Published online Nov 28, 2005. doi: 10.3748/wjg.v11.i44.7001
Revised: April 15, 2005
Accepted: April 18, 2005
Published online: November 28, 2005
AIM: To study the efficacy, safety, and feasibility of a granulocyte adsorptive type apheresis system for the treatment of patients with chronically active ulcerative colitis despite standard therapy.
METHODS: An open label multicenter study was carried out in 39 patients with active ulcerative colitis (CAI 6-8) despite continuous use of steroids (a minimum total dose of 400 mg prednisone within the last 4 wk). Patients received a total of five aphereses using a granulocyte adsorptive technique (Adacolumn®, Otsuka Pharmaceutical Europe, UK). Assessments at wk 6 and during follow-up until 4 mo comprised clinical (CAI) and endoscopic (EI) activity index, histology, quality of life (IBDQ), and laboratory tests.
RESULTS: Thirty-five out of thirty-nine patients were qualified for intent-to-treat analysis. After the apheresis treatment at wk 6, 13/35 (37.1%) patients achieved clinical remission and 10/35 (28.6%) patients had endoscopic remission (CAI<4, EI<4). Quality of life (IBDQ) increased significantly (24 points, P<0.01) at wk 6. Apheresis could be performed in all but one patient. Aphereses were well tolerated, only one patient experienced anemia.
CONCLUSION: In patients with steroid refractory ulcerative colitis, five aphereses with a granulocyte/monocyte depleting filter show potential short-term efficacy. Tolerability and technical feasibility of the procedure are excellent.
- Citation: Kruis W, Dignass A, Steinhagen-Thiessen E, Morgenstern J, Mössner J, Schreiber S, Vecchi M, Malesci A, Reinshagen M, Löfberg R. Open label trial of granulocyte apheresis suggests therapeutic efficacy in chronically active steroid refractory ulcerative colitis. World J Gastroenterol 2005; 11(44): 7001-7006
- URL: https://www.wjgnet.com/1007-9327/full/v11/i44/7001.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i44.7001
Systemically acting corticosteroids are mainstay in the treatment of patients with severely active ulcerative colitis (UC). But steroid free remission cannot be achieved in up to 40% of these selected patients[1,2]. Thus, there is a need of alternative treatment options.
Etiology of inflammatory bowel disease (IBD) is still unknown, but the pathogenesis is thought to comprise interaction between genetic factors, intestinal flora, and immunomediated tissue injury[3] Extravasation of a large number of granulocytes and macrophages into the mucosa plays a major role in the release of proinflammatory cytokines[4,5], reactive oxygen derivatives[6-9], and degradative proteases[10]. Accordingly, immunosuppressive therapy has been introduced for refractory patients. Azathioprine/6-mercaptopurine, cyclosporin, and tacrolimus are widely used at present. But drawbacks such as delayed efficacy, adverse events, and costs are currently still limiting the clinical success in severely ill patients and proctocolectomy is necessary in about one-third of these patients. The search for a better treatment strategy has failed as yet. Trials with methotrexate, infliximab, and interferon have shown no convincing effects.
In view of the pathogenetic key role of granulocytes and macrophages, it makes sense to focus on therapeutic goals here. A recently developed adsorptive carrier-based granulocyte and monocyte apheresis device has demonstrated significant effects on inflammatory processes both in vivo and in vitro[11]. Preliminary data have shown promising therapeutic efficacy in IBD, rheumatoid arthritis, and other conditions[11]. Expanding on these findings, we have reported the results of an open label study on the efficacy of granulocyte apheresis treatment in severely ill patients with chronically active UC despite high doses of systemic corticosteroids.
This was a prospective and open label trial investigating the efficacy, safety, and feasibility of therapeutic apheresis in patients with active UC despite chronic intake of high doses of systemic corticosteroids. The study was conducted in eight European gastrointestinal (GI) referral centers (Germany, Sweden, Italy, Spain) according to the Declaration of Helsinki (as amended in Edinburgh) and the good clinical practice (GCP) guidelines. The study was approved by the “Ethikkommission der Ärztekammer Nordrhein”, Germany as well as local ethics committees of the participating centers. All patients received materials in their own language and gave written consent.
Patients were included if they were aged between 18 and 75 years and diagnosed with active disease (clinical activity index/CAI≥6 and ≤8, and endoscopic index/EI>4)[12]. Additional inclusion criteria were as follows: patients who were dependent on steroids and relapsed despite continuous use of steroids (a minimum total dose of 400 mg prednisone or equivalent within the last 4 wk, dose of steroids was not changed 2 wk prior to the study), history of at least one previous attack with an unsuccessful attempt to taper corticosteroids, immunosuppressants, and aminosalicylates were constant, 3 mo and 4 wk prior to the study, respectively.
Exclusion criteria were patients with severe activity (CAI>8); pregnancy, nursing; arterial hypotension/systolic pressure <11.97 kPa and/or diastolic pressure <8.645 kPa or hypertension (systolic pressure >23.94 kPa or diastolic pressure >15.96 kPa); serious renal, hepatic or cardiovascular disease; laboratory abnormalities such as neutrophils <1×109/L, platelets <100×109/L, hemoglobin <10 g/L, aPT >1.25 times upper range, aPTT >1.66 times upper normal range, AST or ALT >3 times upper normal range, total bilirubin >2.5 times upper normal range, creatinine >1.8 mg/L; allergy to heparin; viral infection within 4 wk prior to the study; positive stool microbiology; major bowel resection.
Each patient was intended to receive a total of five apheresis sessions, each per week for 5 consecutive weeks. An apheresis session lasted for 60 min at a flow rate of 30 mL/min. Most treatments were performed in an outpatient clinic, partly in dialysis units and also in apheresis non-specialized settings.
The apheresis system used consists of a pump with an integrated monitor (Adamonitor®, manufactured by Otsuka Electronics, Japan) and a single use polycarbonate column (Adacolumn®, CE-Mark; EC certificate GI030136676005, TUV) with a capacity of about 335 mL, filled with 220 g cellulose acetate beads (about 35 000 pieces) of 2 mm in diameter (carriers) bathed in physiological saline and steam sterilized (manufactured by JIMRO, Japan).
Concomitant medication according to inclusion criteria was permitted.
The aim of this open label pilot study was to investigate the therapeutic effects of a granulocyte adsorptive type apheresis system in patients with chronically active UC despite high doses of corticosteroids. Clinical efficacy as well as feasibility and safety of the procedure were evaluated.
Assessments were performed at the beginning and end of the study as well as after 1, 2, 3, 4, and 5 wk of treatment. Clinical (clinical activity index, CAI) and endoscopic (endoscopic index, EI) findings were defined according to Rachmilewitz[12]. Endoscopies were performed at the beginning and the end of study. Histology was reviewed by pathologists who were blinded to the study. Clinical remission was defined by a score of CAI≤4, while clinical response was defined as a drop of CAI≥3. Endoscopic remission was defined by an EI≤4, while endoscopic response was defined as a drop of EI≥2. In addition, quality of life was assessed according to the IBDQ scoring system[13]. IBDQ was not assessed in Italian patients(n = 5). Physician’s global assessment was asked for and the consumption of steroids was noted.
Laboratory tests included a differential blood count, hemoglobin, hematocrit, fibrinogen, aPT, aPTT, ESR, CRP, orosomucoid, calcium, sodium, potassium, phosphorus, creatinine, BUN/urea, uric acid, bilirubin, total protein albumin, alpha1-antitrypsin, haptoglobin, alkaline phosphatase, AST, ALT, GGT, LDH, and standard urine analyses.
The primary objective of this open multicenter study was to find the number of patients achieving clinical remission (CAI≤4) at the end of the treatment (wk 6). Because of the uncontrolled pilot characteristics of the study, only descriptive statistical analyses were performed. Sample size was set at 35 patients. Two groups of patients were analyzed. The intent-to-treat (ITT) population consisted of all patients who experienced at least one complete (60 min) treatment of apheresis. Only patients who fulfilled the protocol without major violations were included into the per-protocol (PP) population. Comparisons were made using Wilcoxon signed rank test.
If not otherwise mentioned, data were expressed as mean+SE. P<0.05 was considered statistically significant.
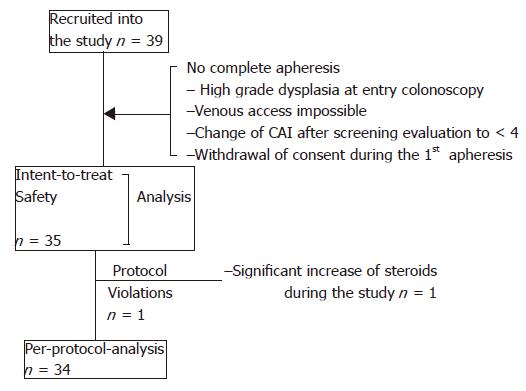
According to the inclusion criteria a total of 39 patients were recruited. Among the patients who underwent proctocolectomy, one patient received surgery before he started apheresis therapy because high grade dysplasia was found in the initial colonoscopy, the other two patients were operated upon because of refractory disease at wk 6 during the follow-up period. Figure 1 displays the different analysis sets and reasons for exclusion. Biographic and clinical characteristics are listed in Table 1. Suitable venous access could not be accomplished in one patient. All the other patients who started treatment received five treatments with apheresis. A total of 176 apheresis procedures could be performed without major technical difficulties. The majority of the patients (18/35) were treated in GI units, while 17/35 patients were treated in specialized dialysis units. Aphereses lasted for 60±3 min in 90.3% of the procedures and were prematurely stopped (50-55 min) in 1.7% procedures and the respective apheresis exceeded (65-91 min) in 8.0% procedures.
| Patients (ITT analysis) | n = 35 |
| Age: median/range (yr) | 35 (20-72) |
| Female n (%) | 10 (28.6) |
| Smoker n (%) | 2 (5.7) |
| Ex-smoker n (%) | 19 (54.3) |
| Disease: duration (mean+SE, yr) 7.2+0.8 | |
| Site of disease: extent n (%): | |
| Proctosigmoiditis | 3 (8.6) |
| Left sided | 9 (25.7) |
| Pancolitis | 23 (65.7) |
| Pretreatment (all patients had steroids) | |
| Aminosalicylates n (%) | 25 (74.40%) |
| Immunosuppressants n (%) | 13 (37.10%) |
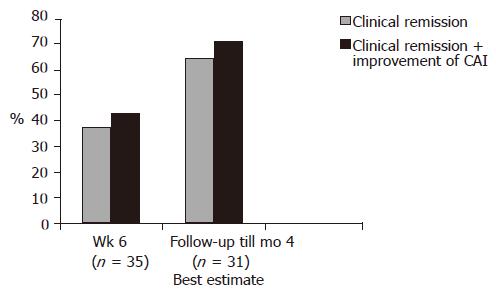
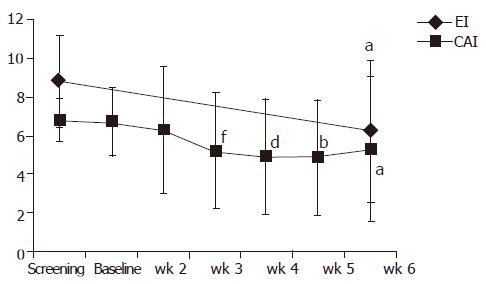
At the end of the treatment period (at wk 6) 13/35 patients (37.1%) achieved clinical remission (CAI≤4). Remission or clinical improvement was observed in 15/35 (42.8%) of the patients. During a follow-up period (up to 4 mo), the number of patients achieving clinical remission increased to 20/31 patients (65%) (best estimate) (Figure 2). Except for doses of steroids no other relevant changes in medication occurred during the follow-up. Four patients lost the follow-up. The clinical remission rate of patients with concomitant immunosuppressants was 23% (3/13 patients) at wk 6 and 54.5% (6/11 patients) at the end of the follow-up. Figure 3 displays the course of clinical (CAI) and endoscopic (EI) activity. Both indices dropped significantly between the beginning and end (at wk 6) of the treatment period.
Endoscopic remission (EI<4) was observed at wk 6 in 10/35 (28.6%) patients. Mucosal healing (vulnerability of mucosa and mucosal damage scores) was improved in 19 patients, unchanged in 13 patients and deteriorated in 3 patients (P<0.001).
Between wk 0 and 6, changes in histology improved in 14 (44%) patients, no change in 16 (50%) and deteriorated in 2 (6%) patients (P<0.002).
Quality of life of the 30 non-Italian patients (IBDQ) improved rapidly by 17 points within 3 wk of treatment (P<0.01) and reached 162±6.4 points at wk 6 which was significantly (P<0.01) better than that at the beginning of the study (138.1±4.8 points).
Physician’s global assessment at wk 6 was ‘very much improved’ (11.4%), ‘much improved’ (37.1%), ‘minimally improved’ (25.7%), ‘no change’ (20.0%), ‘minimally worse’ (5.7%) and ‘much worse’ (0%).
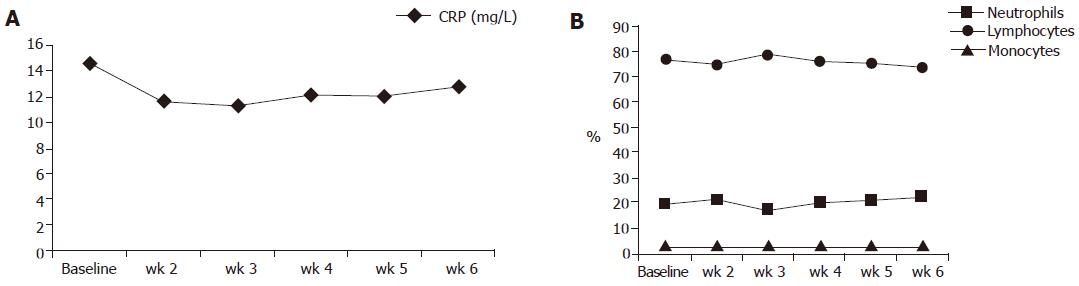
Figure 4 displays the results of blood cell counts and CRP serum concentrations between the beginning and wk 6 of the study. With the exception of the lymphocyte count all other numbers dropped, but none of those changes reached statistical significance.
Consumption of systemic steroids decreased significantly (P<0.05) throughout the whole treatment period. The median total dose of systemic steroids was decreased from 20.0 mg/d at baseline to 15.0 mg/d at wk 6. The means were 26.1+18.04 mg/d and 16.5+15.56 mg/d, respectively. At the end of 4-mo follow-up period, the median daily dose of steroids was 6.5 mg (mean daily dose: 14.6+16.8 mg).
One patient of the ITT patient group experienced several changes of his dose of steroids (increase or decrease >10 mg) throughout the study. He was, therefore, excluded from the per-protocol analysis. The patient did not achieve clinical remission but had clinical improvement.
No unexpected adverse events occurred. The 35 patients included experienced a total of 46 adverse events (AEs) in 65 episodes recorded. Three events were serious: thrombosis of the lower leg, worsening of UC, and a significant drop of hemoglobin which could not be explained despite extensive investigations (the patient recovered quickly). The causality was judged as unlikely, none and possible, respectively. All other AEs were judged as non-serious and mild or moderate. Ten had no causal relationship to the study treatment, 38 had a possible, and 3 had a probable relationship. The three AEs probably related to the therapy were finger edema, right arm phlebitis, and paresthesia of the left hand. All of them were classified as mild. The 38 events judged as possibly related were headache, abdominal disorder, dysesthesia, dizziness, nausea, flush, muscle cramps, fatigue, vegetative dystonia, mood alteration, prostate inflammation, and urinary tract infection. They were judged as mild (28) or moderate (10). Those events classified as unlikely related to the study treatment were oral aphthae, vertigo, headache, common cold, cramps, thrombosis, arthralgia, meralgia, ankle swelling, and bronchopneumonia. Laboratory testing as mentioned in Methods did not reveal any significant alterations.
Granulocyte apheresis application with five scheduled sessions strongly suggests efficacy in chronically active steroid refractory UC in the study reported here. A short treatment period of 5 wk resulted not only in significant clinical but also in significant endoscopic and histological improvements. Moreover, when aphereses were stopped, further beneficial effects could be observed. A follow-up period of 4 mo demonstrated carryover effects of the initial treatment leading to clinical remission in three out of four patients. Apheresis treatment was safe and well tolerated.
These very good results must be critically seen in the light of the open uncontrolled study design. Extracorporal treatment, apparently different from medicinal or surgical therapy, is a new method also for ‘experienced’ patients, a fact which may hold some placebo effects. Other studies demonstrated that the placebo effect is up to 30% in similar patient groups (e.g. 14) but to our knowledge, none of those trials allowed ongoing intensive treatment (dose of steroids, immunosuppressants) at entry to the study as in the present study. All patients kept on having their initial unsuccessful treatment with steroids and in part with immunosuppressants. Recent results of other studies support our findings. Yamamoto et al[15] described 70% clinical remission in 30 patients with active distal UC and Naganuma et al[16] found a remission rate of 55% in either steroid refractory or dependent patients. Both trials used a similar treatment protocol.
The apheresis system is used to remove excess and activated leukocytes from peripheral blood. The release of proinflammatory cytokines in the peripheral blood such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and IL-8 is suppressed as well as the leukocyte adhesion molecules (L-selectin) and neutrophil adhesion to IL-1β-activated endothelial cells are downregulated[17-20]. In addition, it exerts antioxidant effects[21] and the production of anti-inflammatory substances such as IL-1 receptor antagonist (IL-1ra), IL-10, and soluble TNF-α receptors I and II (sTNF-α RI and sTNF-α RII) is increased[22,23]. UC patients with high mucosal content of IL-8mRNA responding well to granulocyte apheresis and mucosal IL-8mRNA can be significantly reduced[24]. Granulocyte apheresis seems also to modulate apoptosis[17]. These far reaching interventions on inflammatory immune reactions may explain the carryover effects of granulocyte apheresis, when its application is stopped and standard treatment is continued.
Adsorption techniques may lead to a decrease of circulating blood cells. Indeed peripheral granulocytes decrease significantly by 22% after a 60-min apheresis application in UC patients, but no granulocytopenia occurs[18]. Measurements of pre- and post-column blood cell counts have demonstrated adsorption of 65% of granulocytes, 55% of monocytes, and 2% of lymphocytes, but the number of peripheral leukocytes does not fall below the normal level[19]. Flow cytometry has shown an increase in the number of immature circulating granulocytes (CD 10- neutrophils) which should be less proinflammatory[17]. The modest effect of apheresis on blood cell counts may prevent dangerous immunosuppressive adverse events seen in systemically acting immunosuppressants.
The protocol of our study prescribed 5-wk apheresis sessions with a duration of 60 min and a flow rate of 30 mL/min, a procedure which is based on empiricism. Intensification of this low exchange technique might create more rapid and/or more favorable therapeutic results. In a large trial in patients with rheumatoid arthritis the protocol here was compared to apheresis twice per week and no therapeutic advantage of intense treatment could be observed[20]. In a small study in patients with UC, intensified apheresis revealed more rapid but not superior therapeutic effects[25]. As yet the most effective procedure remains unclear. Another way to intensify the effects of apheresis is to increase the exchange of blood per time by augmenting the flow rate during the procedure. This may create technical difficulties. The easy technical success of the protocol as it occurred in the present study is based on the low exchange protocol. Simple handling is a prerequisite of the method for its introduction to non specialized (apheresis) units.
In conclusion, granulocyte apheresis seems to be a very promising treatment, particularly in severely ill patients with UC. For patients refractory to standard therapies, it could offer an alternative which is safe and well tolerated. Controlled studies are urgently warranted.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Allison MC, Dhillon AP, Lewis WG, Pounder RE (eds). Inflammatory Bowel Disease. London: Mosby 1998; . [Cited in This Article: ] |
| 2. | Järnerot G, Rolny P, Sandberg-Gertzén H. Intensive intravenous treatment of ulcerative colitis. Gastroenterology. 1985;89:1005-1013. [PubMed] [Cited in This Article: ] |
| 3. | Shanahan F. Crohn's disease. Lancet. 2002;359:62-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 349] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 4. | van Dullemen HM, van Deventer SJ, Hommes DW, Bijl HA, Jansen J, Tytgat GN, Woody J. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology. 1995;109:129-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 770] [Cited by in F6Publishing: 718] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 5. | Lloyd AR, Oppenheim JJ. Poly's lament: the neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol Today. 1992;13:169-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 325] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 6. | Yamada T, Volkmer C, Grisham MB. Antioxidant properties of 5-aminosalicylic acid: Potential mechanism for its anti-inflammatory activity. CN Williams (ed) Boston: Kluwer Academic Publishers 1990; 73-84 PMCid: PMC1257064. [Cited in This Article: ] |
| 7. | Yamada T, Grisham MB. Role of neutrophil-derived oxidants in the pathogenesis of intestinal inflammation. Klin Wochenschr. 1991;69:988-994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 81] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Grisham MB, Yamada T. Neutrophils, nitrogen oxides, and inflammatory bowel disease. Ann N Y Acad Sci. 1992;664:103-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 77] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Kurtel H, Granger DN, Tso P, Grisham MB. Vulnerability of intestinal interstitial fluid to oxidant stress. Am J Physiol. 1992;263:G573-G578. [PubMed] [Cited in This Article: ] |
| 10. | Grisham MB, Granger N. Mechanisms of neutrophil-mediated tissue injury. RP MacDermott, WF Stenson (eds. ) New York: Elsevier 1992; 225-239. [Cited in This Article: ] |
| 11. | Saniabadi AR, Hanai H, Takeuchi K, Umemura K, Nakashima M, Adachi T, Shima C, Bjarnason I, Loefberg R. Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher Dial. 2003;7:48-59. [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 202] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 771] [Cited by in F6Publishing: 782] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 13. | Irvine EJ, Feagan B, Rochon J, Archambault A, Fedorak RN, Groll A, Kinnear D, Saibil F, McDonald JW. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn's Relapse Prevention Trial Study Group. Gastroenterology. 1994;106:287-296. [PubMed] [Cited in This Article: ] |
| 14. | Probert CS, Hearing SD, Schreiber S, Kühbacher T, Ghosh S, Arnott ID, Forbes A. Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomised controlled trial. Gut. 2003;52:998-1002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 270] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 15. | Yamamoto T, Umegae S, Kitagawa T, Yasuda Y, Yamada Y, Takahashi D, Mukumoto M, Nishimura N, Yasue K, Matsumoto K. Granulocyte and monocyte adsorptive apheresis in the treatment of active distal ulcerative colitis: a prospective, pilot study. Aliment Pharmacol Ther. 2004;20:783-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Naganuma M, Funakoshi S, Sakuraba A, Takagi H, Inoue N, Ogata H, Iwao Y, Ishi H, Hibi T. Granulocytapheresis is useful as an alternative therapy in patients with steroid-refractory or -dependent ulcerative colitis. Inflamm Bowel Dis. 2004;10:251-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Kashiwagi N, Sugimura K, Koiwai H, Yamamoto H, Yoshikawa T, Saniabadi AR, Adachi M, Shimoyama T. Immunomodulatory effects of granulocyte and monocyte adsorption apheresis as a treatment for patients with ulcerative colitis. Dig Dis Sci. 2002;47:1334-1341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Shimoyama T, Sawada K, Hiwatashi N, Sawada T, Matsueda K, Munakata A, Asakura H, Tanaka T, Kasukawa R, Kimura K. Safety and efficacy of granulocyte and monocyte adsorption apheresis in patients with active ulcerative colitis: a multicenter study. J Clin Apher. 2001;16:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 207] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Ohara M, Saniabadi AR, Kokuma S, Hirata I, Adachi M, Agishi T, Kasukawa R. Granulocytapheresis in the treatment of patients with rheumatoid arthritis. Artif Organs. 1997;21:989-994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Kashiwagi N, Hirata I, Kasukawa R. A role for granulocyte and monocyte apheresis in the treatment of rheumatoid arthritis. Ther Apher. 1998;2:134-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Hirayama A, Nagase S, Ueda A, Ishizu T, Taru Y, Yoh K, Hirayama K, Kobayashi M, Koyama A. Oxidative stress during leukocyte absorption apheresis. J Clin Apher. 2003;18:61-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Hanai H, Takeuchi K, Iida T, Tanaka T, Tozawa K, Watanabe F, Maruyama Y, Yamada M, Iwaoka Y, Satou Y. Release of IL-10, IL-1 receptor antagonist, soluble TNF-α receptors I and II during adsorptive granulocyte and monocyte/macrophage reduction therapy of patients with active ulcerative colitis. Gastroenterology. 2004;126:A 567. [Cited in This Article: ] |
| 23. | Takeda Y, Hiraishi K, Takeda H, Shiobara N, Shibusawa H, Saniabadi AR, Adachi M, Kawata S. Cellulose acetate beads induce release of interleukin-1 receptor antagonist, but not tumour necrosis factor-alpha or interleukin-1beta in human peripheral blood. Inflamm Res. 2003;52:287-290. [PubMed] [Cited in This Article: ] |
| 24. | Tsukada Y, Nakamura T, Iimura M, Iizuka BE, Hayashi N. Cytokine profile in colonic mucosa of ulcerative colitis correlates with disease activity and response to granulocytapheresis. Am J Gastroenterol. 2002;97:2820-2828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Sakuraba A, Naganuma M, Hibi T. Intensive therapy of granulocyte and adsorption apheresis induces rapid remission in patients with ulcerative colitis. Gastroenterology. 2003;124:A 522. [Cited in This Article: ] |