INTRODUCTION
Liver transplantation is the only therapeutic strategy for many inherited and acquired disorders of the liver. The vulnerability of the transplantation liver to warm ischemia, cold preservation, and reperfusion injury is possibly associated with immediate posttransplant graft function loss, and graft damage caused by ischemia/reperfusion (I/R) is a serious problem after transplantation. Hepatic I/R injury still leads to primary graft nonfunction in about 4% of liver transplantation. Moreover, late consequences of ischemia injury include intrahepatic and extrahepatic biliary strictures, which further increase morbidity and jeopardize graft survival. Therefore, inhibition of I/R injury has become a more serious clinical interest.
Two distinct phases in the development of organ injury have been identified in experimental hepatic I/R injury animal models. During the initial phase of injury, Kupffer cells are activated and release reactive oxygen species and proinflammatory cytokines, including tumor necrosis factor (TNF)-α[1-4]. The enhanced production of TNF-α plays a more important role in the initiation of a cascade of events that causes significant liver injury mediated by neutrophils. One of the main functions of TNF-α is the up-regulation of adhesion molecules and neutrophil-attracting C-X-C chemokines[5,6]. The coordinated efforts of adhesion molecules and C-X-C chemokines mediate the recruitment of neutrophils into the liver. Sequestered neutrophils release protease and reactive oxygen intermediates (ROI), which directly damage hepatocytes and endothelial cells and also to capillary plugging causing hepatic hypoperfusion. The transcription factor nuclear factor (NF)-κB plays a key role in the regulation of genes that function in immune and inflammatory systems such as TNF-α, IFN-γ, chemokines and ICAM-1[7-15]. Previous studies suggested that NF-κB activation plays a deteriorative role during hepatic I/R injury[15-18]. In contrast to these studies, Bradham et al[19] reported that NF-κB activation during orthotopic liver transplantation is protective.
In the present study, we have attempted to evaluate the exact effect of NF-κB activation on the I/R injury in liver graft after orthotopic liver transplantation in rats. For this aim, we used the recently developed technology of decoy ODNs. The original concept of using synthetic double-stranded ODN as “decoy” cis elements to block the binding of nuclear factors to promoter regions of target genes was introduced in 1990 by Sullenger et al and Bielinska et al In 1997, Morishita et al[20] reported the first in vivo application of this technology, they prevented myocardial infarction after reperfusion in rats by direct infusion of synthetic double-stranded 20-bp ODNs containing the NF-κB cis element into cannulated coronary arteries. Subsequently, Kawamura et al[21] showed that the direct injection of NF-κB decoy ODNs into implanted tumors in mice inhibited cachexia, without affecting the tumor growth. In 2000, Abeyama et al[22] reported that intraperitoneal and local administration of NF-κB decoy ODNs reduces the extent of UV-induced skin inflammation, and this is associated with NF-κB activation inhibition and decreased the transcription of inflammatory cytokines TNF-α and IL-1 and IL-6. Here we report that the application of NF-κB decoy ODNs significantly attenuates the liver graft I/R injury in rats.
MATERIALS AND METHODS
NF-κB decoy ODNs and scrambled ODNs
Double-stranded NF-κB decoy ODNs were generated using equimolar amounts of single-stranded sense and antisense phosphorothioate-modified oligonucleotides containing two NF-κB binding sites(sense sequence 5’-AGGGACTTTCCGCTGGGGACTTTCC-3’; NF-κB binding sites bold and underlined)[23]. Scrambled ODNs (treatment control ODNs: 5’-TTGCCGTACCTGACTTAGCC-3’)[22] were generated in the same way. Sense and antisense strands were mixed in the presence of 150 mmol PBS, heated to 100 °C, and allowed to cool to room temperature to obtain double-stranded DNA .
Experimental design and liver transplantation
Male SD rats (220-250 g) were used for the liver graft I/R injury experiments. The animals were maintained with a 12-h light/dark cycle in a conventional animal facility with water and commercial chow provided ad libitum, with no fasting before the transplantation. The following experimental groups were compared: (1) the I/R group, in which orthotopic liver transplantation were performed with two-cuff method and liver graft reperfusion was initiated after 3 h of cold-preservation in chilled (4 °C) University of Wisconsin (UW) solution containing NF-κB decoy ODNs or scrambled ODNs (5 μmol/L/mL); (2) the NF-κB decoy ODNs + I/R group, in which donor and recipient animals were injected intravenously through the penile vein with NF-κB decoy ODNs (1.0 mmol/L solution in PBS, 1 mL/rat ) 6 and 1 h before liver harvesting or orthotopic liver transplantation; (3) the scrambled ODNs + I/R group in which donor and recipient animals were injected intravenously through the penile vein with scrambled ODNs (1.0 mmol/L solution in PBS, 1 mL/rat ) 6 and 1 h before liver harvesting or orthotopic liver transplantation; (4) the sham control group in which rats only underwent a midline laparotomy. Orthotopic liver transplantation was performed according to the method described in our previous study[24]. All operations were performed under ether anesthesia in sterile conditions. The survival rate was >89% at 24 h after liver transplantation. Liver graft tissues and recipient blood samples (n = 5) were harvested at different time points (0, 0.5, 1, 2, 4, 8, and 16 h) after graft reperfusion and were immediately frozen in liquid nitrogen (only liver samples) and kept at -80 °C until use.
Myeloperoxidase assay
Liver myeloperoxidase (MPO) content was assessed by methods described elsewhere[15]. Briefly, liver tissue (50 mg) was homogenized in 2 mL of homogenization buffer (3.4 mmol/L KH2HPO4, 16 mmol/L Na2HPO4, pH 7.4). After centrifugation for 20 min at 10 000 g, 10 volumes of resuspension buffer (43.2 mmol/L KH2HPO4, 6.5 mmol/L Na2HPO4, 10 mmol/L ethylenediaminetetraacetic acid, 0.5% hexadecyltrimethylammonium, pH 6.0) was added to the pellet and the samples were sonicated for 10 s. After heating for 2 h at 60 °C, the supernatant was reacted with 3, 3’, 3, 5’-tetramethylbenzidine (Sigma Chemicall Co.) and read at 655 nm.
Preparation of liver graft nuclear extract
Nuclear proteins from frozen liver graft tissue were extracted according to the method of Nanji[8]. One gram of liver tissue was homogenized in 5 mL buffer [0.32 mol/L sucrose, 50 mmol/L Tris-HCl (pH 7.5), 25 mmol/L KCl, 5 mmol/L MgCl2, 0.5 mmol/L phenylmethylsulfonyl fluoride,10 μg/mL aprotinin, 10 μg/mL tosyllysylchloromethyl ketone(TLCK)] and centrifuged for 10 min at 600 r/min at 40 °C. The pellet was resuspended in 2.5 mL of 2 mol/L sucrose-Tris HCl, KCl, and MgCl2 (TKM) buffer and homogenized. The homogenate was centrifuged at 40 000 g at 4 °C for 2 h. The supernatant was carefully removed and the pellet containing the nuclear extract was resuspended in 40 μL of buffer A [10 mmol/L HEPES/KOH (pH 7.9), 2 mmol/L MgCl2, 0.1 mmol/L ethylenediaminetetraacetic acid, 10 mmol/L KCl, 1 mmol/L dithiothreitol, 0.5 mmol/L phenylmethylsulfonyl fluoride] and left on ice for 10 min, mixed, and centrifuged at 15 000 g at 4 °C for 15 s. The pellet was then resuspended in 1.0 mL of buffer B [10 mmol/L HEPES/KOH (pH 7.9), 50 mmol/L KCl, 300 mmol/L NaCl, 0.1 mmol/L ethylenediaminetetraacetic acid, 10% (v/v) glycerol, 1 mmol/L dithiothreitol, 0.5 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 10 μg/mL TPCK ] and put on ice for 20 min. After centrifugation at 15 000 g at 4 °C for 5 min, the supernatant was stored at -80 °C as a nuclear extract. Before the experiments, the total protein concentration in the samples were determined according to the method of Bradford.
Electrophoretic mobility shift assay (EMSA) for hepatic NF-κB activity
NF-κB DNA binding activity was performed in a 10 μL binding reaction mixture containing 1×binding buffer [50 mg/L of double-stranded poly (dI-dC), 10 mmol/L Tris-HCl (pH 7.5), 50 mmol/L NaCl2, 0.5 mmol/L EDTA, 0.5 mmol/L-1DTT, 1 mmol/L MgCl2, and 100 mL/L glycerol], 5 μg of nuclear protein, and 35 fmol of double-stranded NF-κB consensus oligonucleotide (5’-AGTGAGGGGACTTTCCCAGGC -3’) that was endly labeled with γ-32P (111 TBq/mol/L at 370 GBq-1) using T4 polynucleotide kinase. The binding reaction mixture was incubated at room temperature for 20 min and analyzed by electrophoresis on 7% nondenaturing polyacrylamide gels. After electrophoresis the gels were dried by Gel-Drier (Biol-Rad Laboratories, Hercules, CA, USA) and exposed to Kodak X-ray films at -70 °C. NF-κB DNA binding activity was presented as integral optical density (OD) value.
Semiquantitative RT-PCR assay for hepatic expression of TNF-α, IFN-γ and ICAM-1 mRNA
Analysis of the expression of TNF-α, IFN-γ and ICAM-1 mRNA was determined by semiquantitative RT-PCR amplification in contrast with house-keeping gene β-actin. Total RNA from 10 mg liver graft tissue was extracted using TripureTM reagent. First-strand cDNA was transcribed from 1 μg RNA using AMV and an oligo(dT15)primer. PCR was performed in a 25 μL reaction system. Specific primers used in PCR reaction were as follows: TNF-α, 5’ primer 5’-AGCCCACGTAGCAAACCACCA-3’ and 3’ primer 5’-ACACCCATTCCCTTCACAGAGCAAT-3’, to give a 446-bp product; ICAM-1, 5’ primer 5’-TGGAACTGCACGTGCTGTAT-3’, 3’ primer 5’-ACCATTCTGTTCAAAAGCAG-3’, to give a 513-bp product; IFN-γ, 5’ primer 5’-ACAATGAACGCTACACACTG-3’, 3’ primer 5’-TCAAACTTGGCAATACTCAT-3’, to give a 362-bp product; β-actin, 5’ primer 5’-ATGGATGATGATATCGCCGCG-3’, 3’ primer 5’ -TGAAGGTAGTTTCGTGGATGC-3’, to give a 813-bp product. PCR products of each sample were subjected to electrophoresis in a 15 g/L agarose gel containing 0.5 mg/L ethidium bromide. Densitometrical analysis using NIH image software was performed for semiquantification of PCR products, and mRNA expression was evaluated by the band-intensity ratio of TNF-α, IFN-γ and ICAM-1 to β-actin, and presented as percent of β-actin (%).
Blood assay for serum levels of TNF-α, IFN-γ and ALT
Blood was obtained by cardiac puncture at the time of killing. Serum samples were analyzed for TNF-α and IFN-γ by enzyme-linked immunosorbent assays (ELISA) according to the manufacturer’s instructions. Serum samples were also analyzed for ALT level as indices of hepatocellular injury. Measurements of serum ALT level were made using a diagnostic kit from Sigma Chemical Co.
Statistical analysis
All data were expressed as mean±SE . Statistical analysis of data was performed using the Student’s t-test; P<0.05 was considered statistically significant.
RESULTS
NF-κB activation in the liver graft
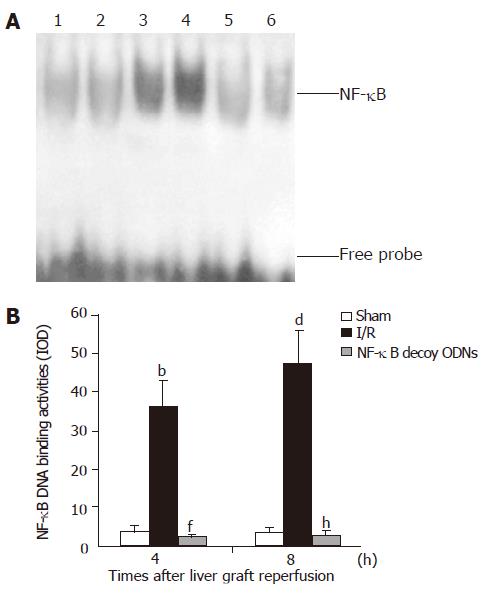
NF-κB activation in the liver graft at seven separate time points after reperfusion was determined by EMSA (as shown in Figure 1). NF-κB activation in the liver graft after reperfusion was induced in a time-dependent manner. Hepatic NF-κB activation was observed to start within 30 min after the initiation of reperfusion and continued for 16 h after liver graft reperfusion. NF-κB activation in the liver graft was significant at 2 to 8 h and slightly decreased at 16 h after graft reperfusion.
Figure 1 NF-κB activation in the liver graft.
Lanes 1: Hepatic nuclear protein extracts from sham control rat; Lanes 2-8: Liver graft nuclear protein extracts on 0, 0.5, 1, 2, 4, 8 and 16 h after reperfusion.
NF-κB decoy ODNs suppresses NF-κB activation in the liver graft
In the I/R + NF-κB decoy ODNs group rats, administration of NF-κB decoy ODNs group almost completely abrogated NF-κB activation in the liver graft after 4 h and 8 h of reperfusion (as shown in Figure 2). Similar effects were observed at 0.5, 1, 2 or 16 h after hepatic reperfusion (data not shown). However, in the I/R + scrambled ODNs group rats, NF-κB activation in the liver graft were not significantly changed compared with the I/R group rats (data not shown).
Figure 2 Hepatic NF-κB activation (A) and NF-κB DNA binding activities presented as IOD value (B).
Lanes 1 and 2: Hepatic RNA extracts from sham control group. Lanes 3 and 4: Hepatic RNA extracts from I/R group. Lanes 5 and 6: Hepatic RNA extracts from I/R + NF-κB decoy ODNs group. b, dP<0.001 vs sham group, f, hP<0.001 vs I/R group.
NF-κB decoy ODNs suppresses hepatic mRNA expression of TNF-α, IFN-γ and ICAM-1
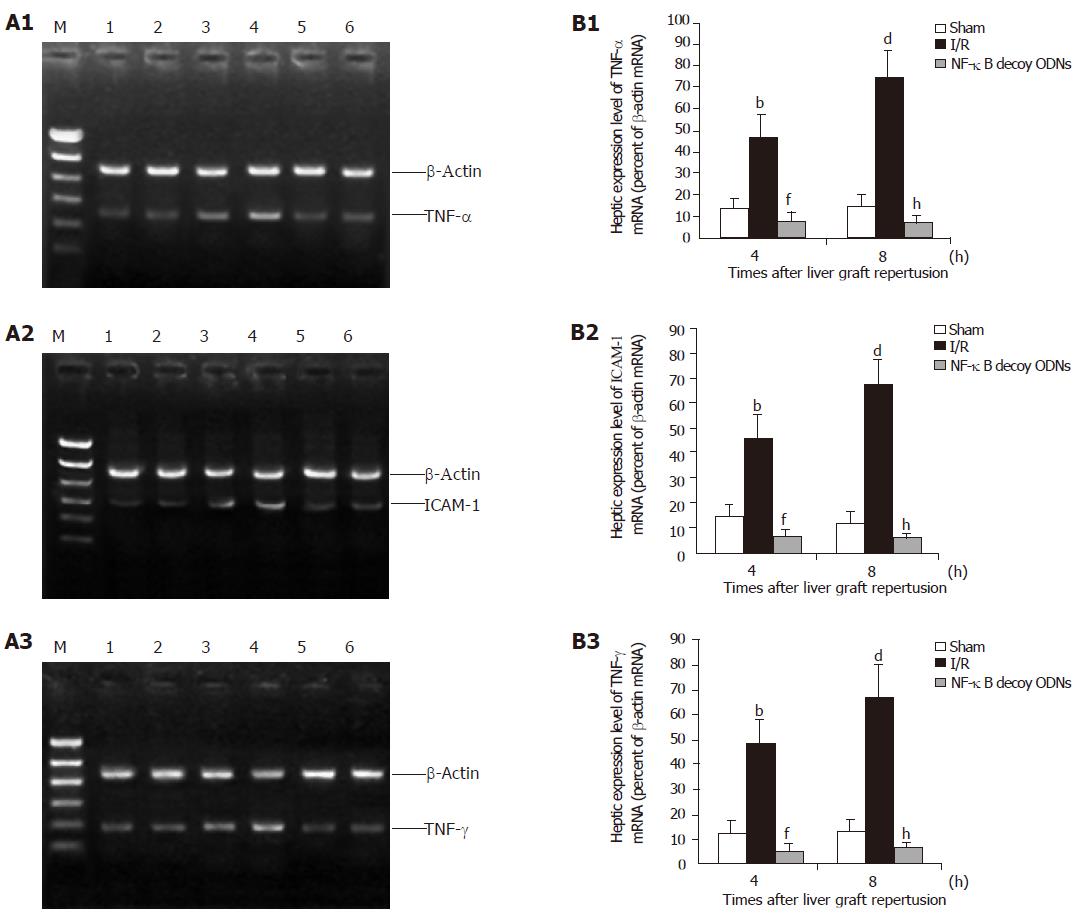
To investigate whether NF-κB decoy ODNs induced suppression of NF-κB was associated with reduced inflammatory mediator expression, mRNA expression of TNF-α, IFN-γ and ICAM-1 in the liver graft were assessed by RT-PCR. Hepatic ischemia/reperfusion increased hepatic mRNA expression of TNF-α, IFN-γ and ICAM-1. Administration of NF-κB decoy ODNs almost completely abrogated hepatic ischemia/reperfusion-induced increases of TNF-α, IFN-γ and ICAM-1 mRNA expression in the liver graft with reperfusion for 4 and 8 h (as shown in Figure 3). Similar effects were observed at 0.5, 1, 2 or 16 h after reperfusion (data not shown). However, administration of scrambled ODNs did not have any significant effect on the hepatic mRNA expression of these inflammatory mediators (data not shown). Thus, NF-κB decoy ODNs-mediated suppression of NF-κB activation in the liver graft is associated with the inhibited hepatic mRNA expression of these proinflammatory mediators.
Figure 3 Hepatic expression (A) and the expression level (B) of cytokine mRNA after 4 and 8 h of reperfusion.
Lanes 1 and 2: Hepatic RNA extracts from sham control group. Lanes 3 and 4: Hepatic RNA extracts from I/R group. Lanes 5 and 6: Hepatic RNA extracts from I/R + NF-κB decoy ODNs group. b, dP<0.001 vs sham group; f, hP<0.001 vs I/R group.
NF-κB decoy ODNs reduces serum level of TNF- α and IFN-γ
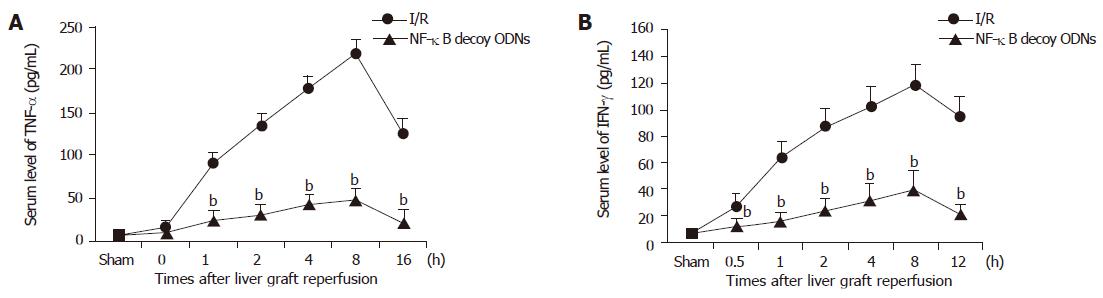
To confirm the inhibitory effects of NF-κB decoy ODNs on the inflammatory mediator production, serum levels of TNF-α and IFN-γ were analyzed by ELISA. Serum levels of TNF-α and IFN-γ were significantly increased within 1 h and were maximal at 8 h after liver graft reperfusion. Administration of NF-κB decoy ODNs markedly reduced serum levels of TNF-α and IFN-γ (P<0.001) at every time point, respectively (Figure 4). However, administration of scrambled ODNs did not have any significant effect on the serum levels of TNF-α and IFN-γ (data not shown).
Figure 4 Serum levels of TNF-α (A) and IFN-γ (B) after hepatic ischemia/reperfusion.
bP<0.001 vs I/R group.
NF-κB decoy ODNs suppresses liver graft neutrophil recruitment and liver graft injury
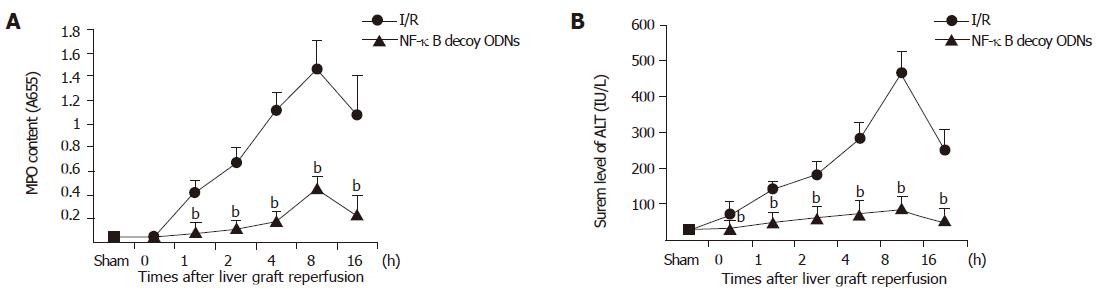
Liver graft neutrophil recruitment was determined by liver MPO content and hepatocellular injury was assessed by serum level of ALT. Liver graft ischemia/reperfusion caused significant increases of liver MPO content and serum level of ALT compared with sham controls. In the presence of NF-κB decoy ODNs, the liver graft MPO content and the serum level of ALT were significantly reduced (P<0.001) (as shown in Figure 5), whereas administration of scrambled ODNs did not have any significant effect on the liver graft MPO content and serum level of ALT (data not shown).
Figure 5 Hepatic MPO content (A) and serum level of ALT (B).
bP<0.001 vs I/R group.
DISCUSSION
Hepatic ischemia/reperfusion injury remains a significant problem and limitation of liver transplantation and may result in liver failure, remote organ failure, and death. Previous studies have identified many proinflammatory mediators (TNF-α, MIP-2, IFN-γ, KC, ENA-78, and ICAM-1) involved in the pathogenesis of hepatic ischemia/reperfusion injury, and production of TNF-α by activated Kupffer cells is central to this process[3,15,6,25-27]. TNF-α enhances the inflammatory response in liver by inducing the expression of adhesion molecules on vascular endothelial cells and stimulating the production and release of neutrophil-attracting CXC chemokines[18,28]. TNF-α[29-33] and ICAM-1[15] also play significant roles in the inflammatory and immune responses that mediate allograft rejection. Thus, they are important as they are against liver graft I/R injury and rejection to suppress the production of these proinflammatory mediators. Each of these mediators is controlled, at least in part, by the transcription factor, NF-κB. Yoshidome et al[15] reported interleukin (IL)-10 protected against hepatic I/R injury by suppressing NF-κB activation and subsequent expression of proinflammatory mediators. Recent studies have confirmed that hepatic ischemic preconditioning (IPC) has protective effect on hepatic cold storage-reperfusion injury, including improved graft function, reduced graft circulatory impairment, enhanced bile production, augmented responses to a bile acid challenge, elevated O2 consumption, improved hepatic tissue blood flow and decreased hepatic vascular resistance, reduced endothelial cell damage, suppressed Kupffer cell activation, decreased apoptosis of hepatocytes, and as a result, graft survival improves after liver transplantation[34-43]. The recent study have found out that attenuation of NF-κB activation and subsequent reduction in TNF-α production after sustained ischemia play important roles in the protective mechanism of IPC against hepatic I/R injury[16,44]. These data suggest a central role of NF-κB activation in the initiation of hepatic I/R injury, and NF-κB activation inhibition could protect against hepatic I/R injury. However, there are contrary reports about the role of NF-κB activation during I/R injury and liver transplantation. Maulik et al[45] showed that IPC activated NF-κB, of which p38 mitogen-associated protein kinase (MAPK) might be upstream before sustained ischemia, and also that inhibitor of p38MAPK abolished preconditioning-induced cardioprotection. Teoh et al[46] recently reported that the hepatoprotective effects of ischemic preconditioning are associated with the activation of NF-κB and SAPKs that are associated with entry of hepatocytes into the cell cycle, a critical biological effect that favors survival of the liver against ischemic and I/R injury. Bradham et al[19] reported that the activation of NF-κB during orthotopic liver transplantation in rats is protective, inhibition of donor hepatic NF-κB activation by adenoviral-mediated IκB alpha superrepressor gene transfer resulted in increased serum ALT levels after 3 h of transplantation. In addition, the blockade of NF-κB resulted in increased histological tissue injury and increased hepatic terminal deoxyribonucleotide transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) staining, indicating apoptosis.
To assess the role of NF-κB activation during liver transplantation, we examined the effect of NF-κB activation inhibition by NF-κB decoy ODNs containing two NF-κB binding sites on the liver graft I/R injury. We found that cold preservation-reperfusion of liver graft rapidly activated hepatic NF-κB and concomitantly elevated hepatic mRNA expression of TNF-α, INF-γ and ICAM-1 as well as serum levels of TNF-α and IFN-γ. Administration of NF-κB decoy ODNs almost completely abrogated the increased hepatic NF-κB activation, the up-regulated hepatic mRNA expression of the proinflammatory mediators, the elevated serum levels of TNF-α and IFN-γ as well as the increased hepatic neutrophil recruitment, and as a result, attenuated liver graft I/R injury. Liver I/R injury is considered to be primarily dependent on the activation of Kupffer cells, these cells are a major source of ROIs, proinflammatory cytokines or chemokines that promote neutrophils recruitment, adhesion, and activation eventually leading to organ injury. Bradham et al[19] reported that the induction of TNF-α mRNA and serum protein during liver transplantation was unaffected by Kupffer cells depletion with GdCl3. These results show that Kupffer cells are not the only major source of TNF-α production after liver transplantation and that stress-signaling protein activation occurs independently of Kupffer cells. A recent study reported that hepatic I/R injury critically depends on liver activated T cells[47,48]. Indeed, both cyclosporine and FK506, which are potent T cell-deactivating agents, were reported to decrease reperfusion injury after liver transplantation or warm ischemia compared with untreated controls. Besides the role of liver reside T cells, lymphocytes recruited from the circulatory cell pool within hours or days after reperfusion are also involved in the cascade of reperfusion in the kidney and in the liver[49,50]. Circulating monocytes/macrophages are the primary systemic sources of TNF-α[51] in response to endotoxin stimulation. Previous studies showed that endotoxin in portal vein blood was significantly increased during portal vein occlusion and after liver transplantation, and the increased endotoxin could stimulat macrophages that reside in spleen, lung, and other organs, as well as circulating monocyte/macrophage to produce TNF-α. Tsoulfas et al[52] have shown the LPS/CD14/LBP/NF-κB signaling pathway activation in hepatic transplantation preservation injury. Thus, far besides Kupffer cells, T lymphocytes and monocytes/macrophages, either residing in the organ, or being recruited from the blood, should definitely be considered effector cells in I/R injury. IFN-γ released by activated T cells is able to prime macrophages/Kupffer cells for the production of proinflammatory cytokines like TNF, and to downregulate the synthesis of IL-10, a protective cytokine which has been identified to protect against hepatic ischemia/reperfusion injury. Conversely, monomacrophages can also activate T cells and promote the synthesis of INF-γ through the release of cytokines like IL-12 and IL-18 and the engagement of costimulatory molecules[53]. In the present study, the administration of NF-κB decoy ODNs may attenuate not only donor hepatic Kupffer cells NF-κB activation, but also recipient monocytes/macrophages and T-cells NF-κB activation, and consequently decreases the hepatic mRNA expression of TNF-α and IFN-γ as well as the protein production of TNF-α and IFN-γ. Although blockade of NF-κB can result in increased hepatocytes apoptosis induced by TNF-α, the significantly decreased production of TNF-α by NF-κB decoy ODNs may attenuates the initiation of hepatocytes apoptosis. Thus, NF-κB decoy ODNs may protect against I/R injury in the transplanted liver graft by suppressing NF-κB activation and the subsequent production of TNF-α, ICAM-1 and IFN-γ.