Published online Nov 28, 2005. doi: 10.3748/wjg.v11.i44.6988
Revised: June 26, 2005
Accepted: July 1, 2005
Published online: November 28, 2005
AIM: To investigate ASCA production over time in CD and murine colitis in order to further our understanding of their etiology.
MATERIALS AND METHODS: Sixty-six CD patients were compared to ulcerative colitis (UC) and irritable bowel syndrome patients with respect to ASCA production as measured by ELISA. ASCA IgG or IgA positivity as well as change in titers over a period of up to 3 years (ΔIgG/A) was correlated with clinical parameters such as CD activity index (CDAI) and C-reactive protein levels (CRP). Moreover, two murine models of colitis (DSS and IL-10 knock out) were compared to control animals with respect to ASCA titers after oral yeast exposure.
RESULTS: ASCA IgG and IgA titers are stable over time in CD and non-CD patients. Fistular disease was associated with a higher rate of ASCA IgA positivity (P = 0.014). Ileal disease was found to have a significant influence on the ΔIgG of ASCA (P = 0.032). There was no correlation found between ASCA positivity or ΔIgG/A and clinical parameters of CD: CDAI and CRP. In mice, neither healthy animals nor animals with DSS-induced or spontaneous colitis exhibited a marked increase in ASCA titers after high-dose yeast exposure. On the other hand, mice immunized intraperitoneally with mannan plus adjuvant showed a marked and significant increase in ASCA titers compared to adjuvant-only immunized controls (P = 0.014).
CONCLUSION: The propensity to produce ASCA in a subgroup of CD patients is largely genetically predetermined as evidenced by their stability and lack of correlation with clinical disease activity parameters. Furthermore, in animal models of colitis, mere oral exposure of mice to yeast does not lead to the induction of marked ASCA titers irrespective of concomitant colonic inflammation. Hence, environment may play only a minor role in inducing ASCA.
-
Citation: Müller S, Styner M, Seibold-Schmid B, Flogerzi B, Mähler M, Konrad A, Seibold F. Anti-
Saccharomyces cerevisiae antibody titers are stable over time in Crohn’s patients and are not inducible in murine models of colitis. World J Gastroenterol 2005; 11(44): 6988-6994 - URL: https://www.wjgnet.com/1007-9327/full/v11/i44/6988.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i44.6988
Much attention has been focused on serologic markers in inflammatory bowel disease (IBD). The anti-Saccharomyces cerevisiae antibody (ASCA) is one such marker, which possesses an intermediate sensitivity and a high specificity for Crohn’s disease (CD)[1-5]. Antibodies to S cerevisiae in CD were first described by Main et al[6], using whole killed yeast cells as antigens. Sendid et al[3] demonstrated greater diagnostic value for CD with S cerevisiae Su1, a strain of brewer’s yeast, and identified the antigenic oligomannosidic epitopes of this organism. Subsequent work demonstrated that CD patients develop antibodies to a variety of baker’s and brewer’s yeast strains[4]. The antigen reacting with ASCA is a phosphopeptidomannan, a component of the S. cerevisiae cell wall[3].
Aside from the diagnostic role performed by ASCA, uncertainty remains as to whether they possess a pathophysiologic significance. One can argue for a genetic origin due to ASCA presence in 20-25% of unaffected first-degree family members[7-10]. Healthy monozygotic twins of CD patients also demonstrate increased IgA, IgG, and IgM ASCA levels[11]. In one study of non-IBD families, ASCA were found to be familial with a vertical transmission pattern[12]. ASCA stability over time and independence from disease activity further indicate a genetic link[11,13]. Moreover, we have shown that T cells from ASCA-positive patients proliferated upon stimulation with mannan[14]. We were able to show that mannan binding lectin (MBL) deficient patients were significantly more frequently ASCA-positive and showed an enhanced T cell proliferation upon mannan stimulation compared to MBL wildtype patients[15]. These results further support the importance of genetic determination of ASCA.
Nevertheless, some of the familial studies have yielded conflicting data. For example, one group showed increased ASCA production in familial vs sporadic CD[7], but others have shown equal or increased ASCA prevalence for sporadic CD[9,16-18]. Thus, the case for an environmental etiology for ASCA has been articulated as well. For instance, a decline in ASCA levels has been observed post-surgically in a pediatric CD population[5]. Another group demonstrated lower ASCA titers in CD patients taking mesalazine than in CD patients not taking mesalazine[19]. Additionally, both brewing and baking strains of S cerevisiae provoke an antibody response in CD, implicating dietary antigens in disease pathogenesis[4]. One group has noted higher ASCA IgG antibody levels in patients with small bowel Crohn’s disease vs those with colonic disease[18]. This same study found high levels of ASCA IgG but not IgA in celiac disease, indistinguishable from levels seen in CD. It was, thus, concluded that ASCA may result from a mucosal permeability defect. However, Vermeire et al[20] were not able to show a correlation between ASCA and intestinal permeability.
In this study, our aim was to characterize ASCA over time in patients with IBD in order to further our understanding of their etiology. Furthermore, we assessed the possibility to induce an ASCA response in animal models.
Sixty-six Crohn’s disease (CD) patients, 29 ulcerative colitis (UC) patients, and 10 irritable bowel syndrome (IBS) patients with informed consent were enrolled in the study, and the study was approved by the ethical committee of the local authorities. Patient serum samples were drawn during routine outpatient visits (Gastroenterology clinic, University Hospital, Bern, Switzerland) for ASCA IgG and IgA analysis. Diagnosis of CD and UC was established by endoscopic, histological, and clinical criteria. Diagnosis of IBS was established by the Rome criteria[21].
A subgroup of CD (73 sera from 29 patients), UC (26 sera from 12 patients) and IBS (22 sera from 10 patients) patients, whose serum samples were drawn on at least 2 consecutive (maximally 4 consecutive) routine out-patient visits, were retrospectively selected for ASCA IgG and IgA titer analysis. An assessment of the Crohn’s disease activity index (CDAI) was obtained at each CD patient's visit to the clinic[22]. The serum CRP levels were obtained at each clinic visit as well. Three patients with stoma were excluded from the CDAI analysis. One patient was excluded from the clinical analysis, since this patient’s CDAI and CRP could not be obtained.
Four to six-weeks-old female interleukin-10 knockout mice (Il10tm1Cgn; henceforth abbreviated as IL-100/0) on a C3H/HeJBir background were obtained from the University of Bern breeding facility (Bern, Switzerland) and housed under specified pathogen-free conditions. Four to six-week-old BALB/c mice were obtained from Harlan (Horst, The Netherlands). All procedures involving animals were performed in compliance with the local authorities on the use of animals in research.
Investigators were blinded to disease status when performing the ELISA. Phosphopeptidomannans from baker’s yeast (Hefe Vital Gold, Deutsche Hefewerke, Nürnberg, Germany) were extracted as previously described[8,23]. Ninety-six well ELISA plates (MaxiSorb, Nunc, Wiesbaden, Germany) were coated with 100 µL of 0.25 µg/mL phosphopeptidomannans in carbonate-bicarbonate buffer, pH 9.6 and incubated overnight at 4 °C. Serum diluted 1/1 000 was applied to the coated plates in triplicates. The plates were incubated for 1 h at 37 °C. Secondary antibody was added and plates were incubated for 1 h at RT. The secondary antibodies (Sigma, Buchs Switzerland) were as follows: goat anti-mouse polyvalent immunoglobulin peroxidase conjugate at 1/1 000; goat anti-human IgA (alpha-chain specific) peroxidase conjugate; and goat anti-human IgG (Fc specific) peroxidase conjugate, both at 1/5 000. The plates were developed using TMB substrate (Sigma) and the reaction was stopped with sulfuric acid. The absorbance at 450 nm (reference filter = 490 nm) was read by an EL 800 microplate reader (Bio-Tek Instruments, Winooski, Vermont). The mean absorbance value for the healthy control sera plus two standard deviations was used to discriminate between positive and negative subjects. Absorbance units equal to or above this value were considered positive for ASCA antibodies. A patient was considered ASCA+, when positive for ASCA IgG, IgA or both.
For the human ELISA, one serum highly positive for ASCA was used to obtain a standard curve at dilutions ranging from 1/100 to 1/102 400. The curve was fitted using a four parameter logistic function to the logarithmically scaled dilutions. Human titers are expressed as dilution units from this curve. We computed the average change of IgG and IgA (∆IgG/A) in human ASCA titer as our main variable of interest. An increase of ∆IgG/A represents a decrease in ASCA levels and vice versa. Of the CD patients, two patient’s IgG titer and one patient’s IgA titer could not be reliably calculated from the standard curve after two repeated assays and thus were excluded from the IgG/A titer analysis. The murine ASCA titers are expressed as optical density (OD).
Baker’s yeast (Hefe Vital Gold, Deutsche Hefewerke, Nürnberg, Germany) was freshly grown in YPD broth (Becton Dickinson, Le Pont de Claix, France) for 12 h at 30 ºC in a shaker at 200 r/min. The yeast solution was centrifuged at 2 000 r/min for 5 min. The cellular pellet was washed twice, resuspended in sterilized water and sonicated at 100 W for 2 min. Protein concentration was determined using the Bradford reagent (Sigma, Buchs, Switzerland).
The previously described model of chronic DSS colitis was adapted from Mähler et al[24]. Briefly, Balb/c mice were given three 5-day cycles of 3% DSS (DSS salt, MW= 36 000-50 000, ICN Biomedicals, Inc., Ohio), each interrupted by the 7th d without DSS. All mice received yeast ad libitum at a protein concentration of 100 or 500 µg/mL for the entire experiment. Murine serum samples were drawn on d 0 and the day of killing (d 36) for ASCA immunoglobulin analysis.
Mice were euthanized via CO2 asphyxiation. Colons were harvested, fixed in 4% phosphate-buffered formaldehyde, embedded in toto and H&E-stained sections were examined by a pathologist (Mähler) blinded to the code. The sections were scored on a previously described[24] scale ranging from 0 (no inflammation) to 4 (severe inflammation). A total colonic inflammation score was obtained by averaging the individual scores of the proximal, middle, and distal colon.
Balb/c mice from our central animal facility were intraperitoneally injected with 50 μg phosphopeptidomannans or ovalbumin (Sigma, Gaithersburg, MD) mixed 1:1 (v/v) with GERBU adjuvant (GERBU, Gaiberg, Germany). Injection was repeated after two weeks and mice were killed for analysis one week later. Serum samples were obtained before primary immunization by tail tip bleeding and after euthanization by puncture of the caval vein.
We analyzed the CD population in regard to ASCA IgG and IgA positivity to test for a significant influence of the following patient variables: age, gender, anatomical location of disease, medication, fistulae, and extra-intestinal manifestations. Significance of each variable was computed via unpaired, two-tailed Student’s t-test assuming equal variances if the additional F-test was non-significant. We analyzed the titer subgroup in respect to ASCA IgG and ASCA IgA positivity to test for a significant influence of the above-mentioned variables and additionally CD activity index (CDAI) and C-reactive protein (CRP) levels.
In order to qualitatively assess the amplitude and distribution of IgG and IgA, the respective ∆IgG/A histograms for the CD, UC, and IBS titer subgroups were computed. The quantitative assessment is shown via the mean, SD and 95% confidence interval of each ∆IgG/A distribution and via unpaired, two-tailed Student’s t-tests. We also employed Student's t-tests for testing ∆IgG/A differences with regard to gender, anatomical location of disease, fistulae, extra-intestinal manifestations, and medication. To assess the differences between the three patient groups with regard to IgG and IgA ∆IgG/A, we applied the one-way ANOVA test. The correlation coefficients between ∆IgG/A and the two clinical parameters, change in CDAI (∆CDAI) and change in CRP (∆CRP), were also computed.
The differences between the mice groups on day 36 were statistically assessed using the unpaired, two-tailed Student’s t-test. Significance level for all tests was 0.05.
Thirty-five of sixty-six CD patients, 4 of 29 UC patients and 3 of 10 IBS patients were ASCA positive. The sensitivity, specificity, and positive predictive value of the ASCA assay in distinguishing CD from UC are 53%, 86%, and 90%, respectively.
The baseline clinical characteristics of the patients and controls can be seen in Table 1. None of the following clinical characteristics had a significant effect on ASCA IgG and IgA positivity in the Crohn’s disease patients: age, gender, anatomical locations of disease, medication, and extra-intestinal manifestations. Patients with fistular disease were significantly more likely to possess ASCA IgA positivity (P = 0.014, 71% vs 32%) than patients with non-fistular disease.
| Study population | Crohn’s disease (n = 66) | Ulcerative colitis (n = 29) | Healthy controls (n = 18) | IBS patients (n = 10) |
| Age, yr | ||||
| Mean±SD | 38±16 | 37±15 | 40±13 | 57±13 |
| Range | 14-82 | 16-78 | 17-74 | 41-81 |
| Male sex | 32 (48) | 15 (52) | 10 (56) | 6 (60) |
| Disease location | ||||
| Colonic | 58 (88) | 29 (100) | ||
| Ileal | 45 (68) | |||
| Upper gastrointestinal involvement | 3 (5) | |||
| Presence of fistulae | 15 (23) | 1 (3) | ||
| Extraintestinal manifestations | 4 (6) | 0 (0) | ||
| Medical therapy | ||||
| Azathioprine | 22 (33) | 9 (31) | ||
| Infliximab | 5 (8) | 3 (10) | ||
| 5-ASA compounds | 4 (6) | 11 (38) | ||
| Methotrexate | 4 (6) | 0 (0) | ||
| Oral steroids | 29 (44) | 15 (52) | ||
| None of the above | 19 (29) | 7 (24) |
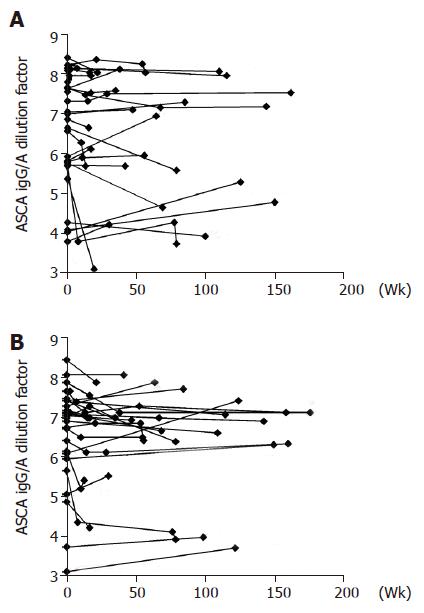
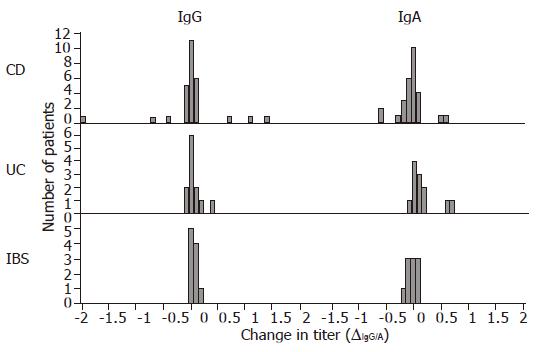
ASCA IgG and IgA titers in the CD subgroup, expressed as logarithmically (ln) scaled dilutions of a high positive control, have been plotted over a time-period of up to 3 years (Figure 1). The change in titers did not vary significantly as can be seen in the Gaussian distribution in the histogram, which is centered close to zero for all patient groups with respect to both IgG and IgA titers (Figure 2). The means, standard deviations and 95% confidence intervals of ∆IgG/A are shown in Table 2 and indicate stable titers over time. Nevertheless, the patient groups are significantly different in regard to ∆IgA values (P<0.05, ANOVA), specifically between CD and UC patients (P<0.05, ANOVA post hoc). This suggests that patients with UC were more likely to experience a decrease in IgA ASCA levels over time than patients with CD. The patient groups are not significantly different with regard to ∆IgG values.
| ∆IgG | ∆IgA | |||||
| Mean | SD | 95%CI | Mean | SD | 95%CI | |
| CD | 0.01 | 0.56 | (-0.20, 0.22) | -0.04 | 0.25 | (-0.13, 0.05) |
| UC | 0.05 | 0.15 | (-0.03, 0.14) | 0.16 | 0.24 | (0.02, 0.29) |
| IBS | 0.05 | 0.08 | (-0.003, 0.09) | -0.01 | 0.12 | (-0.08, 0.06) |
Of the clinical parameters assessed upon entry, ileal disease was found to have a significant influence on the ∆IgG of ASCA (Table 3). The mean ∆IgG value for patients with ileal disease vs those without ileal disease was 0.13 and -0.34, respectively. This suggests that patients without ileal disease were more likely to experience an increase in ASCA level as opposed to patients with ileal disease. All other tested clinical parameters did not significantly influence the ∆IgG/A of ASCA. Regarding the clinical parameters of disease, no strong corre=lation was found between the ∆CDAI and ∆IgG (c = -0.086) and ∆IgA (c = -0.127). Moreover, no strong correlation was found between the ∆CRP and ∆IgG (c = 0.424) and ∆IgA (c = -0.245).
| Clinical characteristics | ASCA IgG | ASCA IgA | ∆IgG | ∆IgA |
| Correlation with age | c = –0.140 | c = 0.198 | c = –0.340 | c = –0.197 |
| Male vs female | 0.881 | 0.881 | 0.089 | 0.571 |
| Disease Location | ||||
| Colonic vs non-colonic | 0.667 | 0.169 | 0.203 | 0.289 |
| Ileal vs non-ileal | 0.285 | 0.285 | 0.032a | 0.395 |
| Fistular vs Non-fistular disease | 0.152 | 0.075 | 0.499 | 0.907 |
| Medical therapy | ||||
| Azathioprine | 0.881 | 0.881 | 0.749 | 0.375 |
| Infliximab | 0.233 | 0.233 | 0.87 | 0.844 |
| 5-ASA compounds | 0.806 | 0.233 | 0.903 | 0.491 |
| Oral steroids | 0.512 | 0.385 | 0.475 | 0.531 |
| None of the above medical | ||||
| therapy vs medical therapy | 0.285 | 0.577 | 0.438 | 0.117 |
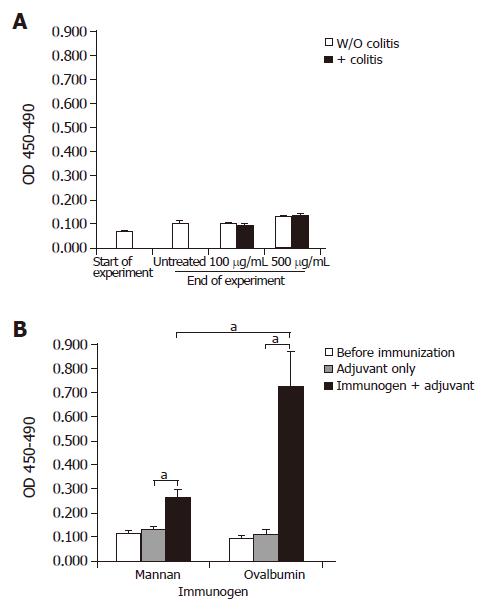
All mice given yeast lysate and unfed controls exhibited slightly increased ASCA titers at the end of the experimental period compared with values obtained at the beginning of the experiment. A slight increase in ASCA titers was observed in mice exposed to 500 μg/mL yeast lysate in their drinking water when compared with mice exposed to 100 μg/mL (Figure 3A). Concomitant DSS-treatment (Figure 3A) or onset of colitis in IL-100/0 mice (data not shown) did not lead to a further increase in ASCA titers. Development of colitis was evidenced by the observation of clinical signs such as soft stools and in some cases anal ulcerations and bloody stools. The average histological score±SD was as follows: 1.056±0.25 for the DSS-treated Balb/c group, 0±0 for the non-DSS-treated Balb/c group, and 1.5±0.19 for the IL-100/0 group.
Groups of Balb/c mice were immunized with adjuvant alone, or with adjuvant plus yeast mannan or, as a positive control, ovalbumin (Figure 3B). Mannan-immunized mice showed an average two-fold increase in ASCA titers when compared with mice receiving adjuvant only (P = 0.014). Mannan was poorly immunogenic compared to ovalbumin (P = 0.038) since specific serum antibody titers after ovalbumin plus adjuvant-immunization increased about seven-fold over adjuvant-only injected controls (P = 0.015).
Antibodies to the S cerevisiae cell wall mannans have been widely studied as a diagnostic tool to distinguish CD from UC. In this study, the rates of CD patient ASCA positivity correlate well with our previous results[8] as well as those cited in the literature[7,10,13]. Of all the clinical variables tested, fistular Crohn’s disease was found to be associated with ASCA IgA positivity. This study is not the first to demonstrate such an association. Others have shown that the subgroup of CD patients with high levels of IgG and IgA ASCA has more aggressive, small-bowel fistulizing disease[25].
Our study was the first to assess time-period streamlined ASCA titers. We found no association between the change in ASCA titers and the following clinical characteristics: age, gender, presence of fistulae, medical therapy, CDAI or CRP levels. Of the clinical parameters tested, patients without ileal disease were more likely to encounter an increase in their ASCA titers over time as compared with CD patients with ileal disease. There is no good explanation why anatomic location of CD should provoke an increase or decrease in ASCA titers. It should be noted that Giaffer et al[18] noted increased ASCA IgG positivity association with small bowel disease as compared to colonic disease. Nevertheless, this study does not explain why these patient’s titers were more likely to change over time. Also of special interest is the lack of association between ΔCDAI and ∆IgG/A and ΔCRP and ∆IgG/A. ASCA stability over time in relation to the changes in CDAI was demonstrated by Landers et al[13], utilizing a time period of at least 4 mo. Recently, Teml et al[26] established ASCA stability during a course of steroid or 5-ASA therapy. Our data further strengthen this stability and lack of association with CDAI or medical therapy with the addition of CRP as a serologic marker of disease. This lack of association with clinical activity leads others and us away from thinking of ASCA as a subclinical marker of disease activity as it does not follow CD clinical status variations.
In line with the results obtained with human sera, the murine ASCA titers were rather stable, at least during the time of the experimental procedure (36 d). This is the first study to investigate murine ASCA production in the setting of DSS and IL-100/0 colitis. After oral treatment with a high antigen dose (500 μg/mL), we observed a slight increase of ASCA titers compared to untreated controls or mice treated with 100 μg/mL. Interestingly, we observed slightly lower ASCA titers in young untreated mice compared to untreated mice at the end of the experiment. It is very likely that ASCA titers in mice may slightly vary between the breeder company and our animal facility as a consequence of differential exposure to yeast-antigens depending on the hygiene status of the animal facility and/or the composition of food pellets. We did not, however, observe a further increase upon induction of colitis. Therefore, the impairment of mucosal barrier during inflammation and/or the enhanced presence of immune competent cells alone does not lead to markedly enhanced ASCA generation.
In contrast, systemic immunization of mice with phosphopeptidomannans in the context of appropriate adjuvant leads to a significant increase in ASCA titers. However, the increase of ASCA titers is much less pronounced than that of anti-ovalbumin antibodies after ovalbumin immunization. Thus, these data show that mannans are not very efficient antigens when used for immunization, further indicating that normally mice and human beings are tolerant to mannans. Local inflammation during colitis is not sufficient to break this tolerance. In our recent study we were able to show that ASCA positivity was associated with MBL deficiency[15]. Furthermore, family studies show an increased incidence of ASCA in healthy family members[7,8,20]. Thus, generation of ASCA in a subgroup of CD patients and healthy family members may be mainly a result of a genetic predisposition, although biochemical and genetic analyses supporting this notion are not currently available.
In conclusion, we have demonstrated the stability of the ASCA titers over time and their lack of association with clinical activity in the form of CDAI and CRP. These findings are in line with the observation that ASCA are only marginally inducible in mice via high dose oral exposure, and are not further influenced by concomitant induction of colitis. Hence, we speculate that the propensity to produce ASCA in a subgroup of CD patients is largely genetically predetermined and, thus, may reflect a predisposition of distinct genetic backgrounds to establish immune responses against normally tolerated antigens.
We are grateful to Dr A Sergew and Dr M Weber for their editorial assistance. Dr MA Styner is acknowledged for insightful discussions regarding statistical analysis.
Co-first-authors: Stefan Müller and Maya Styner
Co-correspondence: Stefan Müller, stefan.mueller@dkf.unibe ch
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Quinton JF, Sendid B, Reumaux D, Duthilleul P, Cortot A, Grandbastien B, Charrier G, Targan SR, Colombel JF, Poulain D. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788-791 DOI : 10.1136/gut.42.6.788. [Cited in This Article: ] |
| 2. | Sandborn WJ, Loftus EV, Colombel JF, Fleming KA, Seibold F, Homburger HA, Sendid B, Chapman RW, Tremaine WJ, Kaul DK. Evaluation of serologic disease markers in a population-based cohort of patients with ulcerative colitis and Crohn's disease. Inflamm Bowel Dis. 2001;7:192-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Sendid B, Colombel JF, Jacquinot PM, Faille C, Fruit J, Cortot A, Lucidarme D, Camus D, Poulain D. Specific antibody response to oligomannosidic epitopes in Crohn's disease. Clin Diagn Lab Immunol. 1996;3:219-226. [PubMed] [Cited in This Article: ] |
| 4. | McKenzie H, Main J, Pennington CR, Parratt D. Antibody to selected strains of Saccharomyces cerevisiae (baker's and brewer's yeast) and Candida albicans in Crohn's disease. Gut. 1990;31:536-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 142] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Ruemmele FM, Targan SR, Levy G, Dubinsky M, Braun J, Seidman EG. Diagnostic accuracy of serological assays in pediatric inflammatory bowel disease. Gastroenterology. 1998;115:822-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 293] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Main J, McKenzie H, Yeaman GR, Kerr MA, Robson D, Pennington CR, Parratt D. Antibody to Saccharomyces cerevisiae (bakers' yeast) in Crohn's disease. BMJ. 1988;297:1105-1106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 242] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Annese V, Andreoli A, Andriulli A, Dinca R, Gionchetti P, Latiano A, Lombardi G, Piepoli A, Poulain D, Sendid B. Familial expression of anti-Saccharomyces cerevisiae Mannan antibodies in Crohn's disease and ulcerative colitis: a GISC study. Am J Gastroenterol. 2001;96:2407-2412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Seibold F, Stich O, Hufnagl R, Kamil S, Scheurlen M. Anti-Saccharomyces cerevisiae antibodies in inflammatory bowel disease: a family study. Scand J Gastroenterol. 2001;36:196-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Sendid B, Quinton JF, Charrier G, Goulet O, Cortot A, Grandbastien B, Poulain D, Colombel JF. Anti-Saccharomyces cerevisiae mannan antibodies in familial Crohn's disease. Am J Gastroenterol. 1998;93:1306-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Sutton CL, Yang H, Li Z, Rotter JI, Targan SR, Braun J. Familial expression of anti-Saccharomyces cerevisiae mannan antibodies in affected and unaffected relatives of patients with Crohn's disease. Gut. 2000;46:58-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 121] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Lindberg E, Magnusson KE, Tysk C, Järnerot G. Antibody (IgG, IgA, and IgM) to baker's yeast (Saccharomyces cerevisiae), yeast mannan, gliadin, ovalbumin and betalactoglobulin in monozygotic twins with inflammatory bowel disease. Gut. 1992;33:909-913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Poulain D, Sendid B, Fajardy I, Danze PM, Colombel JF. Mother to child transmission of anti-S cerevisiae mannan antibodies (ASCA) in non-IBD families. Gut. 2000;47:870-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Landers CJ, Cohavy O, Misra R, Yang H, Lin YC, Braun J, Targan SR. Selected loss of tolerance evidenced by Crohn's disease-associated immune responses to auto- and microbial antigens. Gastroenterology. 2002;123:689-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 330] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 14. | Konrad A, Rütten C, Flogerzi B, Styner M, Göke B, Seibold F. Immune sensitization to yeast antigens in ASCA-positive patients with Crohn's disease. Inflamm Bowel Dis. 2004;10:97-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Seibold F, Konrad A, Flogerzi B, Seibold-Schmid B, Arni S, Jüliger S, Kun JF. Genetic variants of the mannan-binding lectin are associated with immune reactivity to mannans in Crohn's disease. Gastroenterology. 2004;127:1076-1084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Halme L, Turunen U, Helio T, Paavola P, Walle T, Miettinen A, Jarvinen H, Kontula K, Farkkila M. Familial and sporadic inflammatory bowel disease: comparison of clinical features and serological markers in a genetically homogeneous population. Scand J Gastroenterol. 2002;37:692-698. [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Barnes RM, Allan S, Taylor-Robinson CH, Finn R, Johnson PM. Serum antibodies reactive with Saccharomyces cerevisiae in inflammatory bowel disease: is IgA antibody a marker for Crohn's disease? Int Arch Allergy Appl Immunol. 1990;92:9-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Giaffer MH, Clark A, Holdsworth CD. Antibodies to Saccharomyces cerevisiae in patients with Crohn's disease and their possible pathogenic importance. Gut. 1992;33:1071-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 80] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Oshitani N, Hato F, Matsumoto T, Jinno Y, Sawa Y, Hara J, Nakamura S, Seki S, Arakawa T, Kitano A. Decreased anti-Saccharomyces cerevisiae antibody titer by mesalazine in patients with Crohn's disease. J Gastroenterol Hepatol. 2000;15:1400-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Vermeire S, Peeters M, Vlietinck R, Joossens S, Den Hond E, Bulteel V, Bossuyt X, Geypens B, Rutgeerts P. Anti-Saccharomyces cerevisiae antibodies (ASCA), phenotypes of IBD, and intestinal permeability: a study in IBD families. Inflamm Bowel Dis. 2001;7:8-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439-444. [PubMed] [Cited in This Article: ] |
| 23. | Kocourek J, Ballou CE. Method for fingerprinting yeast cell wall mannans. J Bacteriol. 1969;100:1175-1181. [PubMed] [Cited in This Article: ] |
| 24. | Mähler M, Bristol IJ, Leiter EH, Workman AE, Birkenmeier EH, Elson CO, Sundberg JP. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. Am J Physiol. 1998;274:G544-G551. [PubMed] [Cited in This Article: ] |
| 25. | Vasiliauskas EA, Plevy SE, Landers CJ, Binder SW, Ferguson DM, Yang H, Rotter JI, Vidrich A, Targan SR. Perinuclear antineutrophil cytoplasmic antibodies in patients with Crohn's disease define a clinical subgroup. Gastroenterology. 1996;110:1810-1819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 211] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 26. | Teml A, Kratzer V, Schneider B, Lochs H, Norman GL, Gangl A, Vogelsang H, Reinisch W. Anti-Saccharomyces cerevisiae antibodies: a stable marker for Crohn's disease during steroid and 5-aminosalicylic acid treatment. Am J Gastroenterol. 2003;98:2226-2231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |