Published online Nov 28, 2005. doi: 10.3748/wjg.v11.i44.7036
Revised: August 15, 2004
Accepted: August 18, 2004
Published online: November 28, 2005
AIM: To investigate the prediction value of radiosensitivity of hepatocarcinoma cells for apoptosis and micronucleus assay.
METHODS: Clonogenic assay, flow cytometry, and CB micronuclei assay were used to survey the cell survival rate, radiation-induced apoptosis and micronucleus frequency of hepatocarcinoma cell lines SMMC-7721, HL-7702, and HepG2 after being irradiated by X-ray at the dosage ranging 0-8 Gy.
RESULTS: After irradiation, there was a dose-effect relationship between micronucleus frequency and radiation dosage among the three cell lines (P<0.05). A positive relationship was observed between apoptosis and radiation dosage among the three cell lines. The HepG2 cells had a significant correlation (P<0.05) but apoptosis incidence had a negative relationship with micronucleus frequency. There was a positive relationship between apoptosis and radiation dosage and the correlation between SMMC-7721 and HL-7702 cell lines had a significant difference (P<0.01). After irradiation, a negative relationship between cell survival rate and radiation dosages was found among the three cell lines (P<0.01). There was a positive relationship between cell survival rate and micronucleus frequency (P<0.01). No correlation was observed between apoptosis and cell survival rate.
CONCLUSION: The radiosensitivity of hepatocarcinoma cells can be reflected by apoptosis and micronuclei. Detection of apoptosis and micronuclei could enhance the accuracy for predicting radiosensitivity.
- Citation: Liu ZZ, Huang WY, Li XS, Lin JS, Cai XK, Lian KH, Zhou HJ. Prediction value of radiosensitivity of hepatocarcinoma cells for apoptosis and micronucleus assay. World J Gastroenterol 2005; 11(44): 7036-7039
- URL: https://www.wjgnet.com/1007-9327/full/v11/i44/7036.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i44.7036
The prediction of cell radiosensitivity is a main content in radiobiology and carcinoma treatment. Apoptosis of carcinoma cells can be induced by radiotherapy. Micronucleus is a product of abnormal mitosis. Studies have verified that micronucleus analysis can predict radiosensitivity of cells[1,2]. It was reported that apoptosis is correlated with cell survival[3,4]. Apoptosis and micronuclei occur after the first mitosis and are related with the damage in heredity substance. To investigate apoptosis and micronucleus assay is of significance in predicting radiosensitivity.
This study focused on the relationship among apoptosis, micronuclei, and cell survival rate of three hepatic cell lines (HL-7702, HepG2, SMMC-7721). The prediction value of radiosensitivity for apoptosis and micronucleus assay of hepatocarcinoma cells was investigated in vitro.
Three hepatic carcinoma cell lines (HL-7702, HepG2, SMMC-7721) were cultured at 37 °C in RPMI 1640 medium supplemented with 10% fetal bovine serum and divided into irradiated group and non-irradiated group. Cells in experimental growth phase were irradiated by X-ray at a dose of 2-8 Gy (Linear Accelerator Saturn 43 type) with a dose rate of 200 cGy/min. The lucite was placed in dishes.
Cells in the experimental growth phase were digested by 2.5 g/L trypsin into a single-cell suspension. In vitro transduction was performed by plating 1×105 cells in 3-cm2 flasks. Cells were irradiated with 2-8 Gy. After 12-15 d of plating, the culture was stopped. Cells were fixed by formaldehyde and stained with Giemsa. The number of colonies exceeding 50 was defined as positive. The survival fraction rate amounted to colony formation rate in irradiation group. Three parallel samples were set in each dosage point and repeated thrice.
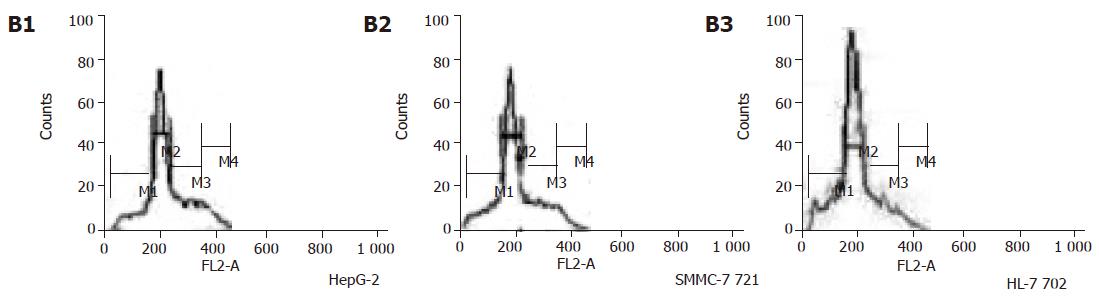
Cells in the experimental growth phase were digested by 2.5 g/L trypsin into single-cell suspension at the concentration of 3×104μg/mL and inoculated in petri dishes. When the cells were attached to the wall, they were cultured for 2 days and then irradiated with 2-8 Gy. After irradiation, samples were added in 2 μg/mL cytochalasin-B, and cultured for 48 h, and then stained in situ. The number of micronuclei in double nuclear cells was counted. At least 1 000 double nuclear cells were calculated. Each dosage had three parallel exponents and the experiment was repeated thrice.
Cells were centrifuged and washed 2-3 times with PBS and then fixed in 80% ethanol at -20 °C. The samples were centrifuged for 5 min (800 r/min) and washed with PBS, mixed into mono cell suspension and diluted at 1:400 in PBS. The cells were treated with RNase for 30 min, resuspended in 10 μg/mL of PI and incubated overnight at 4 °C. Cellular fluorescence was measured by FASort flow cytometry (Becton Dickinson, San Jose, CA, USA). We collected the data by CELLQuest software and analyzed the cell cycle by ModFit2.0.
Correlation analysis was made using the SPSS statistical analysis software.
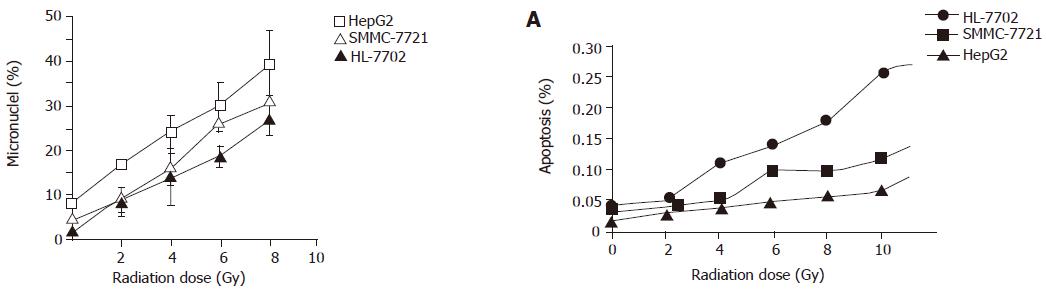
After being irradiated with 2-8 Gy, the micronucleus frequency of HepG2, SMMC-7721, and HL-7702 was related with radiation dosage. The relationship between the micronucleus frequency of HepG2 and radiation dosage was significant (P<0.05, Figure 1).
After being irradiated with 2-8 Gy, a positive relationship was observed between apoptosis incidence and radiation dosages. The relationship between micronucleus frequency of HepG2, SMMC-7721 and radiation dosage was significant (P<0.01, Figure 2). But apoptosis incidence was negatively related with micronucleus frequency.
After being irradiated with 2-8 Gy, the cell survival rate of three cell lines was decreased (P<0.05, Tables 1 and 2).
| Cells | Radiation dose (Gy) | |||
| 2 | 4 | 6 | 8 | |
| HepG2 | 0.45±0.08 | 0.22±0.02 | 0.06±0.02 | 0±0 |
| SMMC-7721 | 0.72±0.05 | 0.29±0.05 | 0.08±0.05 | 0±0 |
| HL-7702 | 0.79±0.02 | 0.31±0.09 | 0.11±0.06 | 0±0 |
| Cells | Do | Dq | n | SF2 |
| HepG2 | 1.5 | 0.8 | 1.7 | 0.45±0.08 |
| SMMC-7721 | 1.8 | 1.5 | 2.5 | 0.72±0.05 |
| HL-7702 | 2 | 1.8 | 3 | 0.79±0.02 |
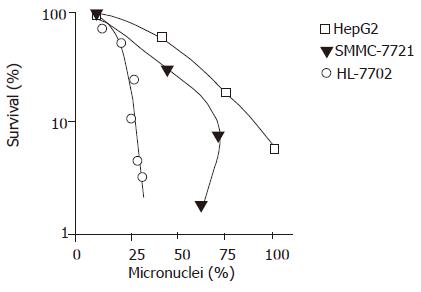
After being irradiated with 2-8 Gy, a positive relationship was found between micronucleus frequency and cell survival rate (P<0.01, Figure 3) .
The relationship between micronucleus frequency and apoptosis of three cell lines was not significant (P>0.05).
Hepatocarcinoma is a common malignant tumor. Stereotactic radiotherapy of hepatocarcinoma can achieve better effects. Hepatocarcinoma cells may have corresponding radiosensitivity.
Micronucleus is a product of abnormal mitosis and consists of chromatosome fragments that cannot enter the core nuclei when cells undergo mitosis. Micronucleus analysis is a method to detect cell radiosensitivity. Widel et al[5] found that micronucleus frequency can be used as a marker to predict prognosis of hepatocarcinoma patients. Kolotas et al[6] showed that the prognosis is better in patients with a higher micronucleus frequency than in those with a lower micronucleus frequency. But we found that micronucleus frequency of radiation-resistant cell lines was not related with cell survival rate. It is usually believed that micronuclei induced by X-ray express only in sensitive cells. Micronucleus frequency of sensitive cells is higher than that of radiation-resistant cells. Therefore, micronuclei as a marker to predict cell radiosensitivity of all cells needs to be studied further.
Apoptosis of carcinoma cells can be induced by radiotherapy. The sensitivity to apoptosis and X-ray of carcinoma cells is coincident. Studies showed that apoptosis index of carcinoma cells increases after radiation. The rate of apoptosis induced by radiotherapy increases after radiation, indicating that apoptosis can be used as a marker to predict radiosensitivity.
In this experiment, radiation dose was related with micronucleus frequency in three cell lines. There was a positive relationship between the micronucleus frequency and cell survival rate, suggesting that micronucleus frequency can be used as a marker to predict the radiosensitivity of hepatic carcinoma cells. Radiation dose was related with apoptosis incidence in three cell lines. The apoptosis incidence of HL-7702 and SMMC-7721 was higher than that of HepG2, suggesting that apoptosis incidence can be used as a marker to predict the radiosensitivity of hepatocarcinoma cells.
In this experiment, no significant difference was found between apoptosis incidence induced by radiotherapy and cell survival rate in three cell lines, indicating that the damage induced by radiotherapy is different from apoptosis of hepatic carcinoma cells and that response of radiation-resistant cells occurs due to apoptosis.
Masunaga et al[10] showed that micronucleus frequency and apoptosis incidence can predict radiosensitivity of carcinoma cells. Our study confirmed that micronucleus frequency was negatively related with apoptosis incidence of three cell lines. Apoptosis resulted from cell damage. The impaired cells express apoptosis or micronuclei. This result supports that once apoptosis occurs, the cells lose the segregating opportunity, thus affecting micronucleus formation. Apoptosis may be an important factor affecting micronucleus frequency.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Vijayalaxmi , Bisht KS, Pickard WF, Meltz ML, Roti Roti JL, Moros EG. Chromosome damage and micronucleus formation in human blood lymphocytes exposed in vitro to radiofrequency radiation at a cellular telephone frequency (847.74 MHz, CDMA). Radiat Res. 2001;156:430-432. [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Bhattathiri VN, Bindu L, Remani P, Chandralekha B, Davis CA, Nair MK. Serial cytological assay of micronucleus induction: a new tool to predict human cancer radiosensitivity. Radiother Oncol. 1996;41:139-142. [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Kirsch-Volders M, Elhajouji A, Cundari E, Van Hummelen P. The in vitro micronucleus test: a multi-endpoint assay to detect simultaneously mitotic delay, apoptosis, chromosome breakage, chromosome loss and non-disjunction. Mutat Res. 1997;392:19-30. [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 213] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Levine EL, Renehan A, Gossiel R, Davidson SE, Roberts SA, Chadwick C, Wilks DP, Potten CS, Hendry JH, Hunter RD. Apoptosis, intrinsic radiosensitivity and prediction of radiotherapy response in cervical carcinoma. Radiother Oncol. 1995;37:1-9. [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 120] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Widel M, Kolosza Z, Jedruś S, Lukaszczyk B, Raczek-Zwierzycka K, Swierniak A. Micronucleus assay in vivo provides significant prognostic information in human cervical carcinoma; the updated analysis. Int J Radiat Biol. 2001;77:631-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Kolotas C, Tonus C, Baltas D, Cernea M, Vogt HG, Martin T, Strassmann G, Zamboglou N. Clinical relevance of tumor ploidy and micronucleus formation for oral cavity cancer. Tumori. 1999;85:253-258. [PubMed] [Cited in This Article: ] |
| 7. | Guo GZ, Sasai K, Oya N, Shibata T, Shibuya K, Hiraoka M. A significant correlation between clonogenic radiosensitivity and the simultaneous assessment of micronucleus and apoptotic cell frequencies. Int J Radiat Biol. 1999;75:857-864. [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Pervan M, Pajonk F, Sun JR, Withers HR, McBride WH. Molecular pathways that modify tumor radiation response. Am J Clin Oncol. 2001;24:481-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Kim JY, Cho HY, Lee KC, Hwang YJ, Lee MH, Roberts SA, Kim CH. Tumor apoptosis in cervical cancer: its role as a prognostic factor in 42 radiotherapy patients. Int J Cancer. 2001;96:305-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Masunaga SI, Ono K, Suzuki M, Nishimura Y, Kinashi Y, Takagaki M, Hori H, Nagasawa H, Uto Y, Tsuchiya I. Radiosensitization effect by combination with paclitaxel in vivo, including the effect on intratumor quiescent cells. Int J Radiat Oncol Biol Phys. 2001;50:1063-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |