Published online Apr 28, 2006. doi: 10.3748/wjg.v12.i16.2569
Revised: October 16, 2005
Accepted: October 26, 2005
Published online: April 28, 2006
AIM: To determine the diagnostic value of the rabeprazole test in patients seen by general practitioners.
METHODS: Eighty-three patients with symptoms suggestive of GERD were enrolled by general practitioners in this multi-centre, randomized and double-blind study. All patients received either rabeprazole (20 mg bid) or a placebo for one week. The diagnosis of GERD was established on the presence of mucosal breaks at endoscopy and/or an abnormal esophageal 24-h pH test. The test was considered to be positive if patients reported at least a “clear improvement” of symptoms on a 7-point Likert scale.
RESULTS: The sensitivities of the test for rabeprazole and the placebo were 83% and 40%, respectively. The corresponding specificity, positive and negative predictive values were 45% and 67%, 71% and 71%, and 62% and 35%, respectively. A receiver operating characteristics (ROC) analysis confirmed that the best discriminatory cut-off corresponded to description of “clear improvement”.
CONCLUSION: The poor specificity of the proton-pump inhibitor (PPI) test does not support such an approach to establish a diagnosis of GERD in a primary care setting.
- Citation: Varannes SBD, Sacher-Huvelin S, Vavasseur F, Masliah C, Rhun ML, Aygalenq P, Bonnot-Marlier S, Lequeux Y, Galmiche JP. Rabeprazole test for the diagnosis of gastro-oesophageal reflux disease: Results of a study in a primary care setting. World J Gastroenterol 2006; 12(16): 2569-2573
- URL: https://www.wjgnet.com/1007-9327/full/v12/i16/2569.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i16.2569
Gastro-esophageal reflux disease (GERD) is one of the most common disorders observed by primary care physicians. In this setting, an accurate, non-invasive and safe diagnostic test would be of great use. Proton-pump inhibitors (PPIs) are the most potent suppressors of gastric acid secretion and represent the mainstay of GERD treatment, with a therapeutic effect throughout the spectrum of the disorder. Therefore, in clinical practice, many physicians consider that rapid symptom relief after a short course of PPI therapy is a valuable marker for a diagnosis of GERD. This represents the basis for the development of so-called PPI tests, the value of which has previously been assessed by a number of different investigators, using various molecules which were tested in assorted referral populations, mainly in a secondary or tertiary care setting. The various PPIs and dosages, the duration of the PPI course and the way the results are interpreted are likely to be responsible for the conflicting results previously reported in the literature[1]. Rabeprazole is a more recently developed PPI with specific pharmacological properties such as a high pKa which may lead to both rapid accumulation in the acidic compartment of the parietal cell and more effective control of acidity during the first day of administration[2,3]. Similarly, when the target population for such PPI tests is considered, the lack of drug interference and the safety profile are both of most importance. In these respects, rabeprazole also displays some pharmacological advantages due to a partly non-hepatic metabolism and a linear response, which result in more predictable effects in terms of acid suppression[4,5].
We therefore aimed to determine the diagnostic value of the rabeprazole test in a population of patients followed up by general practitioners (GPs) for symptoms suspected to be reflux-related.
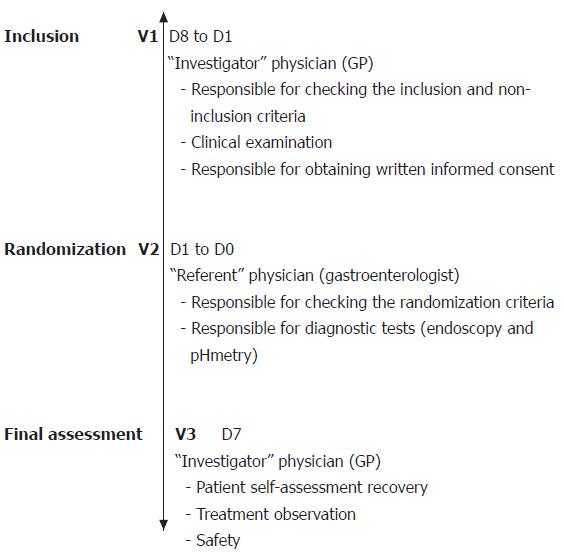
The study was conducted using a multi-centre, randomized and double-blind design. In order to maintain the double-blind nature of the study, each patient was examined by an “investigator” physician and a “referent” one. The investigator was a GP responsible for patient recruitment, inclusion and monitoring at the end of study assessment. The referent was a gastroenterologist with experience of endoscopy and esophageal pH monitoring, responsible for diagnostic testing, checking the randomization criteria, and prescribing treatment. The design of the study consisted three phases (Figure 1).
Phase 1 The investigator enrolled patients into the protocol according to the following inclusion criteria: (a) presence of at least 3 mo of typical (heartburn or regurgitation) or atypical (ascending burning epigastric pain, recurrent nausea, post-prandial digestive discomfort, dysphagia) gastrointestinal or extra-gastrointestinal symptoms suspected to be reflux-related; (b) occurrence of a particular symptom on at least 2 occasions during the 3 d prior to inclusion, the intensity of which being rated as “moderately uncomfortable or worse” using a 7-point Likert verbal analogue scale; (c) lack of previous investigations demonstrating esophagitis, such as esophageal pH monitoring and upper GI endoscopy; (d) absence of previous effective anti-reflux therapy, including PPIs, full-dose H2-receptor antagonists or cisapride during the previous month. Conversely, the following patients were excluded from the trial: (a) women who were either pregnant, breast feeding or not using an effective method of contraception; (b) patients with a malignant condition or an uncompensated chronic disease, particularly uncompensated cardiac, liver or renal disease; (c) patients who had previously undergone a vagotomy or surgery that might alter gastric acid secretion; and (d) patients who were considered unable to comply with the conditions of the protocol, particularly with respect to follow-up and self-assessment questionnaires.
Written consent was obtained from all patients before inclusion. The study was approved by the Local Ethical Committee (‘CCPPRB des Pays de la Loire n°2’).
Patients were given a self-assessment form at the inclusion visit. On each day of the study, patients had to report their dominant symptom (i.e., the symptom which had led them to consult the investigator and that induced most discomfort for the patient). A consultation with the referral doctor was scheduled between 4 and 10 d after the inclusion visit. During this period patients were requested not to take any treatments that could be used to treat GERD, apart from the symptomatic treatment they had been given (Co-magaldrox, Maalox®).
Phase 2 The referent examined patients 4 to 10 d after their inclusion. The inclusion criteria were checked; notably the presence of at least two episodes of the dominant symptom in the 3 d prior to the consultation, rated as being at least “moderately uncomfortable” on the self-assessment form. Endoscopy was performed in all patients according to the usual practice of each centre. In patients with a normal endoscopy from the previous 3 mo, the results were considered not to contribute to a diagnosis of GERD and the investigation was not repeated. Twenty-four hour pH monitoring was performed immediately after the endoscopy. At the end of this second phase, patients who fulfilled the inclusion criteria and without any exclusion factors were randomly allocated to receive either a placebo or rabeprazole (20 mg bid) before breakfast and dinner for 1 wk. The investigators and patients were blinded to the administered treatments. Patients were not informed of the investigation results and the data was sent to a central database until the trial had been completed.
Phase 3 Patients were then received by the investigator at an end of study consultation after 7 ± 1 d of treatment. The investigator was unaware of the results of the endoscopy and pH monitoring. At this visit patients completed the response assessment form, rating any change in their symptoms. A rating according to 3 descriptions (“better”, “roughly the same”, or “worse”) was firstly performed. Secondly, in cases where a symptom had improved, patients assessed the change using a Likert 7-point adjectival scale (“very slight improvement”, “slight improvement”, “clear improvement”, “very great improvement”, “near complete resolution”, and “resolution”). The investigator recorded the number of tablets remaining, any adverse effects and any alteration in the patient’s treatment over the period. According to the results of the rabeprazole test, the investigator classified the patient as a ‘refluxer’ or a ‘non-refluxer’.
Esophageal pH monitoring was conducted using an ambulatory pH recording device (Synectics Mark II or III, Medtronics, Paris, France). The antimony electrode was positioned 5 cm above the cardia, located using the pH step-up method[6] and an ambulatory recording was made. Patients were not given any particular lifestyle or dietary recommendations and were encouraged to behave as normally as possible. Patients were asked to record meals and sleeping periods, and the time of onset of symptoms. Patients returned to the referral centre 24 h later to stop the recording and remove the electrode. The data from the pH monitor were downloaded onto a computer, and the results were analyzed using a specific programme (EsopHogram Synectics software, Medtronics, Paris, France). For symptoms which had occurred during the recording period, analysis included symptom index determination (percentage of the total number of reported symptoms that were reflux-related) and probability of association as previously described[7,8]. Symptoms were considered to be reflux-related if they had occurred during the acid reflux event itself (pH < 4) or within 2 min after it had ended[9].
Patients were classified as ‘refluxers’ or ‘non-refluxers’ according to the results of both upper GI endoscopy and pH monitoring. A diagnosis of reflux was established if one of the following criteria was present: (a) esophageal acid exposure (time below pH 4 during the 24-h period) greater than 4.2%; (b) statistically significant association between symptoms and reflux episodes (P < 0.05 or symptom index > 50%); and (c) presence of mucosal breaks at endoscopy.
The test (rabeprazole or a placebo) was considered to be positive or negative on the basis of the symptom response evaluated by the patient him/herself at the end of the one-week trial period. For this purpose, a 7-point adjectival Likert scale was used and the responses were dichotomised according to the cut-off descriptor of at least a “clear improvement”. To further document the validity of that particular cut-off, a receiver operating characteristics (ROC) analysis of sensitivity was performed using different thresholds for the definition of a positive symptom response (CLINROC software - Metz Software, Chicago, IL, USA).
Sensitivity was defined as the proportion of ‘refluxers’ who had a positive test. Specificity was defined as the proportion of ‘non-refluxers’ for whom the test result was negative. The positive predictive value was defined as the proportion of ‘refluxers’ among patients with a positive test, whereas the negative predictive value was defined as the proportion of ‘non-refluxers’ among patients with a negative test.
Finally, all adverse events reported during the study period were also recorded for safety assessment.
As the study was mainly exploratory in nature, no prior forecast of subject number was made. The study period (January 2001 - May 2002) was also determined in order to reflect clinical practice in a primary care setting. We used mainly descriptive statistics and the results presented on the basis an intention-to-diagnose’ (ITD) analysis. Student’s
t-test was used for quantitative variables and the Chi square test for qualitative variables. P < 0.05 was considered statistically significant.
Ninety-one patients were selected. Of these patients, 83 were included, and 72 were randomized at the phase 2 visit. Among these 72 patients who completed the study and constituted the ITD population, 39 were in the placebo arm and 33 in the rabeprazole arm. Fourteen patients had at least one major deviation either at inclusion or during the course of the study (principally non-compliance with the intervals between visits and/or less than 6-d of treatment). The per-protocol population (PP), therefore, included 58 patients (33 in the placebo arm and 25 in the rabeprazole arm). In the two cohorts (ITD and PP), the most common predominant symptoms were epigastric pain, heartburn and regurgitation. At inclusion, the distribution of the predominant symptoms within the 2 groups was not significantly different. Approximately one third of patients in both groups had esophagitis, which was not of a severe grade in 90% of these subjects. None of the patients included had either stenosis or an ulcer at endoscopy. Compliance with treatment was good as 91% of tablets were taken; there was no significant difference between the two groups (Table 1). Since the results were very similar in the 2 cohorts, only the results from the ITD population are presented here. The characteristics of this population are shown in Table 1.
| Placebo(n = 39) | Rabeprazole(n = 33) | Pvalue | |
| Men/women (%) | 46/54 | 33/67 | 0.269 |
| Mean age (yr)(mean ± SD) | 47.1±11.8 | 49.1±11.9 | 0.4 |
| BMI (kg/m2) (mean ± SD) | 25.6 ± 4.4 | 26.1±5.2 | 0.8 |
| Smoker (%) | 17.9 | 30.3 | 0.219 |
| Previous endoscopy (%) | 33.3 | 30.3 | 0.783 |
| Time from first symptoms1 (mo) | 24.3 ± 51.5 | 20.2 ± 27.0 | 0.956 |
| Post-prandial symptoms (%) | 30.8 | 33.3 | 0.232 |
| Nocturnal symptoms (%) | 12.8 | 15.1 | 0.232 |
| Hiatus hernia (%) | 47.4 | 27.3 | 0.082 |
| Esophagitis (%) | 30.8 | 33.3 | 0.816 |
| Barrett's oesophagus (%) | 2.6 | 6.1 | 0.474 |
| Diagnosis of GERD2 (%) | 67.6 | 62.1 | 0.642 |
Six patients were unclassifiable due to pH monitoring technical failure. As a result, a definitive diagnosis of GERD or absence of GERD was made for 66 patients, 37 in the placebo group and 29 in the rabeprazole group. Forty-three were considered to be ‘refluxers’ (25 in the placebo arm and 18 in the rabeprazole arm) and 23 ‘non-refluxers’ (12 in the placebo arm and 11 in the rabeprazole arm).
In ‘refluxers’, the rabeprazole test was positive in 15 of 18 patients (sensitivity: 83.3%) whilst the placebo test was positive in 10 of 25 (sensitivity: 40.0%) (P = 0.011). In the ‘non-refluxers’, the rabeprazole test was negative in 5 of 11 patients (specificity: 45.5%) whilst placebo test was negative in 8 of 12 (specificity: 66.7%) (P > 0.05).
The positive and negative predictive values for the rabeprazole test were 71.4% and 62.5%, respectively. The corresponding predictive values for the placebo test were 71.4% and 34.8%, respectively (P > 0.05). The percentages of patients who were correctly classified by the rabeprazole test and the placebo test were 69.0% and 48.6%, respectively (P > 0.05).
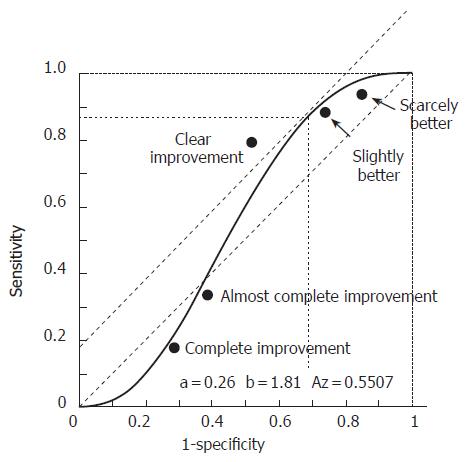
The results of the ROC analysis are illustrated in Figure 2. The categories that were closest to the slope of the tangent to the 45° curve were those of “slight improvement” and “clear improvement”. Nevertheless, the best compromise between sensitivity and specificity corresponded to the description “clear improvement” that was adopted for the former analysis.
Six patients (4 in the placebo arm and 2 in the rabeprazole arm) reported 7 adverse events (placebo: vomiting, diarrhoea, insomnia, dyslipidemia, urinary infection; rabeprazole: lymphadenitis, drug eruption) (non significant). No adverse event was considered to be serious.
The results of this study conducted in a primary care setting and with a placebo-controlled design showed that the rabeprazole test had high sensitivity (83.3%) but low specificity (45.5%). As compared with the placebo test, the rabeprazole test showed a superior sensitivity and negative predictive value, but non-significant differences with regard to specificity and positive predictive value.
This study is one of the first attempts to evaluate the diagnostic yield of a PPI test in primary care conditions with assessment of the results by GPs. The drug was administered for 7 d only for both practical and scientific reasons. In fact, a recent study using esomeprazole showed that although the sensitivity of the test increased each day following the start of the test, a plateau was reached after 5 d of administration, with no further improvement beyond that time point[10]. The reference tests (i.e. 24-h pH monitoring and endoscopy) were performed by independent physicians (referent) and the GP (investigator) remained blind to the results until response to PPI had been determined. In addition to acid exposure measurement, symptom analysis was also an important parameter of pH monitoring interpretation. This seems particularly relevant in a population of patients with normal endoscopy in approximately two thirds of cases. Indeed, nearly half of patients with endoscopy-negative GERD are known to have acid exposure within the normal range[11]. The use of symptom analysis permits an increase in the sensitivity of pH monitoring and therefore decreases the risk of missing genuine ‘refluxers’. In addition, only patients with moderate to severe symptoms present on at least 2 occasions in the 3 d prior to inclusion were enrolled. The outcome of these rather rigorous inclusion criteria and design was a difficulty in fulfilling our initial goal of recruiting a large cohort of patients. Finally, from a number of candidate PPIs, we chose rabeprazole, since this drug has a number of potential advantages: (a) a rapid onset of action as shown by gastric pH monitoring studies[3,12,13]; (b) a good safety profile; (c) a lack of drug interactions due to its specificity in terms of hepatic and non-hepatic metabolic pathways[4,5]; and (d) its effectiveness in the treatment of some symptoms associated with GERD[14].
The results of our study are consistent with other studies of PPI tests which have without exception demonstrated the high sensitivity and poor specificity of this diagnostic approach[1]. This rather disappointing finding has recently been confirmed by the meta-analysis reported by Numans et al[15], which reached a similar conclusion concerning short-term trials of PPIs in GERD. The poor performance of PPI test is further reinforced by the comparison with the placebo-test which adequately ‘classified’ nearly half of the patients (random results). However, these results are not entirely surprising as a good placebo response has also been reported in short-term trials in GERD[10,16-18]. In addition, the above negative conclusions should be further balanced as far as negative predictive values are considered. Indeed, in the test conditions, a negative response to rabeprazole could exclude a diagnosis of GERD in 2 of 3 symptomatic patients (as compared to only one in three following the placebo). As the negative predictive value is influenced by the prevalence of the disease, our results may in fact be an underestimation and higher negative predictive values could be expected in a more representative sample of patients consulting for upper GI symptoms, supposing a 30% prevalence of GERD (i.e. approximately half of that observed in both the placebo and rabeprazole arms of our study). Finally, our study design did not allow an appropriate evaluation of the cost-effectiveness of the PPI test as a whole and of the rabeprazole test in particular. The poor specificity of the test should, however, lead to some caution in terms of guidelines concerning long-term management strategy in GERD; there is a potential risk that ‘non-refluxers’ will continue to receive PPI treatment far beyond the initial test week if the symptomatic response is good. Whether such an empirical approach to acid-sensitive disorders is justified or potentially dangerous is presently unknown. Nevertheless, our data supports the conclusions of the French-Belgian consensus conference, which did not recommend the use of PPI tests for the diagnosis of GERD in clinical practice before the availability of further scientific information[19].
We thank the following practitioners (exercise city indicated): Abdelli N. Chalon en Champagne, Abdelli F. Chalon en Champagne, Aroun JM. La Montagne, Bardoux N. Rouen, Blot E. Rouen, Bonnaud Guillaume. Blagnac, Breban P. Lens, Bressolette B. Rezé, Brevot J.L. Sarry, Brunet H. Meyzieu, Caurier C. Reims, Charbaut E. Juvigny, Clavieres C. Epinal, Colne J.M. Rambervillers, Contamin E. Chalon en Champagne, Delbende H. Rouen, Dietsch P. Chavelot, Fraysse P. Castres, Galmiche J.P. Nantes, Grimaud F. St Père en Retz, Gruber A. Colomier, Haddad-Garcia R. Castres, Hallot V. Bruguières, Jamaux S. Bruguières, Leger B. Castres, Lequeux Y. St Père en Retz, Le Rhun M. Nantes, Loisel M. Rouen, Martin G. Reims, Massonneau A. St Sebastien sur Loire, Mayette P. Reims, Michalski F. Pont à Vendin, Michel E. Courtisols, Mussat P. Vue, Oury P. Rilly La Montagne, Pellissier P.E. Decines-Charpieu, Pasqual J.C. Troyes, Picard A. Rilly La Montagne, Poulat B. Meyzieu, Robiquet Ph. Avion, Sabbagh M. Chalon en champagne, Sanna F. Tournefeuille, Siebler M. Epinal, Vandromme L. Reims, Viault D. Troyes, and Weiss B. Lens.
S- Editor Wang J L- Editor Kumar M E- Editor Liu WF
| 1. | Bruley des Varannes S. The proton-pump inhibitor test: pros and cons. Eur J Gastroenterol Hepatol. 2004;16:847-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Sachs G, Humphries TJ. Rabeprazole: pharmacology, pharmacokinetics, and potential for drug interactions. Introduction. Aliment Pharmacol Ther. 1999;13 Suppl 3:1-2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Pantoflickova D, Dorta G, Ravic M, Jornod P, Blum AL. Acid inhibition on the first day of dosing: comparison of four proton pump inhibitors. Aliment Pharmacol Ther. 2003;17:1507-1514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Horn J. Review article: relationship between the metabolism and efficacy of proton pump inhibitors--focus on rabeprazole. Aliment Pharmacol Ther. 2004;20 Suppl 6:11-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Saitoh T, Fukushima Y, Otsuka H, Hirakawa J, Mori H, Asano T, Ishikawa T, Katsube T, Ogawa K, Ohkawa S. Effects of rabeprazole, lansoprazole and omeprazole on intragastric pH in CYP2C19 extensive metabolizers. Aliment Pharmacol Ther. 2002;16:1811-1817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Pehl C, Boccali I, Hennig M, Schepp W. pH probe positioning for 24-hour pH-metry by manometry or pH step-up. Eur J Gastroenterol Hepatol. 2004;16:375-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Wiener GJ, Richter JE, Copper JB, Wu WC, Castell DO. The symptom index: a clinically important parameter of ambulatory 24-hour esophageal pH monitoring. Am J Gastroenterol. 1988;83:358-361. [PubMed] [Cited in This Article: ] |
| 8. | Shi G, Bruley des Varannes S, Scarpignato C, Le Rhun M, Galmiche JP. Reflux related symptoms in patients with normal oesophageal exposure to acid. Gut. 1995;37:457-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 161] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Weusten BL, Akkermans LM, vanBerge-Henegouwen GP, Smout AJ. Symptom perception in gastroesophageal reflux disease is dependent on spatiotemporal reflux characteristics. Gastroenterology. 1995;108:1739-1744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 89] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Johnsson F, Hatlebakk JG, Klintenberg AC, Román J, Toth E, Stubberöd A, Falk A, Edin R. One-week esomeprazole treatment: an effective confirmatory test in patients with suspected gastroesophageal reflux disease. Scand J Gastroenterol. 2003;38:354-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Fass R. Empirical trials in treatment of gastroesophageal reflux disease. Dig Dis. 2000;18:20-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Warrington S, Baisley K, Boyce M, Tejura B, Morocutti A, Miller N. Effects of rabeprazole, 20 mg, or esomeprazole, 20 mg, on 24-h intragastric pH and serum gastrin in healthy subjects. Aliment Pharmacol Ther. 2002;16:1301-1307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Bruley des Varannes S, Gharib H, Bicheler V, Bost R, Bonaz B, Stanescu L, Delchier JC, Bonnot-Marlier S. Effect of low-dose rabeprazole and omeprazole on gastric acidity: results of a double blind, randomized, placebo-controlled, three-way crossover study in healthy subjects. Aliment Pharmacol Ther. 2004;20:899-907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Oda K, Iwakiri R, Hara M, Watanabe K, Danjo A, Shimoda R, Kikkawa A, Ootani A, Sakata H, Tsunada S. Dysphagia associated with gastroesophageal reflux disease is improved by proton pump inhibitor. Dig Dis Sci. 2005;50:1921-1926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Numans ME, Lau J, de Wit NJ, Bonis PA. Short-term treatment with proton-pump inhibitors as a test for gastroesophageal reflux disease: a meta-analysis of diagnostic test characteristics. Ann Intern Med. 2004;140:518-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Schenk BE, Kuipers EJ, Klinkenberg-Knol EC, Festen HP, Jansen EH, Tuynman HA, Schrijver M, Dieleman LA, Meuwissen SG. Omeprazole as a diagnostic tool in gastroesophageal reflux disease. Am J Gastroenterol. 1997;92:1997-2000. [PubMed] [Cited in This Article: ] |
| 17. | Johnsson F, Weywadt L, Solhaug JH, Hernqvist H, Bengtsson L. One-week omeprazole treatment in the diagnosis of gastro-oesophageal reflux disease. Scand J Gastroenterol. 1998;33:15-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Miner P Jr, Orr W, Filippone J, Jokubaitis L, Sloan S. Rabeprazole in nonerosive gastroesophageal reflux disease: a randomized placebo-controlled trial. Am J Gastroenterol. 2002;97:1332-1339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | French-Belgian Consensus Conference on Adult Gastro-oesophageal Reflux Disease 'Diagnosis and Treatment': report of a meeting held in Paris, France, on 21-22 January 1999. The Jury of the consensus conference. Eur J Gastroenterol Hepatol. 2000;12:129-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |