Published online Mar 21, 2006. doi: 10.3748/wjg.v12.i11.1757
Revised: August 10, 2005
Accepted: October 26, 2005
Published online: March 21, 2006
AIM: To investigate the morphological characterization of tumor infiltrating dendritic cells (TIDCs) and tumor infiltrating lymphocytes (TILs) in human rectal cancer.
METHODS: Light and electron microscopy as well as immunohistochemistry were used to observe the distributive and morphological changes of TIDCs and TILs.
RESULTS: TIDCs were mainly located in tumor-surrounding tissue. The number of TIDCs in the earlier stage was higher than that in the later stage (P < 0.01). TILs were mainly seen in adjacent tissue of cancers and tumor-surrounding tissue. There were more TILs in the earlier stage than that in the later stage (P <0.01). Under electron microscope, TIDCs were irregular in shape and exhibited many dendritic protrusions. It isn’t obvious that cancer cells perforated the basement membrane and TILs were arranged along the basement membrane in the earlier stage. In the later stage, it is explicit that cancer cells perforated the basement membrane and surrounded by TILs. There were contacts among TIDCs, TILs and tumor cell. One TIDCs contacted one or several TILs which clustered around TIDCs. Glycogen granules were seen between TIDCs and TILs.
CONCLUSION: The number of TIDCs and TILs is related with tumor progression There exist close relationships among TIDCs, TILs and tumor cell.
- Citation: Xie ZJ, Jia LM, He YC, Gao JT. Morphological observation of tumor infiltrating immunocytes in human rectal cancer. World J Gastroenterol 2006; 12(11): 1757-1760
- URL: https://www.wjgnet.com/1007-9327/full/v12/i11/1757.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i11.1757
Antitumor effects of human body are mainly achieved by tumor infiltrating immunocytes, including TIDCs, TILs, natural killer(NK) cells and lymphokine-activated killer(LAK) cells. Because of their prominent role in antitumor effects, TIDCs and TILs have drawn more and more attention. DCs are the most powerful professional antigen-presenting cells (APCs) so far. Immunophenotype and function of DCs in biological therapy of tumor are the focus of immunological study [1,2]. Although studies on tumor immunotherapy involving injection of DCs cultured in vitro are reported [3-5], infiltrating DCs in tumor microenvironment have not been fully investigated and studies usually concentrate on the correlation between infiltrating DCs and tumor progression[6,7]. Morphological studies on the relation among TIDCs, TILs and tumor cells, are few. TILs are heterogenous lymphocytes, most of them are T lymphocytes. TILs are a new kind of antitumor effectors after LAK cells. In this study, the morphology of TIDCs and TILs in earlier and later stage of human rectal cancer was investigated, which may provide morphological evidence for antitumor effect of tumor infiltrating immunocytes.
Eighteen rectal cancer patients surgically treated in earlier or later stage in the Second and Third Hospitals of Harbin Medical University were studied. According to their clinical manifestations and pathological features, the patients were divided into earlier stage group (n = 8) and later stage group (n = 10).
Fresh rectal cancer samples were obtained during surgery. Immediately after surgical resection, tissues were rinsed in redistilled water and then in 0.2 mol/L phosphate-buffered saline (PBS). Tumor-surrounding tissues were obtained and fixed in 3% glutaraldehyde for transmission electron microscopy or in 10% neutrally buffered formalin solution. The cut into slices, embedded in paraffin and sectioned at 4 μm for HE and immunohistochemical staining. The deparaffinized sections were washed with PBS. Primary antibodies used were rabbit-anti human S-100 protein and CD3 (Boster Biological Technology Ltd). Secondary antibody used was mouse-anti rabbit IgG. The procedure was performed with SABC kit following the manufacturer’s instructions.
Student t test was used for statistical analysis. P <0.05 was considered statistically significant.
Under the light microscope, the number of infiltrating S-100 (+) and CD3 (+) cells in each section was calculated under 5 high power fields (×400) and statistically analyzed.
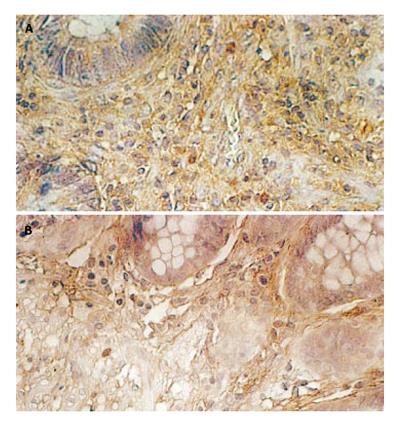
TIDCs were mainly seen in tumor-surrounding area, only a small number of TIDCs were located in cancer tissue scatteredly by HE staining. Using anti-S-100 antibody with the SABC method, Brown S-100 (+) DCs could be observed in each section. Comparatively large DCs exhibited many dendritic protrusions, mainly located in tumor-surrounding tissue. The number of TIDCs was large in the earlier stage (Figure 1A) and comparatively small in the later stage (Figure 1B). The number of TIDCs in earlier stage was significantly higher than that in later stage and the difference was statistically significant (P < 0.01, Table 1).
| Earlier stage | Later stage | P -value | |
| Number of cases | 8 | 10 | |
| Number of TIDCs | 49.32 ± 5.48 | 17.02 ± 3.14 | <0.01 |
| Number of TILs | 107.38 ± 13.61 | 48.21 ± 5.13 | <0.01 |
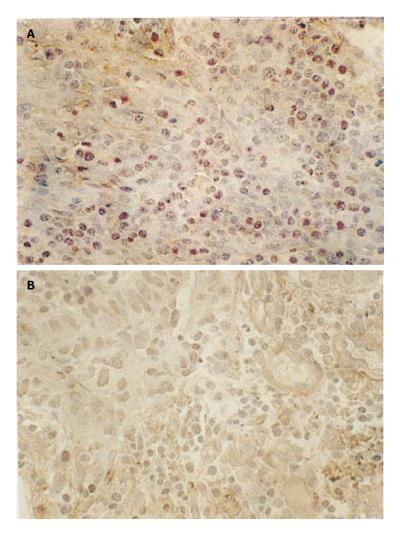
Brown CD3 (+) TILs could be seen in each section. Circular CD3 (+) lymphocytes, little cytoplasm and large nuclei were distributed uniformly and densely. In the earlier stage, the number of TILs was large and TILs were primarily located centrally (Figure 2A). In the later stage, the number of TILs was small and TILs were scattered, only a small number of TILs were distributed centrally (Figure 2B). The number of TILs in earlier stage was significantly higher than that in later stage and the difference was statistically significant (P < 0.01, Table 1).
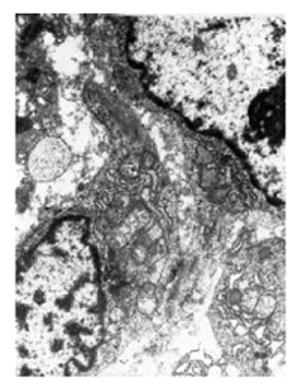
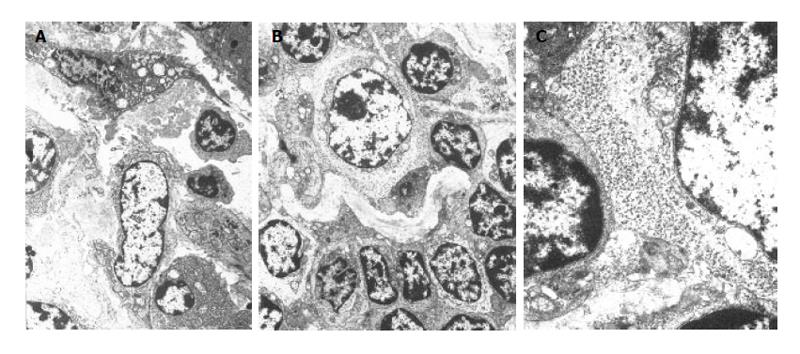
Under electron microscope, TIDCs were seen in tumor-surrounding tissue. TIDCs were irregular in shape and displayed many dendritic cytoplasmic protrusions with varying width. The nuclei were irregular in shape and the nucleoli were small. Heterochromatin was highly marginated along the nuclear membrane. Mitochondria and endoplasmic reticulum were prominent in these cells. A fraction of endoplasmic reticulum and some mitochondria were expanded (Figure 3).
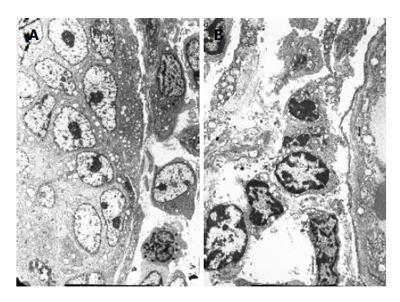
It isn’t obvious that cancer cells perforated the basement membrane. Lymphocytes were arranged serially along the basement membrane in earlier stage (Figure 4A). However, in the later stage, it is explicit that cancer cells perforated the basement membrane and were surrounded by TILs (Figure 4B). Mitochondria in cancer cells perforating the basement membrane degenerated and the cancer cells were surrounded by DCs, lymphocytes and plasma cells (Figure 5).
TIDCs were contacted with TILs in tumor-surrounding tissue, forming DC-lymphocyte clusters (Figure 5A). Clusters of glycogen granules were seen between TIDCs and TILs (Figure 5B). In addition to contacting TILs, TIDCs also contacted cancer cells at the same time. TILs could be seen surrounding the cancer nests, basement membrane of cancer was fragmentary, and TIDCs contacted cancer cells directly (Figure 5C).
DCs are the most powerful professional antigen-presenting cells (APCs)[1]. Studies on immunophenotype and function of DCs are now available[2-5]. Morphological studies on DCs and TIDCs are few. In this study, the morphology of TIDCs and correlation between TIDCs, TILs and cancer cells in human rectal cancer were investigated.
Antitumor immune responses of human body are mainly centralized in local cell-mediated immunity. The infiltrating degree of lymphocytes surrounding tumor tissue reflects the status of antitumor immune responses of human body. Activation of T lymphocytes needs the assistance of APCs. DCs are the most important APCs in human body and are closely related with the function of T lymphocytes. TILs are heterogenous lymphocytes, most of which are T lymphocytes. TILs play an important role in immunity and anti-tumor effects[8,9]. In our study, TILs were closely related with tumor immunity and mainly distributed in adjacent tissue of cancer, suggesting that localization of TILs in tumor-surrounding tissue limits the growth of cancer and confines cancer to a local site, which is the first barrier of anti-tumor effects of human body and contributes to immune response and prevents tumor spreading, which is the second barrier of anti-tumor effects of human body. Infiltration of TILs represents anti-tumor immune responses of human body. The infiltrating degree of TILs plays a decisive role in invasion and metastasis of tumor as well as prognosis of patients. In our study, the number of TILs in earlier stage of rectal cancer was significantly higher than that in later stage (P < 0.01), demonstrating that the number of TILs is related with the progression of rectal cancer.
It was reported that metastasis and prognosis of tumor are related with the infiltrating degree of DCs in tumor tissue [6]. Wright-Browne et al [7] showed that the number of TIDCs in human gastric cancer, colon cancer and carcinoma of esophagus is negatively correlated with lymph node metastasis, size of tumor and survival time, that is, the more the number of TIDCs, the better the patient’s prognosis. In this study, the number of TIDCs in earlier stage was significantly higher than that in later stage (P < 0.01), suggesting that the number of TIDC is related with the progression of rectal cancer.
DCs play an important role in tumor immunity. The powerful antigen-presenting function of DCs can induce efficient and specific anti-tumor immunity activated CTLs can kill tumor cells[10-12]. In this study, TIDCs were closely contacted with TILs. TILs were clustered around TIDCs and cluster of DCs-lymphocytes cluster was formed. Glycogen granules could be seen between TIDCs and TILs. All these suggest that there exists some relation between TIDCs and TILs. It has been reported that DCs mainly present antigen to lymphocytes, stimulate naïve T lymphocyte proliferation and activation to kill tumor cells [13]. The contact of TIDCs and TILs observed in our study may provide morphological evidence and suggests that TIDC can present antigen to TILs and activate TILs. Moreover, fraction of endoplasmic reticulum and some mitochondria in DCs were expanded. These data suggest that these active cells can capture and identify antigens by their cytoplasm protrusions, and subsequently transmit antigens to lymphocytes and prime anti-tumor immunity to kill tumor cells through cytotoxicity.
DCs participate in local anti-tumor immune response and have corresponding changes during oncogenesis and tumor progression, suggesting that DCs are closely related with anti-tumor immunity. DCs are not only professional APCs, but also immunological adjuvant. DCs play an important role in immunoregulation. It has been shown that antigen-sensitized DCs can induce specific CTL in vivo and directly inhibit or kill tumor cells [13-16]. But whether DCs can directly inhibit the growth of tumor in vivo or directly kill tumor cells remains unclear and should be further studied.
S- Editor Wang J L- Editor Wang XL E- Editor Zhang Y
| 1. | Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142-1162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1558] [Cited by in F6Publishing: 1545] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 2. | Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1663] [Cited by in F6Publishing: 1618] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 3. | Tong Y, Song W, Crystal RG. Combined intratumoral injection of bone marrow-derived dendritic cells and systemic chemotherapy to treat pre-existing murine tumors. Cancer Res. 2001;61:7530-7535. [PubMed] [Cited in This Article: ] |
| 4. | Fong L, Brockstedt D, Benike C, Wu L, Engleman EG. Dendritic cells injected via different routes induce immunity in cancer patients. J Immunol. 2001;166:4254-4259. [PubMed] [Cited in This Article: ] |
| 5. | Murakami T, Tokunaga N, Waku T, Gomi S, Kagawa S, Tanaka N, Fujiwara T. Antitumor effect of intratumoral administration of bone marrow-derived dendritic cells transduced with wild-type p53 gene. Clin Cancer Res. 2004;10:3871-3880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Zhang JK, Li J, Chen HB, Sun JL, Qu YJ, Lu JJ. Antitumor activities of human dendritic cells derived from peripheral and cord blood. World J Gastroenterol. 2002;8:87-90. [PubMed] [Cited in This Article: ] |
| 7. | Wright-Browne V, McClain KL, Talpaz M, Ordonez N, Estrov Z. Physiology and pathophysiology of dendritic cells. Hum Pathol. 1997;28:563-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 88] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004;10:4450-4456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2457] [Cited by in F6Publishing: 2498] [Article Influence: 119.0] [Reference Citation Analysis (0)] |
| 10. | Norbury CC, Chambers BJ, Prescott AR, Ljunggren HG, Watts C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur J Immunol. 1997;27:280-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 261] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Movassagh M, Spatz A, Davoust J, Lebecque S, Romero P, Pittet M, Rimoldi D, Lienard D, Gugerli O, Ferradini L. Selective accumulation of mature DC-Lamp+ dendritic cells in tumor sites is associated with efficient T-cell-mediated antitumor response and control of metastatic dissemination in melanoma. Cancer Res. 2004;64:2192-2198. [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Tanaka Y, Dowdy SF, Linehan DC, Eberlein TJ, Goedegebuure PS. Induction of antigen-specific CTL by recombinant HIV trans-activating fusion protein-pulsed human monocyte-derived dendritic cells. J Immunol. 2003;170:1291-1298. [PubMed] [Cited in This Article: ] |
| 13. | Schuler G, Steinman RM. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med. 1997;186:1183-1187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 346] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Asavaroengchai W, Kotera Y, Mulé JJ. Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc Natl Acad Sci U S A. 2002;99:931-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Gatza E, Okada CY. Tumor cell lysate-pulsed dendritic cells are more effective than TCR Id protein vaccines for active immunotherapy of T cell lymphoma. J Immunol. 2002;169:5227-5235. [PubMed] [Cited in This Article: ] |
| 16. | Bedrosian I, Mick R, Xu S, Nisenbaum H, Faries M, Zhang P, Cohen PA, Koski G, Czerniecki BJ. Intranodal administration of peptide-pulsed mature dendritic cell vaccines results in superior CD8+ T-cell function in melanoma patients. J Clin Oncol. 2003;21:3826-3835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |