Published online Mar 21, 2006. doi: 10.3748/wjg.v12.i11.1694
Revised: June 20, 2005
Accepted: October 10, 2005
Published online: March 21, 2006
AIM: To investigate the effects of tachyplesin and n-sodium butyrate on proliferation and gene expression of human gastric adenocarcinoma cell line BGC-823.
METHODS: Effects of tachyplesin and n-sodium
butyrate on proliferation of BGC-823 cells were determined with trypan blue dye exclusion test and HE staining. Effects of tachyplesin and n-sodium butyrate on cell cycle were detected by flow cytometry. Protein levels of c-erbB-2, c-myc, p53 and p16 were examined by immunocytochemistry.
RESULTS: The inhibiting effects were similar after 2.0 mg/L tachyplesin and 2.0 mmol/L n-sodium butyrate treatment, the inhibitory rate of cellular growth was 62.66% and 60.19% respectively, and the respective maximum mitotic index was decreased by 49.35% and 51.69% respectively. Tachyplesin and n-sodium butyrate treatment could markedly increase the proportion of cells at G0/G1 phase and decrease the proportion at S phase. The expression levels of oncogene c-erbB-2, c-myc, and mtp53 proteins were down-regulated while the expression level of tumor suppressor gene p16 protein was up-regulated after the treatment with tachyplesin or n-sodium butyrate. The effects of 1.0 mg/L tachyplesin in combination with 1.0 mmol/L n-sodium butyrate were obviously superior to their individual treatment in changing cell cycle distribution and expression of c-erbB-2, c-myc, mtp53 and p16 protein. The inhibitory rate of cellular growth of BGC-823 cells after combination treatment was 62.29% and the maximum mitotic index was decreased by 51.95%.
CONCLUSION: Tachyplesin as a differentiation inducer of tumor cells has similar effects as n-sodium butyrate on proliferation of tumor cells, expression of correlative oncogene and tumor suppressor gene. It also has a synergistic effect on differentiation of tumor cells.
- Citation: Shi SL, Wang YY, Liang Y, Li QF. Effects of tachyplesin and n-sodium butyrate on proliferation and gene expression of human gastric adenocarcinoma cell line BGC-823. World J Gastroenterol 2006; 12(11): 1694-1698
- URL: https://www.wjgnet.com/1007-9327/full/v12/i11/1694.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i11.1694
Tachyplesin, isolated from acid extracts of hemocytes of Chinese horseshoe crab (Tachypleus tridentatus), a sort of marine arthropod known as “live fossil”, is a low-molecular-weight polypeptide [1]. This bioactive component has antitumor effect and induces tumor cell differentiation [2]. We used the small molecule polar compound n-sodium butyrate[3] as a parallel control to compare the effects on proliferation, cell cycle and expression of correlative oncogene and tumor suppressor gene of BGC-823 cells treated with tachyplesin, n-sodium butyrate and their combination. This research could provide information for further identification of antitumor low-molecular-weight crude bioactive peptides such as tachyplesin that can modulate the proliferation and differentiation of tumor cells.
Tachyplesin was isolated from acid extracts of Chinese horseshoe crab (Tachypleus tridentatus) hemocytes as previously described[4]. The crude extract was separated by SephadexG-50, CM-sepharose CL-6B column chromatography.
BGC-823 cells were cultured in RPMI-1640 medium supplemented with 20% heat-inactivated fetal calf serum,100 units/mL penicillin,100 mg/L streptomycin and 50 mg/L kanamycin at 37°C in atmosphere containing 50 mL/L CO2. BGC-823 cells were treated with culture medium containing inducers after seeded for 24 h.
The powder of tachyplesin obtained from separation, purification and lyophilization was dissolved in D-Hank’s solution to prepare 100 mg/L concentrated solution. The mother liquor was prepared for solution of a given concentration with culture medium. N-sodium butyrate purchased from Sigma Co., was dissolved in appropriate concentration of D-Hank’s solution to prepare 200 mmol/ L concentrated solution. The concentrations of the three treatment solutions were as follows: 2.0 mg/L tachyplesin-treatment (Ta), 2.0 mmol/L n-sodium butyrate-treatment (Tb), and 1.0 mg/L tachyplesin + 1.0 mmol/L n-sodium butyrate for the combination treatment (Ta+Tb). The experimental groups were treated with the three reagents after medium was changed, while the control group was cultured continuously with fresh culture medium for future use.
BGC-823 cells were collected in logarithmic phase, then suspension of BGC-823 cells was made in 5.0×104 cells/mL. The cells were seeded into 15 mL culture flasks with 2 mL per flask. After seeded for 24 h, the experimental groups were treated with the reagents containing different kinds of differentiation-induced gradients while the control group was cultured continuously in fresh culture medium. During the first seven days, untreated or treated cells were harvested from three flasks everyday, and the viable cells were counted by the trypan blue dye exclusion test to get average value. The similar results were found in triplicate experiments, the growth curve was derived from one of the results.
BGC-823 cells (5.0×104 mL) were seeded in to bottles containing little penicillin with cover slips. Treatments were performed after the cells were seeded for 24 h.
During the first seven days, the cover slips were removed from two bottles of the untreated or treated cells everyday, fixed in Bouin-Hollande fixative, and stained with Hematoxylin-Eosin (HE). The mitotic cells in 1000 cells on each cover slip were counted, and the mitotic index curve was drawn.
BGC-823 cells were collected respectively from the treated groups and the control group after digested and centrifuged at 1000 r/min for 5 min. All the cells collected were rinsed three times with D-Hank’s solution. The cells grown on cover slips were fixed in 75% pre-cooled ethanol at 4 °C overnight, centrifuged and resuspended in 100 mg/L RNase at 37°C for 30 min. Then 50 mg/L propidium iodide was added into the suspended cells at 4 °C in dark for 30 min. The cell cycle was analyzed by flow cytometry (Bacton-Dickson Co.) and the data were analyzed by Cell FIT cell cycle analysis software(Version2.01.2).
BGC-823 cells (5.0×104/mL) and cells treated with different inducers were seeded into bottles containing little penicillin with cover slips for 36 h respectively. The cells grown on cover slips were rinsed with D-Hank’s solution at 37°C. The changes in c-erbB-2, c-myc , p53 and p16 expression of treated and untreated BGC-823 cells were determined by SABC immunohistochemical assay. The reagent kit (Wuhan Boster Bioengineering Corporation) was used to determine the expression of these genes. PBS was used to take the place of primary antibody as the negative controls and positive specimens were used as positive controls.
The cell growth curve determination showed that the proliferation of untreated BGC-823 cells was very fast. The cell number increased to 91.29×104/mL on the seventh day which was 18.26 times of that of the original 5.0×104/mL on the first day, with a doubling time of 45.82 h. However, after treated with tachyplesin or n-sodium butyrate, the growth rate of BGC-823 cells was inhibited. After treatment with 2.0 mg/L tachyplesin, the number of cells was 34.09×104/mL which was 6.8 times of that of the original number on the 7th day, with the doubling time prolonged to 75.7 h and the growth inhibitory rate increased to 62.77%. After treatment with 2.0 μg/mL n-sodium butyrate, the number of cells was 36.34×104/mL which was 7.27 times of that of the original number on the 7th day, the doubling time was 67.10 h and the growth inhibitory rate was 60.19%. After treatment with 1.0 mg/L tachyplesin + 1.0 mmol/L n-sodium butyrate, the number of cells was 33.87×104/mL on the 7th day, the doubling time was 69.75 h and the growth inhibitory rate was 62.29% (Figure 1).
The cell mitotic index determination showed that BGC-823 cells had vigorous proliferation capability, which reached to the divided peak on the fourth day and the maximum mitotic index was 38.5‰. However the mitotic index of cells treated with tachyplesin or n-sodium butyrate or their combination was only 19.5‰, 18.6‰ and 18.5‰ respectively at the divided peak, and declined by 49.35%, 51.69% and 51.95% respectively. The divided peak occurred on the third day after inducing treatment (Figure 2A).
Cell cycle of BGC-823 cells was analyzed by flow cytometry. The results showed that the cell cycle distribution of BGC-823 cells changed obviously when the cells were treated with tachyplesin or n-sodium butyrate. The proportion of untreated cells was 50.7% in G0/G1 phase, 39.1% in S phase and 10.2% in G2+M phase. However, the proportion of cells at G0/G1 phase was 59.5%, 26.3% at S phase and 14.2% at G2+M phase after treated with 2.0 mg/L tachyplesin. Similar changes occured in cells treated with 2.0 mmol/L n-sodium butyrate r the proportion of cells in G0/G1 phase was 59.2%, 25.2% in S phase and 15.6% in G2+M phase. Meanwhile, the proportion of BGC-823 cells increased from 50.7% to 64.5% in G0/G1 phase and decreased from 39.1% to 20.9% in S phase(Table 1). The proportion of cells in G2+M phase increased from 10.2% to 14.6%(Figure 2B).
| Group | G0/G1 | S | G2/M |
| BGC-823 | 50.7 | 39.1 | 10.2 |
| Ta | 59.5 | 26.3 | 14.2 |
| Sb | 59.2 | 25.2 | 15.6 |
| Ta+Sb | 64.5 | 20.9 | 14.6 |
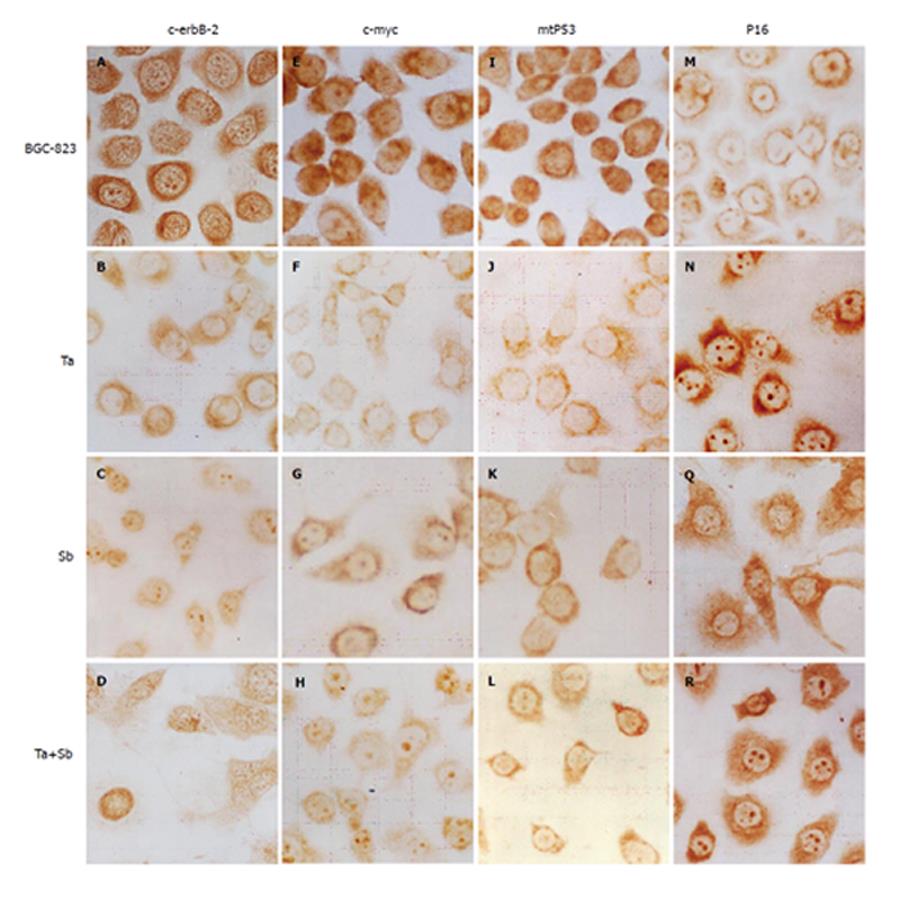
Immunocytochemistry showed that c-erbB-2 protein level in the control group was high. The dark brown-yellow granules were mainly detected in cytoplasm and membrane of BGC-823 cells. But in nuclei, the protein level was low, while a few unevenly-distributed buff granules could be detected (Figure 3A). After treated with tachyplesin, the positive rate of c-erbB-2 expression in BGC-823 cells decreased. The brown-yellow particles were mainly detected in cytoplasm around nuclear membrane but a few in nuclei (Figure 3B). After treated with n-sodium butyrate,relatively bigger brown-yellow granules were mainly detected in nuclei and cytoplasm around the nuclei but a few around the cell membrane(Figure 3C). After treated with the combination of the two inducers, the immunocytochemistry signal became weaker than after treated with n-sodium butyrate alone. The smooth and evenly-distributed brown-yellow granules were mainly detected in nucleoli and nuclear membrane of the cells (Figure 3D).
Immunocytochemistry showed that the c-MYC protein level in the control group was high, the brown granules were mainly detected in nuclei and cytoplasm around the nuclei of BGC-823 cells (Figure 3E). After treated with tachyplesin, the level of c-MYC protein in BGC-823 cells decreased. The light brown-yellow granules were mainly detected in karyoplasms around the nuclear membrane, while in nuclei, the protein level was very low (Figure 3F). After treated with n-sodium butyrate, the brown particles were mainly detected in nucleoli and cytoplasm, while in nuclei of the cells, the signal was almost negative (Figure 3G). After treated with combination of the two inducers, the signal became weaker than after treated with n-sodium butyrate alone. The evenly-distributed brown-yellow granules were mainly detected in nucleoli and cytoplasm while in nuclei of the cells, the signal could be scarcely detected (Figure 3H).
Immunocytochemistry showed that mtP53 protein level in the control group was high. The dark brown-yellow granules were mainly detected in nuclei, while a few granules were detected in cytoplasm of BGC-823 cells. Their distributing was irregular(Figure 3I). After treated with tachyplesin, the rate of positive expression of mtP53 in BGC-823 cells decreased. The light brown-yellow granules were mainly detected in cytoplasm, while in nuclei of the cells the expression was negative (Figure 3J). After treated with n-sodium butyrate, the light brownish-red granules were mainly detected in cytoplasm, while in nuclei of the cells, the mtP53 expression decreased greatly, and the signal became almost negative(Figure 3K). After treated with combination of the two inducers, the immunocytochemistry signal as a whole became weaker than after treated with n-sodium butyrate alone. The light brown granules were mainly detected in cytoplasm and could be hardly detected in nuclei of the cells (Figure 3L).
Immunocytochemistry showed that P16 protein level in the control group was low. The brown-yellow granules were detected in nucleoli and cytoplasm around the nuclear membrane of BGC-823 cells. The signal in cytoplasm and cell membrane was very weak (Figure 3M). However, after treated with tachyplesin, the P16 protein level in BGC-823 cells became very high. The evenly-distributed brown granules were detected in cytoplasm (Figure 3N). After treated with n-sodium butyrate, the P16 protein level became high. The brown granules were mainly detected in cytoplasm in a spare and dispersed manner. Some smaller granules were detected in marginal area of cytoplasm and protuberance of cells (Figure 3Q). After treated with combination of the two inducers, the P16 protein level became very high. The evenly-distributed brown granules were mainly detected in nucleoli and cytoplasm. The protein level in the combination treatment group was higher than in the group treated with n-sodium butyrate alone and the signal in nucleoli became very strong (Figure 3R).
Sine continual division and constant proliferation are important characteristics of tumor cells, the proliferation of tumor cells is one of the significant indexes in identifying exogenous inducers of differentiation [5]. In our study, the cell growth curve, mitotic index and cell cycle indicated that BGC-823 cells had vigorous proliferation capability, the doubling time was 45.82 h, the maximum mitotic index was 38.5%, and the proportion of cells was 50.7% in G0/G1 phase and 39.1% in S phase. However the doubling time of cells treated with tachyplesin or n-sodium butyrate was 75.7 h and 67.1 h respectively, the rate of cell growth inhibition was 62.66% and 60.19% respectively, the maximum mitotic index decreased to 12.6% and 10.9% respectively, the proportion of cells in G0/G1 phase was 59.5% and 59.2% respectively, while the proportion of BGC-823 cells in S phase decreased to 26.3% and 25.2% respectively. The results demonstrated that the inhibitory effects of tachyplesin and n-sodium butyrate on proliferation of BGC-823 cells were significant. n-sodium butyrate, a small molecule polar compound, has been widely used in inducing differentiation of tumor cells [3,6,7]. The effects of n-sodium butyrate on cell growth and cell cycle are coincident with the reports about the anti-proliferative effects of n-sodium butyrate on the cell cycle arrest of human gastric, colonic and endometrial carcinoma cell lines [6-9]. Our experiment showed that the effects of tachyplesin and n-sodium butyrate on cell growth and cell cycle of BGC-823 were similar, indicating that tachyplesin has identical effects on inhibiting proliferation as chemical inducers of cancer cells.
The changes in expression of oncogenes and tumor suppressor genes play a role in cell carcinogenesis and reversal movement, amplification and inactivation of some associated genes such as c-erbB-2, c-myc, p53, p16 [10-11] are a main index in differentiation of human gastric carcinoma cells [11]. In our study, the levels of c-erbB2, c-myc, mtp53 proteins were high but p16 expression was low in untreated BGC-823 cells. However, after treated with tachyplesin or n-sodium butyrate, the level of c-erbB-2, c-myc, mtp53 proteins decreased and p16 protein expression increased significantly in BGC-823 cells, demonstrating that both tachyplesin and n-sodium butyrate can influence the expression of oncogenes and tumor suppressor genes. Previous studies showed that n-sodium butyrate could down-regulate the expression of oncogenes (c-erbB-2, c-myc, p53) and up-regulate the expression of tumor suppressor genes (p16) in endometrial and colonic carcinoma and melanoma[7,8,12,13], confirming that n-sodium butyrate has obvious effects on differentiation of BGC-823 cells. Our results also showed that tachyplesin had the same effect on up-regulating the expression of tumor suppressor genes and down-regulating the expression of oncogenes as n-sodium butyrate in BGC-823 cells.
The combination of different inducers may have a synergistic effect on differentiation and reduce toxicity and side effects by cutting down the dosage. It is not only an important subject in the field of differentiation, but also has a positive impact on the clinical application of different therapies for cancer [14]. Our study showed that at the concentration of 1.0 g tachyplesin + 1.0 mmol/L n-sodium butyrate, the growth inhibitory rate was 62.29%, the mitotic index decreased to 18.5‰ at the divided peak and the proportion of cells in G0/G1 phase increased to 64.5% and decreased to 20.9% in S phase. Immunocytochemistry also showed that the levels of c-erbB-2, c-myc, p53 proteins in BGC-823 cells treated with the combination were lower and the level of p16 protein was higher.
In conclusion, tachyplesin has synergistic effects with n-sodium butyrate and can be used in treatment of cancer.
S- Editor Wang J L- Editor Wang XL E- Editor Zhang Y
| 1. | Iwanaga S, Kawabata S, Muta T. New types of clotting factors and defense molecules found in horseshoe crab hemolymph: their structures and functions. J Biochem. 1998;123:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 231] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 2. | Li QF, Ou Yang GL, Li CY, Hong SG. Effects of tachyplesin on the morphology and ultrastructure of human gastric carcinoma cell line BGC-823. World J Gastroenterol. 2000;6:676-680. [PubMed] [Cited in This Article: ] |
| 3. | Witt O, Schulze S, Kanbach K, Roth C, Pekrun A. Tumor cell differentiation by butyrate and environmental stress. Cancer Lett. 2001;171:173-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Nakamura T, Furunaka H, Miyata T, Tokunaga F, Muta T, Iwanaga S, Niwa M, Takao T, Shimonishi Y. Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus). Isolation and chemical structure. J Biol Chem. 1988;263:16709-16713. [PubMed] [Cited in This Article: ] |
| 5. | Li QF, Wang DY, The differentiation of human gastric adenocarcinoma cell line MGC80-3 induced by dibutyryl cAMP in vitro. Shiyan Shengwu Xuebao. 1990;23:167-175. [PubMed] [Cited in This Article: ] |
| 6. | Hung MW, Tsai LC, Lin YL, Chen YH, Chang GG, Chang TC. Differential regulation of placental and germ cell alkaline phosphatases by glucocorticoid and sodium butyrate in human gastric carcinoma cell line TMK-1. Arch Biochem Biophys. 2001;388:45-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Sasahara Y, Mutoh M, Takahashi M, Fukuda K, Tanaka N, Sugimura T, Wakabayashi K. Suppression of promoter-dependent transcriptional activity of inducible nitric oxide synthase by sodium butyrate in colon cancer cells. Cancer Lett. 2002;177:155-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Rong FN, Guo BY, Zhang GX. Effects of sodium butyrate on the proliferation of human endometrial carcinoma cell lines HHU. Zhongliu Fangzhi Zazhi. 2003;10:611-613. [Cited in This Article: ] |
| 9. | Nørsett KG, Laegreid A, Midelfart H, Yadetie F, Erlandsen SE, Falkmer S, Grønbech JE, Waldum HL, Komorowski J, Sandvik AK. Gene expression based classification of gastric carcinoma. Cancer Lett. 2004;210:227-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Li F, Wu YD, Wang YQ. Some Phenotypic changes in human colorectal carcinoma cells induced by sodium butyrate during differentiation. Zhongguo Yike Daxue Xuebao. 2000;29:404-407. [Cited in This Article: ] |
| 11. | Zhang GQ, Wang K, Zhang YB, Zhao JH. Immunohistochemical analysis of p53 and p16 genes expression in human stomach cancer. Shiyong Zhongliuxue Zazhi. 2003;17:174-176. [Cited in This Article: ] |
| 12. | Demary K, Wong L, Spanjaard RA. Effects of retinoic acid and sodium butyrate on gene expression, histone acetylation and inhibition of proliferation of melanoma cells. Cancer Lett. 2001;163:103-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Mariani MR, Carpaneto EM, Ulivi M, Allfrey VG, Boffa LC. Correlation between butyrate –induced histone hyperacetylation turn-over and c-myc expression. J Steroid Biochem Mol Biol. 2003;86:167-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Verlinden L, Verstuyf A, Mathieu C, Tan BK, Bouillon R. Differentiation induction of HL60 cells by 1, 25(OH)2D3, all trans retinoic acid, rTGF-beta2 and their combinations. J Steroid Biochem Mol Biol. 1997;60:87-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |