Published online Dec 7, 2005. doi: 10.3748/wjg.v11.i45.7192
Revised: April 23, 2005
Accepted: April 30, 2005
Published online: December 7, 2005
AIM: To investigate the diagnostic accuracy of potent serum biochemical fibrosis markers in children with chronic hepatitis B evaluated by receiver operating characteristics (ROC) analysis.
METHODS: We determined the serum level of apolipoprotein A-I (APO A-I), haptoglobin (HPT) and a-2 macroglobulin (A2M) with an automatic nephelometer in 63 children (age range 4-17 years, mean 10 years) with biopsy-verified chronic HBeAg-positive hepatitis B. Fibrosis stage and inflammation grade were assessed in a blinded fashion according to Batts and Ludwig. We defined mild liver fibrosis as a score ≤2 and advanced fibrosis as a score equal to 3. ROC analysis was used to calculate the power of the assays to detect advanced liver fibrosis (AccuROC, Canada).
RESULTS: Serum concentrations of APO A-I, HPT and A2M were not significantly different in patients with chronic hepatitis B compared to controls. However, APO A-I level of 1.19 ng/L had a sensitivity of 85.7% and a specificity of 60.7% (AUC = 0.7117, P = 0.035) to predict advanced fibrosis. All other serum biochemical markers and their combination did not allow a useful prediction. None of these markers was a good predictor of histologic inflammation.
CONCLUSION: Apolipoprotein A-I may be a suitable serum marker to predict advanced liver fibrosis in children with chronic hepatitis B.
- Citation: Lebensztejn DM, Skiba E, Tobolczyk J, Sobaniec-Lotowska ME, Kaczmarski M. Diagnostic accuracy of serum biochemical fibrosis markers in children with chronic hepatitis B evaluated by receiver operating characteristics analysis. World J Gastroenterol 2005; 11(45): 7192-7196
- URL: https://www.wjgnet.com/1007-9327/full/v11/i45/7192.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i45.7192
Liver biopsy is regarded as the gold standard for the determination of the stage of liver fibrosis. However, in clinical practice, the use of invasive liver biopsy has several limitations such as complications, sampling variability, low reproducibility and it only provides static information about the fibrotic process[1-3]. There is a clinical need for noninvasive measurement of liver fibrosis both to diagnose significant hepatic fibrosis and to monitor the effects of antiviral or antifibrotic therapy.
Several matrix-derived surrogate markers of extracellular matrix (ECM) turnover have been studied in adults so far and some were found to be correlated with matrix deposition, while their role as predictors of ECM turnover remains unclear[4-6]. This applies particularly for children whose serum levels of ECM-derived parameters are usually influenced by body growth[7,8]. Therefore, there is an urgent need for noninvasive parameters that better define those children who should undergo liver biopsy in order to assess fibrosis stage, to decide whether they should receive antiviral therapy or not, to predict fibrosis progression or to monitor potential antifibrotic treatment. Serum levels of type IV collagen[9,10], hyaluronic acid[10-13], laminin[14], collagen VI[15], transforming growth factor beta 1 (TGF beta 1)[16] and metalloproteinases (MMPs) or tissue inhibitors of metalloproteinases (TIMPs)[10,17] have recently been studied in children, but almost exclusively in patients with secondary biliary fibrosis due to biliary atresia and cystic fibrosis.
To our knowledge, serum fibrosis markers predicting liver fibrosis have not been assessed before in children with chronic hepatitis B (HBV) except our previous studies[18-20]. From a broad panel of matrix-derived serum markers (collagen IV, collagen VI, PIIINP, laminin-2, hyaluronan, MMP-2, TIMP-1, MMP-9/TIMP1 complex, tenascin-C), the combination of serum hyaluronan and laminin-2 can accurately predict significant liver fibrosis[18]. In our previous studies, we have reported that serum TGF beta 1 and cystatin C level did not predict advanced liver fibrosis in children with chronic hepatitis B[19,20].
In the present study, the serum levels of apolipoprotein A-I (APO A-I), haptoglobin (HPT) and a-2 macroglobulin (A2M) were measured in children with chronic hepatitis B and compared to liver histological features to determine whether the measurement of these biochemical tests have any clinical usefulness as markers of liver fibrosis. Receiver operating characteristics (ROC) analysis was used to determine the sensitivity and specificity of the assays in detecting advanced liver fibrosis.
The study was carried out in 63 children, including 41 boys and 22 girls (range 4-17 years, mean age 10 years) with biopsy-verified chronic HBeAg-positive hepatitis B prior to antiviral therapy. Other causes of chronic liver diseases, such as HCV coinfection, autoimmune hepatitis and metabolic liver disorders, were excluded. Children with diagnosed liver cirrhosis and evidence of other acute or chronic infections were also excluded from this study. Informed consent was obtained from all the parents of patients and the protocol used was approved by the Ethics Committee of the Medical University of Bialystok, Poland. As a control group, 16 children (mean age 10 years) were included without anamnestical, clinical or laboratory signs of liver or other systemic diseases.
APO A-I, HPT and A2M were measured in serum samples (obtained after an overnight fast) with an automatic nephelometer (BNII, Dade Behring; Marburg, Germany).
All children underwent liver biopsy on the day after serum sampling. Liver specimens were fixed in buffered formalin and embedded in paraffin. Histological sections were stained using hematoxylin-eosin, Masson’s-Goldner, Masson’s trichrome and reticulin. Fibrosis stage and inflammation grade were assessed in a blinded fashion by a single pathologist who was without the knowledge of the patients’ laboratory or clinical data. In order to determine specificity and sensitivity of the assay, we arbitrarily defined mild liver fibrosis or inflammation as a score ≤2and advanced liver disease as fibrosis or inflammation score equal to 3 according to Batts and Ludwig[21].
Serum concentrations of biochemical tests were expressed as mean±SD. Statistical analysis was performed using the Mann-Whitney two-sample test for nonparametric data. The relationship between the enzymes and liver histology scores was analyzed by the Spearman’s rank-correlation test for nonparametric data. Tests were considered statistically significant at P<0.05. Receiver operating characteristics (ROC) analysis (AccuROC, Montreal, Canada) was used to calculate the power of the assays to detect advanced liver fibrosis. The best cut-off points for the diagnosis of advanced fibrosis are those which maximize the sum of sensitivity and specificity. Sensitivity of the assays was plotted against the false positivity (1-specificity). Comparison of the area under the curve (AUC) was performed using a P-test, which compares the AUC to the diagonal line of no information (AUC 0.5)[22].
Selected biochemical and histological data are presented in Table 1.
| Parameters | Mean | SD | Minimum | Maximum |
| Age (yr) | 10 | 3.41 | 4 | 17 |
| ALT (IU/L) | 84 | 57 | 12 | 312 |
| AST (IU/L) | 68 | 37 | 27 | 264 |
| GGT (IU/L) | 15 | 9 | 3 | 69 |
| Bilirubin (μmol/L) | 10.26 | 4.45 | 2.74 | 32.83 |
| APO A-I (g/L) | 1.14 | 0.3 | 0.5 | 1.77 |
| HPT (g/L) | 0.58 | 0.36 | 0.31 | 1.69 |
| A2M (g/L) | 2.27 | 0.57 | 1 | 3.59 |
| Staging | 1.9 | 0.56 | 1 | 3 |
| Grading | 1.49 | 0.56 | 1 |
Serum concentrations of APO A-I, HPT and A2M were not significantly different in patients with chronic hepatitis B as compared with the controls (1.14±0.3 vs 1.13±0.2; 0.58±0.36 vs 0.71±0.62, 2.27±0.57 vs 2.53±0.3, respectively). There were no significant correlations of examined markers with liver fibrosis and inflammation according to Batts and Ludwig[21].
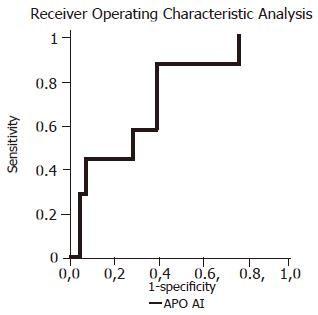
Seven children (11.1%) had advanced liver fibrosis. The ability of examined biochemical markers to differentiate the children with advanced liver fibrosis from those with mild fibrosis was not significant except APO A-I (Table 2 and Figure 1). Using the APO A-I and the Batts and Ludwig score, 34 and 6 out of 63 children could be correctly allocated either to the group with mild or advanced fibrosis, respectively, potentially avoiding biopsy in 40 (63.5%) of the examined children. HPT and A2M did not reach the predictive power of APO A-I (Table 2). None of the combination of examined markers resulted in a significant increase in sensitivity and specificity for the identification of patients with advanced liver fibrosis. None of these markers was a good predictor of histologic inflammation (Table 3).
| Markers | Cut-off | Se | Sp | PPV | NPV | AUC | P values |
| (g/L) | (%) | (%) | (%) | (%) | |||
| APO A-I | 1.19 | 85.7 | 60.7 | 21.4 | 97.1 | 0.7117 | 0.035 |
| HPT | 1.01 | 14.3 | 87.5 | 12.5 | 89.1 | 0.3878 | NS |
| A2M | 1.79 | 100 | 23.2 | 14 | 100 | 0.5268 | NS |
| Markers | Cut-off | Se | Sp | PPV | NPV | AUC | P values |
| (g/L) | (%) | (%) | (%) | (%) | |||
| APO A-I | 0.87 | 89.7 | 20.6 | 49.1 | 70.0 | 0.3803 | NS |
| HPT | 0.59 | 34.5 | 70.6 | 50.0 | 55.8 | 0.4701 | NS |
| A2M | 1.6 | 96.6 | 14.7 | 49.1 | 83.3 | 0.3813 | NS |
Infection with the hepatitis B virus remains one of the most important epidemiological problems all over the world. It was estimated that chronic HBV infection, which is the single most common cause of cirrhosis and hepatocellular carcinoma (HCC), affects more than 400 million people worldwide[23,24].
During the early phase of chronic hepatitis B, the underlying disease is usually subclinical and can be quite mild, particularly in children[25]. This is referred to as the immune-tolerant phase of infection[26]. While the natural history of chronic hepatitis B in children is usually marked by an indolent course, advanced disease with significant fibrosis can be found in up to one-third of the children, and decompensated cirrhosis and even HCC have been reported[27,28]. For this reason, noninvasive diagnosis and monitoring of liver fibrosis in children are urgently needed.
There are several features required for an ideal serum fibrosis marker. It should be liver-specific, independent of metabolic alterations, minimally influenced by impaired urinary and biliary excretion, easy to perform and it should measure either the dynamic processes of fibrogenesis or fibrolysis and reflect the degree of fibrosis. Unfortunately, no current assay fulfills enough of these criteria[5].
Recently, algorithms of non-ECM-derived serum markers have been proposed as the predictors of fibrosis stage in adult patients with chronic hepatitis C[29-31]. APO A-I, HPT and A2M are the components of FibroTest[32] and APO A-I and A2M are the components of PGAA index[33,34]; they have also been validated as single serum fibrosis markers[6,35-38].
In the present study, we did not find significantly different levels of examined serum fibrosis markers in children with chronic hepatitis B as compared with the controls. There were also no significant correlation of APO A-I, HPT, and A2M levels with histologically assessed stage of liver fibrosis and inflammation grade. Because liver biopsy is not necessarily a gold standard for assessing liver histology, noninvasive markers will not have complete concordance with histological staging. Only 11.1% children had advanced fibrosis according to Batts and Ludwig[21] and probably for this reason, we also did not observe statistically significant correlation between the examined markers and fibrosis stage.
These findings confirmed previous observation in children by Selimoglu et al[35,37] who also demonstrated that mean APO A-I level of the patients with chronic hepatitis B was not different from controls and did not correlate with fibrosis and inflammation scores. To our knowledge, A2M and HPT as potent serum fibrosis markers have not been assessed in children yet.
In adults with chronic hepatitis B, Oberti et al[6] and Huang and Gong[36] observed that APO A-I and A2M were significantly correlated with the staging of liver fibrosis. Lu et al[34] confirmed that A2M but not APO A-I was significantly correlated with fibrosis stage. However, HPT was negatively associated with fibrosis[38]. These opposing correlation of A2M and HPT with fibrosis staging could be explained by the different roles of hepatocyte growth factor (HGF) and TGF beta 1 in fibrogenesis and acute phase response[39-41]. As seen in experimental fibrosis, transduction with HGF gene suppresses increase of TGF beta 1[39] and the factor stimulates synthesis of A2M[40] and reduces synthesis of haptoglobin[40,41].
Recently, ROC analysis has been recommended to calculate the power of the assays to detect advanced liver fibrosis[42-46]. In this study, the ability of examined biochemical markers to differentiate children with advanced liver fibrosis from those with mild fibrosis was not significant except APO A-I. The high negative predictive value of this marker could potentially be useful in the selection of patients without significant fibrosis and in avoiding invasive liver biopsy in this group of children. Our study is not consistent with the findings of Selimoglu et al[35], because they did not find diagnostic performance of APO A-I for discriminating patients with advanced liver disease. Usefulness of APO A-I as well as HPT and A2M as potent serum fibrosis markers in patients with chronic liver disease needs to be evaluated in large controlled studies.
Science Editor Kumar M and Guo SY Language Editor Elsevier HK
| 1. | Kirchner GI, Wagner S, Flemming P, Bleck JS, Gebel M, Schedel I, Schüler A, Galanski M, Manns MP. COACH syndrome associated with multifocal liver tumors. Am J Gastroenterol. 2002;97:2664-2669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1193] [Cited by in F6Publishing: 1334] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 3. | Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 850] [Cited by in F6Publishing: 769] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 4. | Plebani M, Burlina A. Biochemical markers of hepatic fibrosis. Clin Biochem. 1991;24:219-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Schuppan D, Stölzel U, Oesterling C, Somasundaram R. Serum assays for liver fibrosis. J Hepatol. 1995;22:82-88. [PubMed] [Cited in This Article: ] |
| 6. | Oberti F, Valsesia E, Pilette C, Rousselet MC, Bedossa P, Aubé C, Gallois Y, Rifflet H, Maïga MY, Penneau-Fontbonne D. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology. 1997;113:1609-1616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 310] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Trivedi P, Risteli J, Risteli L, Tanner MS, Bhave S, Pandit AN, Mowat AP. Serum type III procollagen and basement membrane proteins as noninvasive markers of hepatic pathology in Indian childhood cirrhosis. Hepatology. 1987;7:1249-1253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Danne T, Grüters A, Schuppan D, Quantas N, Enders I, Weber B. Relationship of procollagen type III propeptide-related antigens in serum to somatic growth in healthy children and patients with growth disorders. J Pediatr. 1989;114:257-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Kobayashi H, Miyano T, Horikoshi K, Tokita A. Prognostic value of serum procollagen III peptide and type IV collagen in patients with biliary atresia. J Pediatr Surg. 1998;33:112-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Pereira TN, Lewindon PJ, Smith JL, Murphy TL, Lincoln DJ, Shepherd RW, Ramm GA. Serum markers of hepatic fibrogenesis in cystic fibrosis liver disease. J Hepatol. 2004;41:576-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Kobayashi H, Horikoshi K, Yamataka A, Yamataka T, Okazaki T, Lane GJ, Miyano T. Hyaluronic acid: a specific prognostic indicator of hepatic damage in biliary atresia. J Pediatr Surg. 1999;34:1791-1794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Hasegawa T, Sasaki T, Hoki M, Okada A, Mushiake S, Yagi M, Imura K. Measurement of serum hyaluronic acid as a sensitive marker of liver fibrosis in biliary atresia. J Pediatr Surg. 2000;35:1643-1646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Wyatt HA, Dhawan A, Cheeseman P, Mieli-Vergani G, Price JF. Serum hyaluronic acid concentrations are increased in cystic fibrosis patients with liver disease. Arch Dis Child. 2002;86:190-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Sasaki F, Hata Y, Hamada H, Takahashi H, Uchino J. Laminin and procollagen-III-peptide as a serum marker for hepatic fibrosis in congenital biliary atresia. J Pediatr Surg. 1992;27:700-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Gerling B, Becker M, Staab D, Schuppan D. Prediction of liver fibrosis according to serum collagen VI level in children with cystic fibrosis. N Engl J Med. 1997;336:1611-1612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Rosensweig JN, Omori M, Page K, Potter CJ, Perlman EJ, Thorgeirsson SS, Schwarz KB. Transforming growth factor-beta1 in plasma and liver of children with liver disease. Pediatr Res. 1998;44:402-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Kobayashi H, Li ZX, Yamataka A, Lane GJ, Miyano T. Clinical evaluation of serum levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases as predictors of progressive fibrosis in postoperative biliary atresia patients. J Pediatr Surg. 2002;37:1030-1033 DOI : 10.1053/jpsu.2002.33836. [Cited in This Article: ] |
| 18. | Lebensztejn DM, Kaczmarski M, Sobaniec-Łotowska M, Bauer M, Voelker M, Schuppan D. Serum laminin-2 and hyaluronan predict severe liver fibrosis in children with chronic hepatitis B. Hepatology. 2004;39:868-869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Lebensztejn DM, Sobaniec-Lotowska M, Kaczmarski M, Werpachowska I, Sienkiewicz J. Serum concentration of transforming growth factor (TGF)-beta 1 does not predict advanced liver fibrosis in children with chronic hepatitis B. Hepatogastroenterology. 2004;51:229-233. [PubMed] [Cited in This Article: ] |
| 20. | Lebensztejn DM, Skiba E, Kaczmarski M, Tobolczyk J, Koput A, Sobaniec-Łotowska ME. Serum cystatin C concentration does not predict advanced liver disease in children with chronic hepatitis B. Clin Chim Acta. 2004;347:227-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 823] [Cited by in F6Publishing: 800] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 22. | Vida S. A computer program for non-parametric receiver operating characteristic analysis. Comput Methods Programs Biomed. 1993;40:95-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1728] [Cited by in F6Publishing: 1678] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 24. | Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000--summary of a workshop. Gastroenterology. 2001;120:1828-1853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 540] [Cited by in F6Publishing: 490] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 25. | Tang JR, Hsu HY, Lin HH, Ni YH, Chang MH. Hepatitis B surface antigenemia at birth: a long-term follow-up study. J Pediatr. 1998;133:374-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Lok AS, Lai CL. A longitudinal follow-up of asymptomatic hepatitis B surface antigen-positive Chinese children. Hepatology. 1988;8:1130-1133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 132] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Bortolotti F, Jara P, Crivellaro C, Hierro L, Cadrobbi P, Frauca E, Camarena C, De La Vega A, Diaz C, De Moliner L. Outcome of chronic hepatitis B in Caucasian children during a 20-year observation period. J Hepatol. 1998;29:184-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Fujisawa T, Komatsu H, Inui A, Sogo T, Miyagawa Y, Fujitsuka S, Sekine I, Kosugi T, Inui M. Long-term outcome of chronic hepatitis B in adolescents or young adults in follow-up from childhood. J Pediatr Gastroenterol Nutr. 2000;30:201-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1066] [Cited by in F6Publishing: 1113] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 30. | Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 672] [Cited by in F6Publishing: 751] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 31. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2762] [Cited by in F6Publishing: 2988] [Article Influence: 142.3] [Reference Citation Analysis (0)] |
| 32. | Halfon P, Imbert-Bismut F, Messous D, Antoniotti G, Benchetrit D, Cart-Lamy P, Delaporte G, Doutheau D, Klump T, Sala M. A prospective assessment of the inter-laboratory variability of biochemical markers of fibrosis (FibroTest) and activity (ActiTest) in patients with chronic liver disease. Comp Hepatol. 2002;1:3 DOI : 10.1186/1476-5926-1-3 PMCid: PMC149429. [Cited in This Article: ] |
| 33. | Naveau S, Poynard T, Benattar C, Bedossa P, Chaput JC. Alpha-2-macroglobulin and hepatic fibrosis. Diagnostic interest. Dig Dis Sci. 1994;39:2426-2432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 94] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Lu LG, Zeng MD, Mao YM, Li JQ, Qiu DK, Fang JY, Cao AP, Wan MB, Li CZ, Ye J. Relationship between clinical and pathologic findings in patients with chronic liver diseases. World J Gastroenterol. 2003;9:2796-2800. [PubMed] [Cited in This Article: ] |
| 35. | Selimoglu MA, Yagcl RV, Yüce G. Low plasma apolipoprotein A-I level is not a reliable marker of fibrosis in children with chronic hepatitis B. World J Gastroenterol. 2004;10:2864-2866. [PubMed] [Cited in This Article: ] |
| 36. | Huang W, Gong FY. [Diagnostic value of serum biochemical markers for liver fibrosis in patients with hepatitis B virus]. Di Yi Jun Yi Da Xue Xue Bao. 2002;22:1034-1036. [PubMed] [Cited in This Article: ] |
| 37. | Selimoğlu MA, Aydoğdu S, Yağci RV. Low plasma apolipoprotein A-I level: new prognostic criterion in childhood cirrhosis? Turk J Pediatr. 2001;43:307-311. [PubMed] [Cited in This Article: ] |
| 38. | Bacq Y, Schillio Y, Brechot JF, De Muret A, Dubois F, Metman EH. [Decrease of haptoglobin serum level in patients with chronic viral hepatitis C]. Gastroenterol Clin Biol. 1993;17:364-369. [PubMed] [Cited in This Article: ] |
| 39. | Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 456] [Cited by in F6Publishing: 483] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 40. | Guillén MI, Gómez-Lechón MJ, Nakamura T, Castell JV. The hepatocyte growth factor regulates the synthesis of acute-phase proteins in human hepatocytes: divergent effect on interleukin-6-stimulated genes. Hepatology. 1996;23:1345-1352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Moshage H. Cytokines and the hepatic acute phase response. J Pathol. 1997;181:257-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 42. | Zheng M, Cai WM, Weng HL, Liu RH. ROC curves in evaluation of serum fibrosis indices for hepatic fibrosis. World J Gastroenterol. 2002;8:1073-1076. [PubMed] [Cited in This Article: ] |
| 43. | Lu LG, Zeng MD, Wan MB, Li CZ, Mao YM, Li JQ, Qiu DK, Cao AP, Ye J, Cai X. Grading and staging of hepatic fibrosis, and its relationship with noninvasive diagnostic parameters. World J Gastroenterol. 2003;9:2574-2578. [PubMed] [Cited in This Article: ] |
| 44. | Zhang BB, Cai WM, Weng HL, Hu ZR, Lu J, Zheng M, Liu RH. Diagnostic value of platelet derived growth factor-BB, transforming growth factor-beta1, matrix metalloproteinase-1, and tissue inhibitor of matrix metalloproteinase-1 in serum and peripheral blood mononuclear cells for hepatic fibrosis. World J Gastroenterol. 2003;9:2490-2496. [PubMed] [Cited in This Article: ] |
| 45. | Patel K, Gordon SC, Jacobson I, Hézode C, Oh E, Smith KM, Pawlotsky JM, McHutchison JG. Evaluation of a panel of non-invasive serum markers to differentiate mild from moderate-to-advanced liver fibrosis in chronic hepatitis C patients. J Hepatol. 2004;41:935-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 251] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 46. | Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704-1713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 756] [Cited by in F6Publishing: 706] [Article Influence: 35.3] [Reference Citation Analysis (0)] |