1007-9327/full/v12/i32/5168.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i32.5168
It is widely accepted that cancer is the endpoint of an accumulation of genetic mutations that result in a cellular phenotype characterized by uncontrolled growth and reduced apoptosis. Consequently, therapeutic strategies, which address the genetic lesions and thus kill cancer cells, are reasonable. This concept has made virus-mediated gene therapy an ideal candidate for therapeutic approaches either alternative or complementary to chemotherapy or radiotherapy. Human adenoviruses are widely used as delivery systems but adenovirus (Ad)3 uptake is dependent on expression of Coxsackievirus-adenovirus receptor (CAR). Unfortunately, tumor cells are usually characterized by a reduced expression of CAR that binds the fiber knob domain of the Ad serotypes 2 and 5[1]. Cells lacking this receptor are more resistant to adenoviral infection and, consequently, they are poor targets for Ad-associated tumor therapies[2-5]. Further, it has been shown that the treatment of colorectal cancer with the replication-selective Ad dl1520 in combination with 5-Fluorouracil (5-FU) was more efficient in inducing apoptosis than the administration of the two agents separately[6-9]. Therefore we evaluated the mechanism underlying the effects of 5-FU in the context of adenoviral infection in this study. To better understand the role of 5-FU in adenoviral infection of tumor cells, we used two E1- and replication-deficient adenoviral mutants expressing GFP (Ad-GFP) to infect colorectal cancer cell lines that show different CAR expression. A significantly higher number of GFP-expressing cells were observed after treatment with 5-FU and Ad-GFP compared to Ad-GFP alone. The effect of 5-FU was even more striking in a cell line with low CAR expression (SW480) indicating that a CAR-independent mechanism may be responsible for the transport of Ad through the cell membrane. This enhancement of infection was dose-dependent and maximal with simultaneous application of 5-FU and Ad-GFP. To assess this effect in a CAR-independent pathway, Ad-GFP was encapsulated in liposomes, which were treated with 5-FU. Supernatants of these liposomes contained 2.4 times more Ad-GFP after 5-FU treatment compared to controls. In addition, morphological changes in the lipid membranes were seen by electron microscopy. In conclusion, we could demonstrate that simultaneous treatment with 5-FU enhances adenoviral uptake into tumor cells. More importantly, this effect could be observed irrespective of CAR expression and 5-FU favors the crossing of the viral protein capsid through the lipid membranes. Regardless of the underlying mechanism, the combination of adenoviral and 5-FU treatment might be of significant importance in the context of gene transfer.
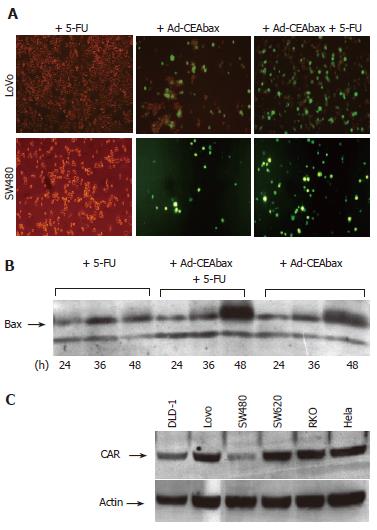
All cell lines used in this study were purchased from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany) and were grown in the appropriate media containing 100 mL/L FCS, 10 g/L Pen/Strep (PS) and 10 g/L glutamine. The human embryonic kidney 293 cells were grown in DMEM, the human colon adenocarcinoma cells DLD-1, LOVO, SW480 and SW620 in RPMI 1640, and the RKO cells in McCoy.
The adenoviral vectors for gene expression were constructed with the AdEasy system[10]. Briefly, Ad-CEAbax was constructed with the pAdTrack vector containing the CEA promoter in front of the bax gene and the gene for GFP under the control of the CMV promoter, whereas Ad-GFP was constructed with the pAdTrack vector containing GFP under the control of the CMV promoter. The resulting plasmids were then transformed into Escherichia coli cells with pAdEasy-1. The recombinant adenoviruses were generated in the 293 cells (E1-transformed) and purified by CsCl gradient ultracentrifugation[11]. The titer was determined by counting the green cells after 48 h.
For Ad infection of SW480 the cells were seeded onto 96-well plates at 1500 cells/well and grown for 2 d. For the 5-FU pretreatment, the cells were first incubated at 37°C in RPMI/FCS/PS containing 5-FU at concentrations of 2, 10, 30 and 50 μmol/L. After 2 h the medium was removed, the cells were washed and infected with Ad-GFP at 300 pfu/well in 200 μL medium without the drug. For the co-treatment, the cells were infected with Ad-GFP diluted in 200 μL medium containing 5-FU at the concentrations reported above. Controls were incubated with medium without 5-FU. The number of green cells was counted after 24, 48 and 72 h with a fluorescence microscope for GFP expression. For the infection of the Lovo and SW480 cells with Ad-CEAbax, the cells were plated onto 60 mm dishes at a density of 2 × 105 cells/dish. After 1 d, the cells were infected with the virus (1 MOI) in 1 mL medium without FCS and PS. After 30 min incubation at 37°C, the cells were treated with the medium containing FCS, PS and 5-FU at the final concentrations of 2 μmol/L for Lovo and 20 μmol/L for SW480. For the treatment of the colon cancer cells with Ad-GFP and 5-FU, 5-BrU, 5-FC, DOC, or taxol, the cells were seeded onto 6-well plates at 2 × 105 cells/well. After 1 d, Ad-GFP was added at 3 × 104 pfu/well in 1 mL medium without FCS and PS. After 30 min incubation at 37°C, 2 mL growth medium were added containing FCS, PS and the drug at the following final concentration: 4 μmol/L for 5-FU, 5-BrU, 5-FC and DOC, and 5 μg/L for taxol. The number of green cells was counted 48 h after infection.
To assess the effects of 5-FU of lipid membranes and the ability of Ad to penetrate the membranes, we encapsulated Ad in liposomes with and without 5-FU. The release of Ad was measured by PCR for E4orf6. In addition, the number of infective particles was evaluated by infection of 293 cells with the supernatant. The liposomal formulation of Ad was prepared as follows: Ad-GFP (3 × 104 pfu) was mixed with lipofectamine (Invitrogen) that was brought to a final concentration of 0.4 g/L with PBS. After 2 h incubation at room temperature, 5-FU was added to the final concentration of 20 μmol/L. The mixture was incubated overnight at room temperature and then centrifuged at 2060 g for 50 min at 20°C. The supernatant (100 μL/well) was diluted to 1 mL with DMEM without FCS and PS and added to the cells that were then incubated for 30 min at 37°C. Afterwards, 2.5 mL DMEM containing FCS and PS were added and the cells were further incubated. For the experiment with the liposome-encapsulated Ad-GFP, the 293 cells were plated into 6-well plates at 2 × 105 cells/well and were grown overnight.
The following adenoviral DNA samples (each 2 µg) were prepared for PCR: DNA alone, DNA in the presence of 9.6 μmol/L 5-FU, DNA/lipofectamine (0.2 g/L), DNA/lipofectamine (0.2 g/L) in the presence of 9.6 μmol/L 5-FU. The samples were incubated overnight at room temperature, then centrifuged at 2060 g for 35 min at 18°C. For control, an additional sample of DNA/lipofectamine (0.2 g/L) was prepared and treated with a lysis buffer for 1 h at 4°C prior to centrifugation. The DNA was precipitated from the supernatants upon addition of NaOAc and isopropanol, isolated by centrifugation, washed with 750 mL/L ethanol and dried. The PCR was performed by using the Taq PCR Master Mix (Qiagen). The resultant PCR products were then resolved on a 1.5% agarose gel containing 0.25 mg/L of ethidium bromide.
Single cell suspensions were fixed in 700 mL/L ethanol and incubated with 50 g/L PI and 20 g/L RNAse for 15 min. at 37°C. Flow analysis was done at 488 nm excitation and > 525 nm Em range collected for GFP fluorescence. Elite Software 4.0 (Coulter Corp, Miami, FL) and Multi Cycle DNA Analysis program software (Phoenix Flow Systems, San Diego, CA).
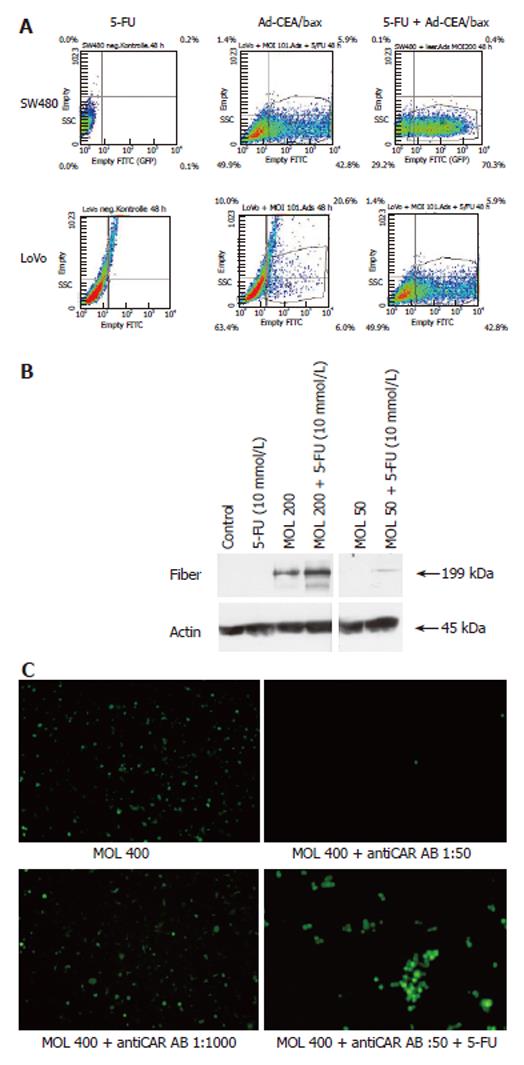
Protein concentration was determined by using the BC assay (Interchim, Montluçon, France). The protein samples [(40 μg for the CAR detection, 60 μg for the bax detection and 50 μg for the fiber detection (samples were not cooked)] were separated on SDS-polyacrylamide gels (100 g/L for the CAR detection, 150 g/L for the bax detection and 75 g/L for the fiber detection), and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). The blots were blocked with 50 mL/L nonfat dry milk in 1 g/L Tween 20 PBS (TPBS-MLK) for 1 h at room temperature, then incubated overnight at 4°C with the appropriated antibodies: CAR (goat polyclonal antibody, Santa Cruz) at the dilution of 1:285, bax (polyclonal rabbit anti-human, PharMingen) at the dilution of 1:1000, fiber (polyclonal mouse, NeoMarkers, Fremont, CA) at the dilution of 1:1000 and actin (goat polyclonal antibody, Santa Cruz) at the dilution of 1:1000. After washing with TPBS, the following secondary antibodies were added at the dilution of 1:500 and incubated for 1 h at room temperature: HRP-conjugated IgG from donkey anti-goat for CAR and actin detection, and from goat anti-rabbit for bax detection. The proteins were finally visualized by enhanced chemiluminescence (ECL, Amersham Pharmacia Biotech).
SW480 cells were plated into 6 well plates at 2 × 105 cells/well and incubated with CAR antibody (goat polyclonal antibody, Santa Cruz) at the dilution of 1:1000, 1:750, 1:500, 1:250, 1:100 and 1:50 for 24 h. The cells were incubated with Ad-GFP and the number of green cells was counted 48 h after infection.
The following solutions were analyzed by electron microscopy (EM): (A) lipofectamine at 0.5 g/L, (B) lipofectamine at 0.5 g/L treated overnight with 20 μmol/L 5-FU, (C) Ad-GFP (4 × 108 pfu/L) complexed with lipofectamine at 0.5 g/L, (D) Ad-GFP (4 × 105 pfu/mL) complexed with lipofectamine at 0.5 g/L and incubated for 1 h at room temperature prior to treatment overnight with 20 μmol/L 5-FU. The EM samples were prepared by using the negative stain procedure. Briefly, a drop of each sample was deposited on a copper grid and coated by a formvar/carbon film. The film was then stained with 20 g/L tungsten phosphoric acid and dried on air. The EM images were recorded on a Zeiss instrument operating at 80 kV.
Several cell lines (DLD-1, Lovo, SW480 and SW620) were treated with Ad-GFP or Ad-CEAbax at a MOI that infected 50% of the cells. The infection of all cell lines was surprisingly efficient (up to 98%) when the adenoviral constructs were used in combination with 5-FU, as indicated by the high population of green cells in Lovo and SW480 cell examples (Figure 1A and 2A). In accordance with GFP expression, an increased expression of bax was detected (Figure 1B) after Ad-CEAbax infection and 5-FU. The RKO cells died after simultaneous treatment and thus the number of green cells decreased after 24 h (Figure 4). As control 293 cells were transfected with the CMV Promoter/GFP DNA and treated with 10 μmol/L 5-FU. No difference in the amount of green cells was seen after 5-FU indicating that 5-FU does not interfere with the transcription of the reporter gene (data not shown). In addition, SW480 cell lysates of 5-FU treated (10 μmol/L) and control cells were blotted for adenoviral fiber protein after treatment with two different adenoviral MOI (200 and 50). In both cases more fiber protein could be detected in 5-FU treated cells (Figure 2B) indicating a higher amount of intracellular adenovirus. 5-FU enhanced the uptake of Ad not only in cell systems expressing CAR at high levels, such as the Lovo cells, but even more in cells with a low-level CAR expression (Figure 1C), indicating that the effect might not be dependent on CAR expression. To block the function of CAR SW480 cells were incubated with antibody against CAR (Santa Crux, N-17) in increasing concentrations from 1:1000 to 1:50 for 24 h and incubated with Ad (MOI 400). Viral uptake was blocked at higher concentrations than 1:500 but treatment with 5-FU still enhanced viral uptake (Figure 2C) accounting for a CAR independent mechanism of 5-FU.
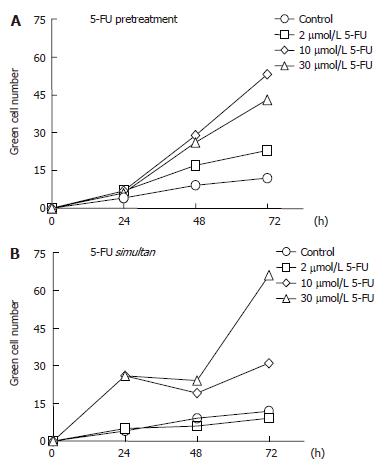
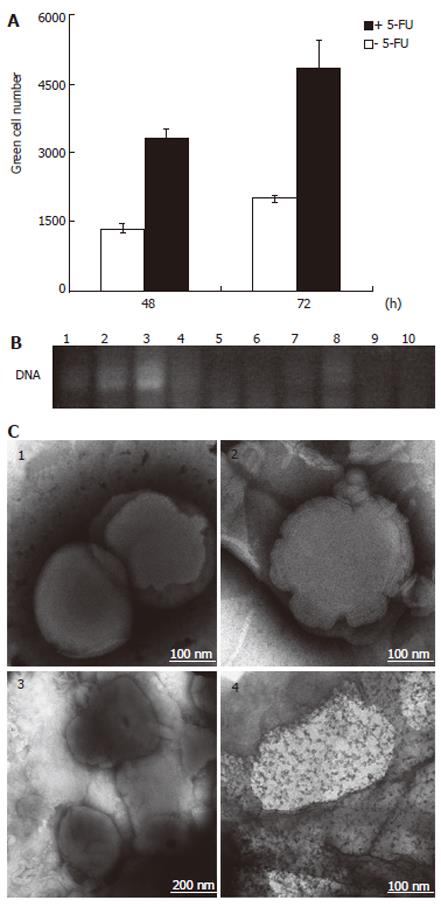
The GFP expression in 5-FU-pretreated SW480 cells was significantly higher than in untreated cells, and the number of cells producing the fluorescent protein increased with increasing concentrations of the anticancer agent up to 10 μmol/L. Indeed, after 48 and 72 h the rate of Ad infection in cell pretreated with 10 µmol/L 5-FU was more than twice the infection rate obtained with 2 μmol/L 5-FU (Figure 3A). At the higher concentrations of 30 μmol/L, however, the drug was shown to be moderately less effective, a phenomenon that could be due to an inhibitory effect of 5-FU on the viral DNA replication (data not shown). All experiments were done in triplicate and the standard deviation was less then 15%. A superior efficacy of 5-FU was observed by its simultaneous application with Ad, which led to an improvement not only in the yield but also in the rate of the infection, especially when the drug was used at 30 μmol/L. Under these conditions the number of green cells counted after 24 h was three times higher than in the case of the drug pretreatment and there was an increment of more than 50% in the density of green cells after 72 h (Figure 3B).
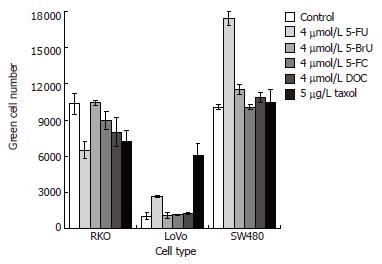
The positive effect of 5-FU on Ad infection could be related to its function as a chemotherapeutic agent or to its structural and chemical characteristics. To assess which structural and chemical features of 5-FU might play a role in the entry of Ad into the cells, the two compounds 5-BrU and 5-FC, which are pyrimidine derivatives as 5-FU, were used in combination with Ad. In 5-BrU the halogen is bigger and less electronegative than in 5-FU. In 5-FC an amino group is present at position 4 instead of a carbonyl group as in 5-FU, thus conferring a higher hydrophilicity to the molecule. In both cases, the level of the infection did not change with respect to Ad alone, indicating that the ability of 5-FU to favor Ad infection is specific and not common to other molecules structurally-related to 5-FU. Additionally to other chemotherapy drugs, such as taxol[12], the cell membrane-destabilizing bile salt sodium deoxycholate[13] was used in combination with Ad. In the case of the DLD-1 and SW480 cells only 5-FU positively influenced the cellular entry of Ad, whereas taxol did not show any effect. In contrast, the infection of the Lovo cells was improved by both anticancer drugs, with taxol being twice more effective than 5-FU (Figure 4). The effect of these drugs was only moderate in the RKO cells, which do express high levels of CAR.
Based on the observation that 5-FU positively affected the Ad uptake independently of CAR, we postulated a 5-FU-mediated transfer of Ad through lipid membranes. To investigate whether 5-FU exerts any effect on ordered lipid structures, a liposome mixture of DOSPA/DOPE at a 3:1 ratio was used to encapsulate the Ad, and the resulting complex was then treated with 5-FU overnight. After centrifugation, the number of infective particles in the supernatant was tested on 293 cells, which provide a system for the replication of E1B deleted Ad mutants. As shown in Figure 5A, the green cell number obtained from the infection with the supernatant of the 5-FU-treated Ad-liposome preparation was at least 2.4 times higher than that obtained from the infection with the supernatant of the same preparation but without 5-FU. This is indicative of an augmented viral concentration in the supernatant as the result of the incubation of the liposome-entrapped Ad with 5-FU. In order to control whether the treatment with 5-FU could induce the release of adenoviral DNA from the liposomes, samples of DNA-liposome solutions with and without 5-FU were subjected to PCR and then analyzed by agarose gel electrophoresis. No adenoviral DNA was visualized by ethidium bromide staining, indicating that there was no release of the DNA component from the liposome complex in the presence of 5-FU. As a control, the DNA-liposome complex was subjected to liposome disruption prior to PCR and the resultant PCR product was visualized on the agarose gel, as it was expected (Figure 5B).
The EM images of the liposomal formulations upon 5-FU treatment showed a multilayer motif that was characterized by an irregular thickness of the liposome as a result of the disappearance of an ordered layer structure in some regions (Figure 5C). Similarly, in the case of the Ad-liposome complex treated with 5-FU, the lipid surface was not uniform, but showed some bright spots that are probably indicative of perturbations in the packing and ordering of the multilayers.
In this study we could demonstrate that 5-FU increases the effectiveness of adenoviral uptake into colorectal cancer cells, thus overcoming the resistance of several colorectal cancer cells to adenoviral treatment. This effect is independent of CAR expression on the cell surface and could be confirmed in a receptor free system. In addition this effect is associated with changes in lipid membranes. Thus, the combination of the anti-tumor drug 5-FU with adenovirus enhances gene transfer capabilities of the virus in colon cancer cells. The synergistic effect of oncolytic Ad (dl1520) and chemotherapeutic agents, such as 5-FU and cisplatin[6-9], is already known, but the reason for such behavior is not completely elucidated. One proposed explanation is the enhancement of cell chemosensitivity induced by viral replication, probably through the expression of the E1A gene that occurs after Ad infection and increases tumor cell killing[14]. Nevertheless, this mechanism does not apply to all cell systems, because it can occur only in those cells that are not resistant to the Ad entry and, more strikingly, our data demonstrate that even E1-deleted constructs, which are replication-deficient, are more effective in the context of 5-FU treatment.
As stated before, a replication defect of human Ad was used to transfer CMV promoter/GFP DNA but 5-FU could probably increase transcription of the CMV promoter similar to FDXR induction by p53 in cells treated with 5-FU[15]. Therefore we transfected cells with CMV promoter/GFP plasmid and could not see any enhancement of GFP expression with of 5-FU. In addition, higher amounts of adenoviral fiber protein were detected in cell lysates after Ad and 5-FU treatment indicating more Ad particles within the cells and suggesting that the higher amount of green cells is a result of higher Ad uptake into cells. Furthermore, it was proposed that the loss of sensitivity of cancer cells to Ad treatment is generally a consequence of loss of CAR representing a severe limitation of the application of gene therapeutic strategies with Ad as a gene delivery system[11]. Interestingly, although the binding of Ad to its receptor is suggested to be the first step for infection, our results indicated that the presence of CAR does not provide a guarantee for efficient viral infection. Indeed, not only cells expressing low levels of CAR, such as the SW480 cells, but also cells expressing normal levels of CAR, such as the DLD-1, Lovo and SW620 cells, were found to be Ad-resistant. However, simultaneous treatment with 5-FU and Ad could enhance the sensitivity to adenoviral infection in all tested cell systems but the effects of 5-FU treatment was more impressing in cell lines which are difficult to infect with Ad. To assess the effect of 5-FU we blocked CAR and could still see enhanced viral uptake. Interestingly, the positive effect of 5-FU was superior when both Ad and 5-FU were added simultaneously in comparison to the pre-incubation of the cells with the drug before the addition of Ad. This suggests that the entry of Ad into cells may occur independently of the production or degradation of effectors caused by 5-FU treatment. Therefore we assessed the capability of Ad to pass through liposomal membranes in the presence of 5-FU and, in accordance with this hypothesis, we observed an increased adenoviral release from liposomes treated with 5-FU.
In light of our experiments on cells differing in CAR expression, we postulate that Ad uptake could be based on a mechanism alternative to that requiring the binding to the Ad receptor. In the presence of CAR, Ad is delivered into the cell via an internalization process involving receptor-mediated endocytosis[16]. Alternatively, in the absence of CAR, the adenoviral protein capsid is likely to interact directly with the phospholipid layers of the cell membrane; however, such interaction results in an effective intracellular transfer only in the presence of 5-FU. The intra- and extra-cellular drug diffusion across the membrane could be coupled to temporary changes in the packing and ordering of the lipid bilayers building the cell membrane, which, in turn, could become more accessible to external agents. Nevertheless, the potential effects of 5-FU on the membrane must be different from those of other amphiphylic molecules that are known to exert a lytic action on membranes, such as DOC[17], as suggested by the observation that this bile salt did not increase the Ad uptake. Previous studies have reported on morphological changes of cells after treatment with adenoviruses[18] or with detergents[17]. Moreover, the interactions between biological or model membranes and hydrophobic drugs, such as 1, 4-dihydropyridines[19] and benzocaine[20], have been investigated in detail, but the exact mechanism of 5-FU with cell membranes remains elusive. The human colon cancer cells tested in this work generally became bigger and adopted a spindle shape upon incubation with 5-FU. This supports our hypothesis that, beside its well-known anti-cancer action, 5-FU may exert a potential disturbing effect at the cell membrane. Interestingly, 5-FU changes intestinal absorption in rat of dextran[21]. However, the exact mechanism of adenoviral passage through lipid membrane in the presence of 5-FU remains unclear.
In conclusion, we suggest that 5-FU might play a role in increasing the sensitivity of cells for environmental influences by changes in the phospholipid bilayers of cell membranes in addition to its chemotherapeutic property. This would be especially useful for the transport of therapeutic compounds independently of the presence or absence of specific cell surface receptors, as in the case of the transfer of Ad into CAR-negative cells. On the other hand, side effects of adenoviral infections during high-dose chemotherapy might not only be based on a suppressed immune system, but, if 5-FU enhances viral uptake, flue-like side effects of the 5-FU chemotherapy might be caused by a higher adenoviral uptake into cells.
S- Editor Pan BR L- Editor Alpini GD E- Editor Bi L
| 1. | Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320-1323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2385] [Cited by in F6Publishing: 2263] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 2. | Li Y, Pong RC, Bergelson JM, Hall MC, Sagalowsky AI, Tseng CP, Wang Z, Hsieh JT. Loss of adenoviral receptor expression in human bladder cancer cells: a potential impact on the efficacy of gene therapy. Cancer Res. 1999;59:325-330. [PubMed] [Cited in This Article: ] |
| 3. | Pearson AS, Koch PE, Atkinson N, Xiong M, Finberg RW, Roth JA, Fang B. Factors limiting adenovirus-mediated gene transfer into human lung and pancreatic cancer cell lines. Clin Cancer Res. 1999;5:4208-4213. [PubMed] [Cited in This Article: ] |
| 4. | Li D, Duan L, Freimuth P, O'Malley BW Jr. Variability of adenovirus receptor density influences gene transfer efficiency and therapeutic response in head and neck cancer. Clin Cancer Res. 1999;5:4175-4181. [PubMed] [Cited in This Article: ] |
| 5. | Okegawa T, Li Y, Pong RC, Bergelson JM, Zhou J, Hsieh JT. The dual impact of coxsackie and adenovirus receptor expression on human prostate cancer gene therapy. Cancer Res. 2000;60:5031-5036. [PubMed] [Cited in This Article: ] |
| 6. | Reid T, Galanis E, Abbruzzese J, Sze D, Andrews J, Romel L, Hatfield M, Rubin J, Kirn D. Intra-arterial administration of a replication-selective adenovirus (dl1520) in patients with colorectal carcinoma metastatic to the liver: a phase I trial. Gene Ther. 2001;8:1618-1626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 181] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Petit T, Davidson KK, Cerna C, Lawrence RA, Von Hoff DD, Heise C, Kirn D, Izbicka E. Efficient induction of apoptosis by ONYX-015 adenovirus in human colon cancer cell lines regardless of p53 status. Anticancer Drugs. 2002;13:47-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Heise C, Lemmon M, Kirn D. Efficacy with a replication-selective adenovirus plus cisplatin-based chemotherapy: dependence on sequencing but not p53 functional status or route of administration. Clin Cancer Res. 2000;6:4908-4914. [PubMed] [Cited in This Article: ] |
| 9. | Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 820] [Cited by in F6Publishing: 753] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 10. | He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509-2514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2861] [Cited by in F6Publishing: 3006] [Article Influence: 115.6] [Reference Citation Analysis (0)] |
| 11. | Graham FL, Prevec L. Adenovirus-based expression vectors and recombinant vaccines. Biotechnology. 1992;20:363-390. [PubMed] [Cited in This Article: ] |
| 12. | Mann J. Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer. 2002;2:143-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 456] [Cited by in F6Publishing: 440] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 13. | Lasch J, Hoffmann J, Omelyanenko WG, Klibanov AA, Torchilin VP, Binder H, Gawrisch K. Interaction of Triton X-100 and octyl glucoside with liposomal membranes at sublytic and lytic concentrations. Spectroscopic studies. Biochim Biophys Acta. 1990;1022:171-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Sánchez-Prieto R, Quintanilla M, Cano A, Leonart ML, Martin P, Anaya A, Ramón y Cajal S. Carcinoma cell lines become sensitive to DNA-damaging agents by the expression of the adenovirus E1A gene. Oncogene. 1996;13:1083-1092. [PubMed] [Cited in This Article: ] |
| 15. | Hwang PM, Bunz F, Yu J, Rago C, Chan TA, Murphy MP, Kelso GF, Smith RA, Kinzler KW, Vogelstein B. Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nat Med. 2001;7:1111-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 335] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 16. | Nemerow GR, Stewart PL. Role of alpha(v) integrins in adenovirus cell entry and gene delivery. Microbiol Mol Biol Rev. 1999;63:725-734. [PubMed] [Cited in This Article: ] |
| 17. | Shiao YJ, Chen JC, Wang CT. The solubilization and morphological change of human platelets in various detergents. Biochim Biophys Acta. 1989;980:56-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Habib NA, Mitry RR, Sarraf CE, Jiao LR, Havlík R, Nicholls J, Jensen SL. Assessment of growth inhibition and morphological changes in in vitro and in vivo hepatocellular carcinoma models post treatment with dl1520 adenovirus. Cancer Gene Ther. 2002;9:414-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Boffi F, Caminiti R, Sadun C, Capuani S, Giovanelli A, Congiu Castellano A. A structural and kinetic study by energy dispersion X-ray diffraction: interaction between 1,4-dihydropyridines and biological membranes. Chem Phys Lett. 1998;286:473-478. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Pinto LM, Yokaichiya DK, Fraceto LF, de Paula E. Interaction of benzocaine with model membranes. Biophys Chem. 2000;87:213-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Hirata K, Horie T. Changes in intestinal absorption of 5-fluorouracil-treated rats. Pharmacol Toxicol. 1999;85:33-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |