Published online Aug 21, 2006. doi: 10.3748/wjg.v12.i31.4959
Revised: April 15, 2006
Accepted: April 21, 2006
Published online: August 21, 2006
AIM: To determine the incidence of Epstein Barr virus associated gastric carcinoma (GC) in Brazil and compare the expressions of apoptosis related proteins and nitric oxide synthases between EBV positive and negative gastric carcinoma.
METHODS: In situ hybridization of EBV-encoded small RNA-1 (EBER-1) and PCR was performed to identify the presence of EBV in GCs. Immunohistochemistry was used to identify expressions of bcl-2, bcl-xl, bak, bax, p53, NOS-1, NOS-2, and NOS-3 proteins in 25 EBV positive GCs and in 103 EBV negative GCS.
RESULTS: 12% of the cases of GC (25/208) showed EBER-1 and EBNA-1 expression. The cases were preferentially of diffuse type with intense lymphoid infiltrate in the stroma. EBV associated GCs showed higher expression of bcl-2 protein and lower expression of bak protein than in EBV negative GCs. Indeed, expressions of NOS-1 and NOS-3 were frequently observed in EBV associated GCs.
CONCLUSION: Our data suggest that EBV infection may protect tumor cells from apoptosis, giving them the capacity for permanent cell cycling and proliferation. In addition, EBV positive GCs show high expression of constitutive NOS that could influence tumor progression and aggressiveness.
- Citation: Begnami MD, Montagnini AL, Vettore AL, Nonogaki S, Brait M, Simoes-Sato AY, Seixas AQA, Soares FA. Differential expression of apoptosis related proteins and nitric oxide synthases in Epstein Barr associated gastric carcinomas. World J Gastroenterol 2006; 12(31): 4959-4965
- URL: https://www.wjgnet.com/1007-9327/full/v12/i31/4959.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i31.4959
Gastric carcinoma (GC) is one of the most common malignant tumors in the world, although its incidence has gradually declined in recent years. In Brazil, gastric cancer remains a prevalent neoplasm with high mortality, ranked as the third highest cause of cancer-related deaths[1].
The correlation between Epstein-Barr virus (EBV) infection and GC is well known. EBV infection is found in 2%-16% of GCs[2-4]. However, the pathogenic role of EBV infection in GCs remains uncertain and little is known regarding the molecular characteristics of these tumors.
Apoptosis is a biological phenomenon of critical importance in the regulation of a number of physiological and pathological situations, such as cancer development. In general, it is thought that viral infection and its associated proteins protect against apoptosis, which would normally cause cancer regression[5]. Among the genes that regulate apoptosis are the members of the bcl-2 family. Expression of bcl-2 protein in gastric tumor cells is frequent and may differ according the histological type[6]. There are few studies that compare the relationship between EBV infection in gastric carcinomas with apoptosis related protein expression[7-9].
Nitric oxide (NO) is a short-lived biomolecule with various biological functions. It is an important bioactive agent and signaling molecule that mediates a diverse array of actions such as vasodilatation, neurotransmission, and iron metabolism. NO is also involved in the multi-step process of carcinogenesis by mediating DNA damage, inducing angiogenesis, and suppressing immune responses[10]. It has been demonstrated that low concentrations of NO can protect tumor cells against apoptosis[11]; alternatively, high concentrations of NO can induce tumor cell death[12]. This small molecule is a product of the conversion of L-arginine to L-citruline by nitric oxide synthases (NOS). There are two groups of NOS, inducible (iNOS or NOS-2) and constitutive (nNOS or NOS-1 and eNOS or NOS-3). Recent studies have examined the expression and activity of NOS in human cancer[13,14]. In gastric carcinomas, many studies addressed the issue, and most of them showed an increased activity and expression of NOS in tumor tissue when compared with normal gastric mucosa[15-18].
To the best of our acknowledgement, the relationship between NOS expression and EBV related gastric cancer has not been investigated. Thus, the aim of the present study is to determine the prevalence of EBV infection in GCs in a Brazilian population and investigate the expression of apoptosis related proteins and nitric oxide synthases in EBV positive and EBV negative gastric carcinomas.
Two hundred and eight gastric carcinomas, surgically resected from 1995 to 1998, were analyzed for EBV status using in situ hybridization and PCR. To compare EBV positive and negative carcinomas, we selected 103 EBV negative GC, which were samples that were previously studied for expression of apoptosis related proteins and NOS. The cases were reviewed, and representative formalin-fixed, paraffin-embedded blocks from the tumors were selected. Clinicopathological findings were obtained from surgical records and pathology reports. New paraffin sections were stained with hematoxylin and eosin (H&E), and they were assessed independently by two pathologists (MDB & FAS). Histologically, the tumors were classified according to Lauren’s classification system in intestinal or diffuse types[19]. Also we graded the amount of lymphoid infiltrate within or between the tumor cells as minimal/mild or moderate/intense.
Sections (5-6 μm thick) on glass slides were prepared for ISH with a fluorescein-conjugated oligonucleotide probe for EBER-1 (Novocastra, Newscastle- Upon- Tyne, U.K.). Briefly, after deparaffinization and dehydration the sections were treated with proteinase K (Sigma, St. Louis, MO) for 15 min at 37°C. After washing and dehydration, the FITC- conjugated probe was applied and hybridized overnight at 37°C. The hybridization was further detected by rabbit anti- FITC antibody conjugated with alkaline phosphatase (Novocastra) for 30 min at room temperature. The slides were counterstained with a light Mayer’s haematoxylin. Negative controls had no EBER-1 probe applied.
Sections (5-6 μm thick) from the same tumor blocks, used for EBER detection, were immunohistochemically analyzed using the standard streptavidin- biotin- peroxidase method. The staining was done using the microwave antigen retrieval technique (95°C for 30 min in citrate buffer pH 6.0) for all antibodies. Sections were incubated with antibodies against p53 (DO-7, 1:100, Dako, Copenhagen, Denmark), Bcl-2 (124, 1:50, Dako, Copenhagen, Denmark), Bax (bax, 1:50, Dako, Copenhagen, Denmark), Bcl-xl (bcl-xl, 1:50, Dako, Copenhagen, Denmark), Bak (bak, 1:400, Dako, Copenhagen, Denmark), NOS-1 (nNOS, 1:200, Transduction Laboratories, USA), NOS-2 (iNOS, 1:40, Transduction Laboratories, USA), and NOS-3 (eNOS, 1:50, Transduction Laboratories, USA) at 4°C overnight. After reagenting them with biotinylated secondary anti-mouse antibodies, the antigen-antibody reactions were visualized using streptavidin-horseradish peroxidase conjugate (Dako LSAB Kit, Los Angeles, CA). The peroxidase activity was localized by 0.05% 3, 3’-diaminobenzidine and 0.03% hydrogen peroxide in Tris- buffered saline. The slides were counterstained with Mayer’s hematoxylin. The percentage of positively stained tumor cells in each tumor section was blinded evaluated by counting at least 1000 cells in 10 randomly selected high-power fields. Brown staining for p53 protein was located in nuclei; staining for bcl-2, bcl-xl, bak, bax, NOS-1, NOS-2, and NOS-3 protein was located in cytoplasm. The section was considered as expressing the protein if cellular staining was ≥ 5%[5].
The DNA of cancerous tissues from 25 patients with EBV positive gastric carcinomas was obtained from paraffin-embedded surgical blocks. The DNA was extracted by prolonged proteinase K digestion. Briefly, after deparaffinization with xylene and rehydration in ethanol, the tissue was resuspended in 300 μL of SDS-Proteinase K solution (1% SDS; 9 mmol/L Tris-HCl, pH 9.0; 2.25 mmol/L EDTA; 56.5 mmol/L NaCl; 1 μg/μL Proteinase K (invitrogen) and incubated at 48°C for 48 h. Each 12 h, 20 μL of 20 μg/μL Proteinase K were added. After extraction with an equal volume of phenol-chloroform, the aqueous layer was transferred to a new tube and the DNA was precipitated by the addition of 2 μL of 20 mg/mL glycogen, 100 μL of ammonium acetate (7.5 mol/L) and 800 μL of 100% ethanol followed by incubation overnight at -20°C. After centrifugation, the DNA was resuspended in 50 μL of water. PCR was performed in a reaction final volume of 25 μL, containing 10 μL of DNA; 2.5 μL of 10 × PCR buffer; 1.5 μL of 25 mmol/L MgCl2; 2 μL of 2.5 mmol/L dNTP mix; 1 μL of 10 μmol/L EBNA1F and EBNA1R primers; and 0.3 μL of Taq DNA polymerase. The following thermal cycle conditions were used: 94°C for 5 min, 35 cycles of 94°C for 30 s, 60°C for 45 s, and 72°C for 45 s. After the last cycle, a final extension at 72°C for 7 min was carried out. The sequences of the primers were: EBNA-1F (5’- GTCATCATCATCCGGGTCTC-3’) and EBNA-1R (5’-TTCGGGTTGGAACCTCCTTG-3’). The amplified products were visualized by electrophoresis in 8% poliacrylamide gels.
Statistical comparison among groups was performed using χ2 test and Fisher’s exact test when appropriate. P values less than 0.05 were considered statistically significant.
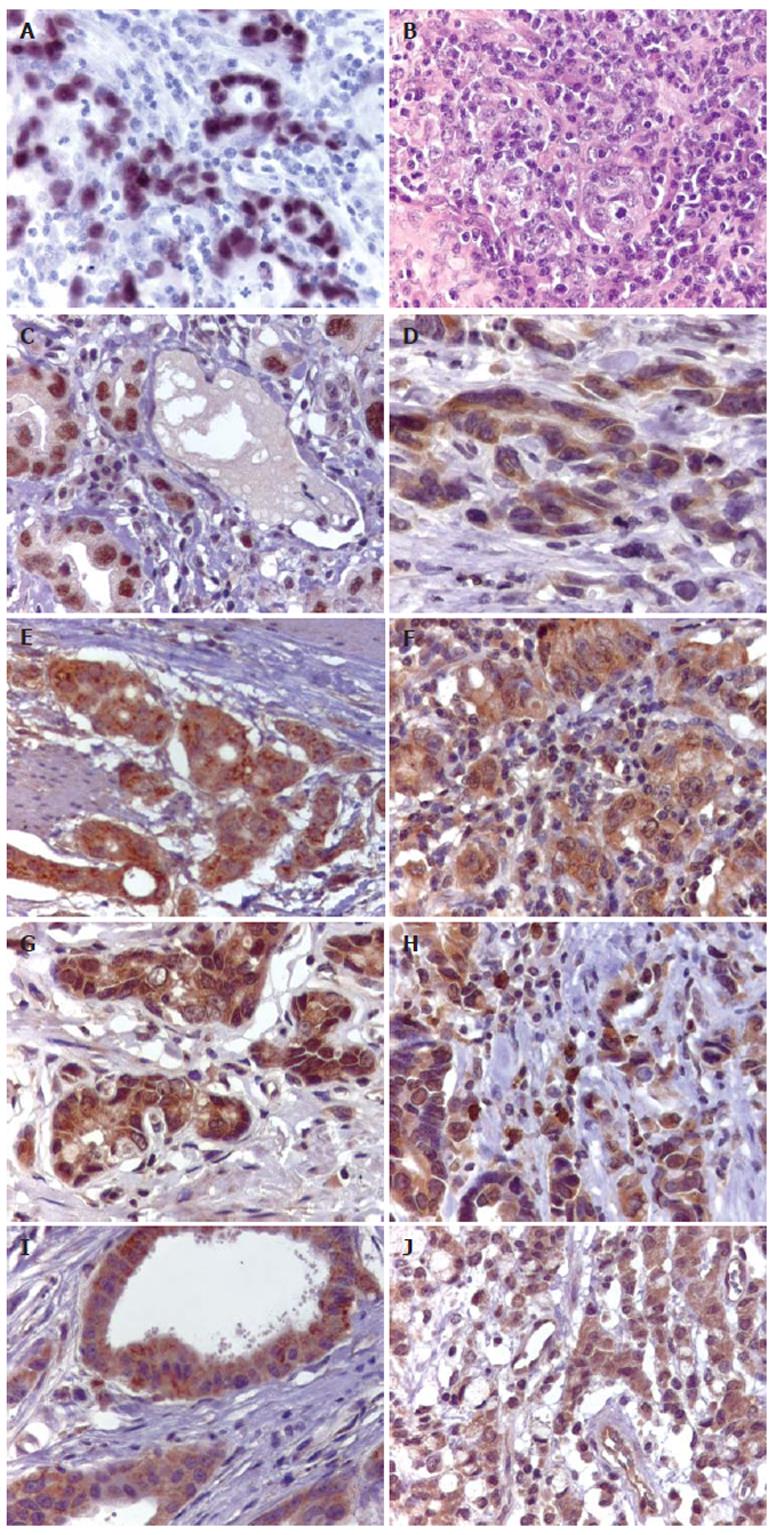
EBER-1 expression and EBNA-1 products were visualized in 25 out of 208 (12%) cases of gastric carcinomas (Figure 1A).
The clinicopathological characteristics of EBV positive and negative GCs are given in Table 1. The male/female ratio in patients with EBV positive GCs and those with EBV negative GCs was 4:1 and 2:1, respectively, and no difference was seen in the average age at 60.5 and 61.2 years, respectively. 68% of EBV positive GCs were in the body and cardia and 32% of them were in the antrum. In the group of EBV negative GCs, 80% were in the body and cardia and only 20% of them were in the antrum, showing predilection for the body and cardia region in both groups. Regarding histological type, 18 of 25 (72%) EBV positive GCs were diffuse type and 7 (28%) were intestinal. 60% of EBV negative GCs were intestinal type and 41 of 103 (40%) were diffuse, resulting in a strong association between histological type and EBV infection in gastric carcinomas (P < 0.01). EBV associated GCs also correlated with tumor infiltrating lymphocytes. 19 of 25 (76%) EBV positive GCs showed marked lymphoid stroma evenly distributed with the malignant epithelial cells (Figure 1B). In the EBV negative GCs only 30% of the cases showed marked lymphocytic infiltration (P < 0.01). Metastases in lymph nodes were found in 18 of 25 (72%) EBV positive GCs and in 60 of 103 (59%) EBV negative cases. This difference was not statistically significant.
| EBV positive gastric carcinomas (n = 25) | EBV negative gastric carcinomas (n = 103) | P value | |
| Gender | NS | ||
| Male | 20 (80%) | 68 (66%) | |
| Female | 5 (20%) | 35 (34%) | |
| Age: range(mean)1 | 35-78 (60.5) | 25-84 (61.2) | NS |
| Site of tumor | NS | ||
| Body and Cardia | 8 (32%) | 21 (20%) | |
| Antrum | 17 (68%) | 82 (80%) | |
| LN metastasis | NS | ||
| Absent | 7 (28%) | 43 (41%) | |
| Present | 18 (72%) | 60 (59%) | |
| Histological type | < 0.05 | ||
| Diffuse | 18 (72%) | 41 (40%) | |
| Intestinal | 7 (28%) | 62 (60%) | |
| Lymphoid stroma | < 0.05 | ||
| Minimal/mild | 6 (24%) | 71 (69%) | |
| Moderate/intense | 19 (76%) | 32 (31%) |
Table 2 shows the pattern of protein expression in EBV positive and negative GCs. P53 protein expression was observed in the nucleus of the tumor cells with a uniform staining (Figure 1C). 11 of 25 (44%) EBV positive GCs and 55 of 103 (51%) EBV negative GCs were positive for p53 and no difference was observed between the groups. Expression of bcl-2 protein was observed in 28% of EBV positive GCs and in only 11% of the EBV negative GCs (Figure 1D), showing a statistically significant correlation between EBV status and bcl-2 expression in GCs (P < 0.05). The rates of the bcl-xl and bax expressions in EBV positive and negative GCs were 88% and 93%, and 96% and 86%, respectively (Figures 1E and 1F). None of the differences were statistically significant. Bak expression was lower in the EBV positive GCs than in EBV negative tumors (Figure 1G) and it was detected in 11 of 25 (44%) EBV positive GCs, and in 71 of 103 (69%) EBV negative GCs. This difference was statistically significant (P < 0.01).
| EBV positive gastric carcinoma (n = 25) | EBV negative gastric carcinoma (n = 103) | P value | |
| P53 | NS | ||
| Positive | 11 (44) | 55 (54) | |
| Negative | 14 (56) | 48 (46) | |
| Bcl-2 | < 0.05 | ||
| Positive | 7 (28) | 11 (11) | |
| Negative | 18 (72) | 92 (89) | |
| Bcl-x | NS | ||
| Positive | 22 (88) | 96 (93) | |
| Negative | 3 (12) | 7 (7) | |
| Bak | < 0.05 | ||
| Positive | 11 (44) | 71 (69) | |
| Negative | 14 (56) | 32 (31) | |
| Bax | NS | ||
| Positive | 24 (96) | 88 (86) | |
| Negative | 1 (4) | 15 (14) |
NOS were observed in the cytoplasm of the tumor, epithelial, endothelial, smooth muscle and inflammatory cells. For final analysis, we scored the expression of these enzymes only in the tumor cells (Figures 1H, 1I, and 1J). NOS expressions were significantly different in EBV positive and negative GCs. Overexpression of constitutive forms of NOS (NOS-1 and NOS-3) were more frequently observed in EBV positive than in EBV negative GCs (P < 0.01). The majority of GC cases were negative for an inducible form of NOS (NOS-2), independently of EBV status. Comparisons of NOS expressions and EBV status are shown in Table 3.
| EBV positive gastric carcinoma (n = 25) | EBV negative gastric carcinoma (n = 103) | P value | |
| NOS1(nNOS) | < 0.05 | ||
| Positive | 23 (92) | 69 (67) | |
| Negative | 2 (8) | 34 (33) | |
| NOS2 (iNOS) | NS | ||
| Positive | 5 (20) | 31(30) | |
| Negative | 20 (80) | 72 (70) | |
| NOS3 (eNOS) | < 0.05 | ||
| Positive | 18 (72) | 36 (35) | |
| Negative | 7 (28) | 67 (65) |
The relationship between Epstein-Barr virus and various epithelioid diseases has already been demonstrated. Involvement of EBV has been described in the etiopathogenesis of not only the nasopharyngeal carcinoma but other carcinomas as well, including gastric carcinomas[20-22].
It is estimated that EBV infection can be found in about 10% of GCs worldwide, especially those with marked lymphocytic infiltration[23-27]. We observed EBV infection in 12% of GCs. Previous studies showed the incidence of EBV infection in Brazilian GCs ranging between 5%-11.32%[28-30]. Some reports described the presence of EBV and its products as restricted on the carcinoma cells[31-33], whereas others found this virus in the precursor epithelial lesions as well as in the lymphoid cells[2,7]. In our study the virus was detected in the neoplastic cells but not in the normal, dysplastic gastric epithelium, or in inflammatory cells. This finding indicates that EBV affects the gastric epithelium at a late stage of the multistep process of gastric carcinogenesis.
The role of EBV in neoplastic transformation of gastric epithelial cells is not completely understood. It has been suggested that EBV positive GCs display specific clinicopathological features compared with EBV negative GCs. EBV associated GCs had been characterized by male predominance, preferential location in proximal stomach, and a high prevalence of diffuse types[2,34-36]. In our series, EBV positive GCs were frequently seen in males, in the body and cardia region, and in diffuse carcinomas in agreement with previous reports. Indeed we observed a strong association between EBV infection and lymphoid stroma. The presence of marked lymphoid infiltrate has already been demonstrated and there is growing evidence that extensive lymphocyte infiltration is a consistent characteristic of EBV positive carcinomas and correlates with less aggressive behavior, although no additional information is available regarding follow-up of the patients in the present study.
Molecular analysis of EBV positive carcinoma has been shown to have distinct characteristics including different chromosomal aberrations[37], different and lower frequencies of microsatellite instability[35] and allelic loss[38], and more CpG methylation[39]. However, studies have focused on the relationship between EBV and oncogenes or tumor suppressor genes in EBV associated GCs, but no conclusive results have been reported[5,7,9,40,41]. In this study, expression of apoptosis related proteins and nitric oxide synthases was examined in EBV associated and EBV negative gastric carcinomas. In this report, we observed a correlation between bcl-2 and bak expressions and EBV status. Bcl-2 protein expression was observed more frequently in EBV positive than in EBV negative GCs. On the other hand, positivity for bak protein was more common in EBV negative than in EBV positive GCs. It is already known that the balance between anti-apoptotic and pro-apoptotic members of the bcl-2 family genes determine the outcome of patients with tumors. Bak protein is shown to induce cell death, while bcl-2 can protect cells from apoptosis. Previous reports indicated that EBV infection may protect tumor cells from apoptosis inducing expression of the anti-apoptotic proteins[41]. Our results showed additional information and confirm that EBV infection and apoptosis related proteins interact to negatively influence apoptosis, through high expression of bcl-2 and low expression of bak in gastric tumor cells. Bax and bcl-xl protein expressions were found in both EBV positive and negative GCs and there was no statistical difference between the groups.
There is some indication that EBV modulates and mutates p53 for its own survival[42], however earlier reports showed contradictory results[8,38,43]. In turn, p53 gene mutations are rarely identified in EBV associated carcinomas[44]. The obtained results in our study showed disturbed function of p53 in almost all cases of EBV positive and EBV negative GCs. Although p53 expression seemed to be slightly lower in EBV positive GCs, that difference was not statistically significant. These results indicated that abnormalities in p53 observed in GCs are independent of EBV infection.
It has been reported that nitric oxide synthases are present in human tumor cell lines and solid tumor tissues[14,16,45-47]. Increased NOS expression has been observed in different tumors tissues when compared with normal tissues[14,48,49].
To the best of our knowledge, this is the first report showing NOS expression is associated with EBV infection gastric cancer. In EBV positive GCs, NOS-1 and NOS-3 proteins were more frequent positive in tumor cells than NOS-2. The results agree with previous data that showed higher activity of constitutive rather than inducible NOS in human gastric tumors[15]. In a recent study, the authors demonstrated significantly high NOS-3 expression in gastric tumors and a directly relationship with tumor angiogenesis and the overall aggressive biology of gastric cancer[50]. Our study showed higher NOS-3 expression in EBV positive GCs than in EBV negative GCs. Increasing evidence suggests that NOS-3 expression, although constitutive, can be regulated by various hormones, cytokines, and growth factors, and by genetic alterations, such as oncogene activation and tumor suppressor inactivation[51-54]. Our data indicate that EBV infection and its products can affect the regulation of this enzyme resulting in elevated NO production. It has been showed that elevated NO production enhances the growth of some tumors through the suppression of anti-tumor immune responses[55-57].
NOS-2 expression was decreased in the whole group of gastric tumors in our study. This feature has been demonstrated by immunohistochemistry in other tumors and there is a possible relationship between loss of NO and carcinogenenesis[15,16]. However, many publications showed elevated NOS expression and increased activity in gastric cancer[58-60]. Although the mechanisms for the antiviral action of NO have not been clarified, the multiplicity of host enzymes it targets makes it possible that multiple alterations in host cell proteins are involved. An apparent role of NO in inhibiting EBV reactivation from latency was demonstrated in a study using gastric cells lines in culture[61]. This finding led us to propose that EBV latency can be directly and/or indirectly associated with NOS gene expression and also lead us to postulate that NO might also be an important factor in the host EBV relationship. Our data suggest that the increased expression of NOS-1 and NOS-3 in EBV positive GC renders a major contribution to high activity of NOS in this specific group of tumors.
In conclusion, it is clear that EBV is associated with gastric carcinomas. EBV positive GCs have a distinct protein expression profile, as well as distinct clinicopathological features. This present data suggest that EBV infection may protect tumor cells from apoptosis, giving them the capacity for permanent cell cycling and proliferation. Indeed, EBV positive GCs have high expression of constitutive NOS, which might be associated with tumor progression or aggressiveness. A larger number of cases and long-term follow-up are necessary to further investigate this possibility.
S- Editor Wang J L- Editor Lutze M E- Editor Bai SH
| 1. | Brasil.Ministério da Saúde. Secretaria Nacional de Assistência à Saúde. Instituto Nacional do Câncer. Estimativa da Incidência e Mortalidade por Câncer no Brasil. Rio de Janeiro: INCA 2006; Available from: http://www.inca.gov.br/estimativa/2006. [Cited in This Article: ] |
| 2. | Shibata D, Weiss LM. Epstein-Barr virus-associated gastric adenocarcinoma. Am J Pathol. 1992;140:769-774. [PubMed] [Cited in This Article: ] |
| 3. | Rowlands DC, Ito M, Mangham DC, Reynolds G, Herbst H, Hallissey MT, Fielding JW, Newbold KM, Jones EL, Young LS. Epstein-Barr virus and carcinomas: rare association of the virus with gastric adenocarcinomas. Br J Cancer. 1993;68:1014-1019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 78] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Osato T, Imai S. Epstein-Barr virus and gastric carcinoma. Semin Cancer Biol. 1996;7:175-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Ishii H, Gobé G, Kawakubo Y, Sato Y, Ebihara Y. Interrelationship between Epstein-Barr virus infection in gastric carcinomas and the expression of apoptosis-associated proteins. Histopathology. 2001;38:111-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | van der Woude CJ, Kleibeuker JH, Tiebosch AT, Homan M, Beuving A, Jansen PL, Moshage H. Diffuse and intestinal type gastric carcinomas differ in their expression of apoptosis related proteins. J Clin Pathol. 2003;56:699-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Gulley ML, Pulitzer DR, Eagan PA, Schneider BG. Epstein-Barr virus infection is an early event in gastric carcinogenesis and is independent of bcl-2 expression and p53 accumulation. Hum Pathol. 1996;27:20-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 119] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Ishii HH, Gobe GC, Yoneyama J, Mukaide M, Ebihara Y. Role of p53, apoptosis, and cell proliferation in early stage Epstein-Barr virus positive and negative gastric carcinomas. J Clin Pathol. 2004;57:1306-1311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Ohfuji S, Osaki M, Tsujitani S, Ikeguchi M, Sairenji T, Ito H. Low frequency of apoptosis in Epstein-Barr virus-associated gastric carcinoma with lymphoid stroma. Int J Cancer. 1996;68:710-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 10. | Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109-142. [PubMed] [Cited in This Article: ] |
| 11. | Brüne B, von Knethen A, Sandau KB. Nitric oxide and its role in apoptosis. Eur J Pharmacol. 1998;351:261-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 345] [Cited by in F6Publishing: 330] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Moochhala S, Chhatwal VJ, Chan ST, Ngoi SS, Chia YW, Rauff A. Nitric oxide synthase activity and expression in human colorectal cancer. Carcinogenesis. 1996;17:1171-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 81] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Ambs S, Hussain SP, Harris CC. Interactive effects of nitric oxide and the p53 tumor suppressor gene in carcinogenesis and tumor progression. FASEB J. 1997;11:443-448. [PubMed] [Cited in This Article: ] |
| 14. | Ambs S, Merriam WG, Ogunfusika MO, Bennett WP, Ishibe N, Hussain SP, Tzeng EE, Geller DA, Billiar TR, Harris CC. p53 and vascular endothelial growth factor regulate tumor growth of NOS2-expressing human carcinoma cells. Nat Med. 1998;4:1371-1376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 223] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Koh E, Noh SH, Lee YD, Lee HY, Han JW, Lee HW, Hong S. Differential expression of nitric oxide synthase in human stomach cancer. Cancer Lett. 1999;146:173-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Rajnakova A, Goh PM, Chan ST, Ngoi SS, Alponat A, Moochhala S. Expression of differential nitric oxide synthase isoforms in human normal gastric mucosa and gastric cancer tissue. Carcinogenesis. 1997;18:1841-1845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Son HJ, Kim YH, Park DI, Kim JJ, Rhee PL, Paik SW, Choi KW, Song SY, Rhee JC. Interaction between cyclooxygenase-2 and inducible nitric oxide synthase in gastric cancer. J Clin Gastroenterol. 2001;33:383-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Yamaguchi K, Saito H, Oro S, Tatebe S, Ikeguchi M, Tsujitani S. Expression of inducible nitric oxide synthase is significantly correlated with expression of vascular endothelial growth factor and dendritic cell infiltration in patients with advanced gastric carcinoma. Oncology. 2005;68:471-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | LAUREN P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] [Cited in This Article: ] |
| 20. | Mori M, Watanabe M, Tanaka S, Mimori K, Kuwano H, Sugimachi K. Epstein-Barr virus-associated carcinomas of the esophagus and stomach. Arch Pathol Lab Med. 1994;118:998-1001. [PubMed] [Cited in This Article: ] |
| 21. | Leoncini L, Vindigni C, Megha T, Funtò I, Pacenti L, Musarò M, Renieri A, Seri M, Anagnostopoulos J, Tosi P. Epstein-Barr virus and gastric cancer: data and unanswered questions. Int J Cancer. 1993;53:898-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Harn HJ, Chang JY, Wang MW, Ho LI, Lee HS, Chiang JH, Lee WH. Epstein-Barr virus-associated gastric adenocarcinoma in Taiwan. Hum Pathol. 1995;26:267-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 70] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Burke AP, Yen TS, Shekitka KM, Sobin LH. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod Pathol. 1990;3:377-380. [PubMed] [Cited in This Article: ] |
| 24. | Matsunou H, Konishi F, Hori H, Ikeda T, Sasaki K, Hirose Y, Yamamichi N. Characteristics of Epstein-Barr virus-associated gastric carcinoma with lymphoid stroma in Japan. Cancer. 1996;77:1998-2004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 25. | Cho YJ, Chang MS, Park SH, Kim HS, Kim WH. In situ hybridization of Epstein-Barr virus in tumor cells and tumor-infiltrating lymphocytes of the gastrointestinal tract. Hum Pathol. 2001;32:297-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Kijima Y, Hokita S, Takao S, Baba M, Natsugoe S, Yoshinaka H, Aridome K, Otsuji T, Itoh T, Tokunaga M. Epstein-Barr virus involvement is mainly restricted to lymphoepithelial type of gastric carcinoma among various epithelial neoplasms. J Med Virol. 2001;64:513-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Shibata D, Tokunaga M, Uemura Y, Sato E, Tanaka S, Weiss LM. Association of Epstein-Barr virus with undifferentiated gastric carcinomas with intense lymphoid infiltration. Lymphoepithelioma-like carcinoma. Am J Pathol. 1991;139:469-474. [PubMed] [Cited in This Article: ] |
| 28. | Hayashi K, Chen WG, Chen YY, Murakami I, Chen HL, Ohara N, Nose S, Hamaya K, Matsui S, Bacchi MM. Deletion of Epstein-Barr virus latent membrane protein 1 gene in Japanese and Brazilian gastric carcinomas, metastatic lesions, and reactive lymphocytes. Am J Pathol. 1998;152:191-198. [PubMed] [Cited in This Article: ] |
| 29. | Koriyama C, Akiba S, Iriya K, Yamaguti T, Hamada GS, Itoh T, Eizuru Y, Aikou T, Watanabe S, Tsugane S. Epstein-Barr virus-associated gastric carcinoma in Japanese Brazilians and non-Japanese Brazilians in São Paulo. Jpn J Cancer Res. 2001;92:911-917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Lopes LF, Bacchi MM, Elgui-de-Oliveira D, Zanati SG, Alvarenga M, Bacchi CE. Epstein-Barr virus infection and gastric carcinoma in São Paulo State, Brazil. Braz J Med Biol Res. 2004;37:1707-1712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Yamamoto N, Tokunaga M, Uemura Y, Tanaka S, Shirahama H, Nakamura T, Land CE, Sato E. Epstein-Barr virus and gastric remnant cancer. Cancer. 1994;74:805-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 32. | Imai S, Koizumi S, Sugiura M, Tokunaga M, Uemura Y, Yamamoto N, Tanaka S, Sato E, Osato T. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc Natl Acad Sci USA. 1994;91:9131-9135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 345] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 33. | Zur Hausen A, van Rees BP, van Beek J, Craanen ME, Bloemena E, Offerhaus GJ, Meijer CJ, van den Brule AJ. Epstein-Barr virus in gastric carcinomas and gastric stump carcinomas: a late event in gastric carcinogenesis. J Clin Pathol. 2004;57:487-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Lee HS, Chang MS, Yang HK, Lee BL, Kim WH. Epstein-barr virus-positive gastric carcinoma has a distinct protein expression profile in comparison with epstein-barr virus-negative carcinoma. Clin Cancer Res. 2004;10:1698-1705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Chang MS, Lee HS, Kim CW, Kim YI, Kim WH. Clinicopathologic characteristics of Epstein-Barr virus-incorporated gastric cancers in Korea. Pathol Res Pract. 2001;197:395-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | van Beek J, zur Hausen A, Klein Kranenbarg E, van de Velde CJ, Middeldorp JM, van den Brule AJ, Meijer CJ, Bloemena E. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol. 2004;22:664-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 37. | zur Hausen A, van Grieken NC, Meijer GA, Hermsen MA, Bloemena E, Meuwissen SG, Baak JP, Meijer CJ, Kuipers EJ, van den Brule AJ. Distinct chromosomal aberrations in Epstein-Barr virus-carrying gastric carcinomas tested by comparative genomic hybridization. Gastroenterology. 2001;121:612-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | van Rees BP, Caspers E, zur Hausen A, van den Brule A, Drillenburg P, Weterman MA, Offerhaus GJ. Different pattern of allelic loss in Epstein-Barr virus-positive gastric cancer with emphasis on the p53 tumor suppressor pathway. Am J Pathol. 2002;161:1207-1213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Kang GH, Lee S, Kim WH, Lee HW, Kim JC, Rhyu MG, Ro JY. Epstein-barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. Am J Pathol. 2002;160:787-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 262] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 40. | Leung SY, Chau KY, Yuen ST, Chu KM, Branicki FJ, Chung LP. p53 overexpression is different in Epstein-Barr virus-associated and Epstein-Barr virus-negative carcinoma. Histopathology. 1998;33:311-317. [PubMed] [Cited in This Article: ] |
| 41. | Kume T, Oshima K, Shinohara T, Takeo H, Yamashita Y, Shirakusa T, Kikuchi M. Low rate of apoptosis and overexpression of bcl-2 in Epstein-Barr virus-associated gastric carcinoma. Histopathology. 1999;34:502-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Moritani S, Sugihara H, Kushima R, Hattori T. Different roles of p53 between Epstein-Barr virus-positive and -negative gastric carcinomas of matched histology. Virchows Arch. 2002;440:367-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Szkaradkiewicz A, Majewski W, Wal M, Czyzak M, Majewski P, Bierła J, Kuch A. Epstein-Barr virus (EBV) infection and p53 protein expression in gastric carcinoma. Virus Res. 2006;118:115-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Wang Y, Luo B, Yan LP, Huang BH, Zhao P. Relationship between Epstein-Barr virus-encoded proteins with cell proliferation, apoptosis, and apoptosis-related proteins in gastric carcinoma. World J Gastroenterol. 2005;11:3234-3239. [PubMed] [Cited in This Article: ] |
| 45. | Thomsen LL, Miles DW, Happerfield L, Bobrow LG, Knowles RG, Moncada S. Nitric oxide synthase activity in human breast cancer. Br J Cancer. 1995;72:41-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 481] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 46. | Cobbs CS, Brenman JE, Aldape KD, Bredt DS, Israel MA. Expression of nitric oxide synthase in human central nervous system tumors. Cancer Res. 1995;55:727-730. [PubMed] [Cited in This Article: ] |
| 47. | Rosbe KW, Prazma J, Petrusz P, Mims W, Ball SS, Weissler MC. Immunohistochemical characterization of nitric oxide synthase activity in squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 1995;113:541-549. [PubMed] [Cited in This Article: ] |
| 48. | Fujimoto H, Ando Y, Yamashita T, Terazaki H, Tanaka Y, Sasaki J, Matsumoto M, Suga M, Ando M. Nitric oxide synthase activity in human lung cancer. Jpn J Cancer Res. 1997;88:1190-1198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Radomski MW, Jenkins DC, Holmes L, Moncada S. Human colorectal adenocarcinoma cells: differential nitric oxide synthesis determines their ability to aggregate platelets. Cancer Res. 1991;51:6073-6078. [PubMed] [Cited in This Article: ] |
| 50. | Wang L, Shi GG, Yao JC, Gong W, Wei D, Wu TT, Ajani JA, Huang S, Xie K. Expression of endothelial nitric oxide synthase correlates with the angiogenic phenotype of and predicts poor prognosis in human gastric cancer. Gastric Cancer. 2005;8:18-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1-12. [PubMed] [Cited in This Article: ] |
| 52. | Li H, Wallerath T, Münzel T, Förstermann U. Regulation of endothelial-type NO synthase expression in pathophysiology and in response to drugs. Nitric Oxide. 2002;7:149-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 53. | Wu KK. Regulation of endothelial nitric oxide synthase activity and gene expression. Ann N Y Acad Sci. 2002;962:122-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | Geller DA, Billiar TR. Molecular biology of nitric oxide synthases. Cancer Metastasis Rev. 1998;17:7-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 243] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 55. | Takahashi Y, Bucana CD, Akagi Y, Liu W, Cleary KR, Mai M, Ellis LM. Significance of platelet-derived endothelial cell growth factor in the angiogenesis of human gastric cancer. Clin Cancer Res. 1998;4:429-434. [PubMed] [Cited in This Article: ] |
| 56. | Morisaki T, Katano M, Ikubo A, Anan K, Nakamura M, Nakamura K, Sato H, Tanaka M, Torisu M. Immunosuppressive cytokines (IL-10, TGF-beta) genes expression in human gastric carcinoma tissues. J Surg Oncol. 1996;63:234-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 57. | Bredt DS, Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci USA. 1989;86:9030-9033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1196] [Cited by in F6Publishing: 1295] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 58. | Rajnakova A, Moochhala S, Goh PM, Ngoi S. Expression of nitric oxide synthase, cyclooxygenase, and p53 in different stages of human gastric cancer. Cancer Lett. 2001;172:177-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | Li LG, Xu HM. Inducible nitric oxide synthase, nitrotyrosine and apoptosis in gastric adenocarcinomas and their correlation with a poor survival. World J Gastroenterol. 2005;11:2539-2544. [PubMed] [Cited in This Article: ] |
| 60. | Wang GY, Ji B, Wang X, Gu JH. Anti-cancer effect of iNOS inhibitor and its correlation with angiogenesis in gastric cancer. World J Gastroenterol. 2005;11:3830-3833. [PubMed] [Cited in This Article: ] |
| 61. | Gao X, Tajima M, Sairenji T. Nitric oxide down-regulates Epstein-Barr virus reactivation in epithelial cell lines. Virology. 1999;258:375-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |