Published online Nov 28, 2005. doi: 10.3748/wjg.v11.i44.6954
Revised: June 3, 2005
Accepted: June 9, 2005
Published online: November 28, 2005
AIM: To evaluate the effects of a portocaval shunt on the decrease of excessive portal flow for the prevention of sinusoidal microcirculatory injury in extremely small-for-size liver transplantation in pigs.
METHODS: The right lateral lobe of pigs, i.e. the 25% of the liver, was transplanted orthotopically. The pigs were divided into two groups: graft without portocaval shunt (n = 11) and graft with portocaval shunt (n = 11). Survival rate, portal flow, hepatic arterial flow, and histological findings were investigated.
RESULTS: In the group without portocaval shunt, all pigs except one died of liver dysfunction within 24 h after transplantation. In the group with portocaval shunt, eight pigs survived for more than 4 d. The portal flow volumes before and after transplantation in the group without portocaval shunt were 118.2±26.9 mL/min/100 g liver tissue and 270.5±72.9 mL/min/100 g liver tissue, respectively. On the other hand, in the group with portocaval shunt, those volumes were 124.2±27.8 mL/min/100 g liver tissue and 42.7±32.3 mL/min/100 g liver tissue, respectively (P<0.01). As for histological findings in the group without portocaval shunt, destruction of the sinusoidal lining and bleeding in the peri-portal areas were observed after reperfusion, but these findings were not recognized in the group with portocaval shunt.
CONCLUSION: These results suggest that excessive portal flow is attributed to post transplant liver dysfunction after extreme small-for-size liver transplantation caused by sinusoidal microcirculatory injury.
- Citation: Wang HS, Ohkohchi N, Enomoto Y, Usuda M, Miyagi S, Asakura T, Masuoka H, Aiso T, Fukushima K, Narita T, Yamaya H, Nakamura A, Sekiguchi S, Kawagishi N, Sato A, Satomi S. Excessive portal flow causes graft failure in extremely small-for-size liver transplantation in pigs. World J Gastroenterol 2005; 11(44): 6954-6959
- URL: https://www.wjgnet.com/1007-9327/full/v11/i44/6954.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i44.6954
Since the first successful living donor liver transplantation (LDLT) in a child[1] patient and an adult[2] patient, LDLT has become the established method to reduce the number of patients on the waiting list and is considered as an alternative to standard liver transplantation[3-7]. The survival rate of adults is significantly lower than that in children[8] and the key to a successful LDLT, especially in adult recipients, is the adequacy of the size of the graft that can be safely harvested from the donor[9-11]. In some cases, graft weight ratio of the recipient native liver weight (GWRLW) of 30% or less has been transplanted successfully, but in general, graft weight per recipient’s body weight (GWBW) less than 0.8% or GWRLW less than 40% has been considered marginal or small-for-size. These small-for-size grafts are associated with an increased incidence of complications and graft failure[9-12]. A small-for-size graft cannot supply the metabolic demand of an adult recipient in the early posttransplant period. Poor early graft function is characterized by protracted cholestasis, coagulopathy and ascites, and these findings are proposed to be the essential symptoms of small-for-size syndrome[13]. However, the precise mechanism for this dysfunction remains unclear.
Previously, we have reported that portal hypertension after reperfusion is one of the most important factors aggravating the microcirculatory injury of the graft[14]. In the present study, we hypothesized that the increment of portal flow played a major role in graft injury and poor function of small-for-size grafts, and investigated the effects of portocaval shunt (PCS) on the excessive portal flow for the prevention of the sinusoidal microcirculatory injury after extremely small-for-size liver transplantation using pigs.
Landrace white pigs, weighing 18-28 kg, were used as donors and recipients. All experiments were conducted according to Principles of Laboratory Animal Care (NIH publication No. 86-23, revised in 1985). Food was withheld for 24 h before the operation. Anesthesia was induced by intramuscular administration of ketamine (5 mg/kg) and atropine sulfate (1.0 mg/body) followed by endotracheal intubation and maintenance with oxygen and isoflurane by positive pressure mechanical ventilation. A catheter was placed in the internal jugular vein for fluid administration and central venous pressure (CVP) monitoring and fixed at the back of the neck for postoperative venous sampling. A carotid arterial line was also placed for intraoperative blood sampling and monitoring mean arterial pressure (MAP). In recipients, an opposite internal jugular vein was used for the venovenous bypass from the portal vein and femoral vein. Eleven transplantations were carried out in each group.
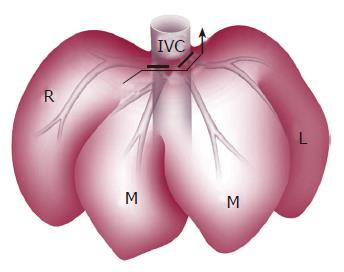
After laparotomy, the left hepatic artery supplying the left lateral and left median lobes was ligated and divided. Glisson’s sheaths of the left lobe and the right median lobe were identified and resected. After mobilization of the liver, by dissecting all ligamentous attachments, the left and middle hepatic veins were ligated with transfixing sutures (Figure 1). Parenchymal transection of the left lobe and the right median lobe was performed and finally left tri-segmentectomy of the liver was done.
Following intravenous administration of heparin, catheters were cannulated in the lower abdominal aorta and splenic vein. The diaphragm was widely opened and the supradiaphragmatic aorta was divided and encircled. Cold University of Wisconsin (UW) solution (4°C) was flushed in from both the aorta and the splenic vein after aortic clamp. The right lateral lobe was removed and preserved in UW solution.
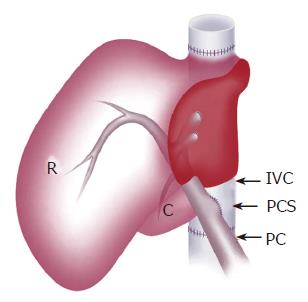
In the group with shunt, the PCS was made on the back table and placed by means of side-to-side anastomosis between the portal vein and the infra-hepatic inferior vena cava (IVC) (Figure 2). The diameter of the PCS was 6 mm. The graft was weighed after the back table preparation.
Total hepatectomy was performed under ilioportaljugular venovenous bypass[15]. The reduced-size graft with or without PCS was implanted orthotopically with end to end vascular anastomosis of the suprahepatic IVC, the portal vein, the infra-hepatic IVC, and the hepatic artery. Before anastomosis of the portal vein, rinse solution was perfused into the portal vein of the graft. Hepatic arterial reconstruction was performed under a microscope. A catheter was inserted into the common bile duct for bile drainage.
After transplantation, the pigs were placed in a warmed cage with free access to water and food. FK506 (0.1 mg/kg·d) was injected for immunosuppression from postoperative day (POD) 1-7. When recipients died, autopsy was performed to exclude the possibility of technical complications and to confirm the patency of all anastomoses. Liver grafts were also weighed.
Systemic hemodynamic monitoring of MAP and CVP was carried out continuously during the operation, as well as portal vein pressure was monitored. Portal flow and hepatic arterial flow (HAF) were measured before hepatectomy in donors and after arterial reperfusion in recipients using ultrasound transit time flow probes and a flow meter (Transonic Systems Inc., Ithaca, NY, USA). The diameter of the probe was 8 mm for the portal vein and 3 mm for the artery. Total liver blood flow (TLBF) was calculated as the sum of portal flow and HAF. Liver biopsies were performed before hepatectomy, after reperfusion, and on every other day up to POD 7, and tissue specimens were examined under light microscope and Ki-67 staining was performed for the determination of proliferating hepatocytes. Transmission electron microscopical findings were also investigated in the specimen after reperfusion. Arterial blood samples were obtained hourly for gas analysis during the operation and determining alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (T-Bil), and anti-thrombin III (AT-III) before the operation, at 3 and 6 h after reperfusion and on every POD up to POD 7.
The graft weight at the time of transplantation expressed as a percentage of GWRLW and GWBW was calculated.
Values of parameters were expressed as mean±SD. Statistical significance was determined by Student’s t-test. The survival rate was calculated by the Kaplan-Meier method. Values between the two groups were statistically analyzed by generalized Wilcoxon test. P values less than 0.05 were regarded as statistically significant.
Body weight, graft volume, and operation time are shown in Table 1. The graft volume was approximately 25% of the recipient native liver volume, and 0.6% of the recipient body weight in both groups. The cold ischemic time in the group with PCS was slightly longer than that in the group without PCS because of the time required for the PCS at the back table, but there was no statistical difference. There was no significant difference in MAP or CVP between the two groups (data not shown).
| Parameters | Group without PCS | Group with PCS |
| (n = 11) | (n = 11) | |
| DBW (kg) | 21.8±2.7 | 20.8±1.1 |
| RBW (kg) | 22.8±1.2 | 22.1±2.6 |
| GW (g) | 131.8±18.3 | 139.3±32.0 |
| RLW (g) | 525.4±41.4 | 548.3±63.8 |
| GWRLW (%) | 25.1±2.7 | 25.4±3.4 |
| GWBW (%) | 0.58±0.09 | 0.63±0.13 |
| Anhepatic time (min) | 43±2 | 41±4 |
| Operation time (min) | 261±28 | 263±44 |
| Total ischemic time (min) | 151±51 | 161±20 |
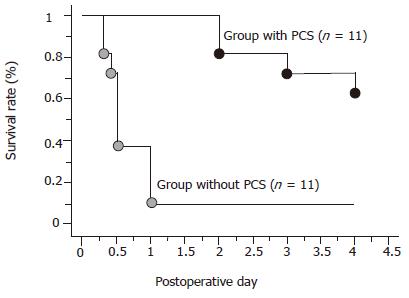
In the group without PCS, all pigs except one died of liver dysfunction within 24 h after reperfusion. On the other hand, in the group with PCS, eight pigs survived for more than 4 d and the remaining three died of portal vein thrombosis at the anastomotic site of PCS or perforated gastric ulcer within 3 d. The PCS significantly improved the survival rate of the animals in comparison to the animals without the shunt after transplantation (P<0.05) (Figure 3).
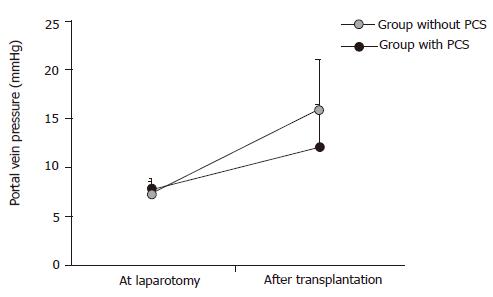
There were no significant differences in portal vein pressures at laparotomy in both groups. However, after reperfusion, portal vein pressures significantly increased up to 2.2±0.7 KPa in the group without PCS and 1.6±0.6 KPa in the group with PCS (Figure 4).
Hepatic hemodynamic parameters including portal flow, HAF and TLBF at laparotomy and after reperfusion in both groups are shown in Table 2. The portal flow after reperfusion in the group without PCS increased significantly more than that at laparotomy, whereas it decreased in the group with PCS (P<0.01). The HAF after reperfusion decreased compared to that at laparotomy in the group without PCS, while it increased more than that at laparotomy in the group with PCS (P<0.05).
| Parameters | Group | At laparotomy | After transplantation |
| Portal flow | Group without PCS | 118.2±26.9 | 270.5±72.9b,a |
| (mL/min/100 g liver tissue) | Group with PCS | 124.2±27.8 | 42.7±32.3a |
| Hepatic arterial flow | Group without PCS | 50.1±8.3 | 37.7±21.0a |
| (mL/min/100 g liver tissue) | Group with PCS | 50.5±11.6 | 75.1±39.5 |
| Total liver blood flow | Group without PCS | 168.3±30.9 | 308.2±52.8b,a |
| (mL/min/100 g liver tissue) | Group with PCS | 174.8±29.1 | 117.8±42.1c |
| Ratio of PF/TLBF (%) | Group without PCS | 70.2±4.2 | 87.8±8.2b,a |
| Group with PCS | 71.1±4.9 | 36.3±19.5a |
The TLBF after reperfusion increased significantly higher than that at laparotomy in the group without PCS, whereas the TLBF slightly decreased after reperfusion in the group with PCS. After reperfusion, the TLBF in the group without PCS was significantly greater than that in the group with PCS (P<0.01). In the group without PCS, the contribution of portal flow to TLBF increased from 70.2% to 87.8%, while in the group with PCS the contribution of the portal flow to TLBF decreased from 71.1% to 36.3%.
The light microscopical findings of the liver graft after reperfusion are shown in Figure 5. In the group without PCS, enlargement of the sinusoidal lumen and bleeding in the peri-portal triads were observed but these abnormal findings were not recognized in the group with PCS.
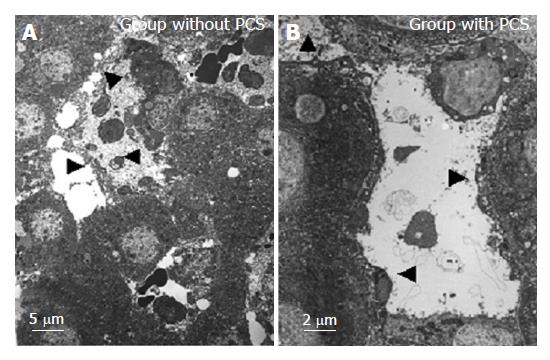
Transmission electron microscopic findings of the sinusoid after reperfusion are shown in Figure 6. In the group without PCS, the sinusoidal endothelial cells were destroyed and detached into the sinusoidal space with destruction of the Disse’s spaces, while in the group with PCS the sinusoidal endothelial cells were well preserved, the Disse’s spaces were intact, and the structure of endothelial lining was well preserved.
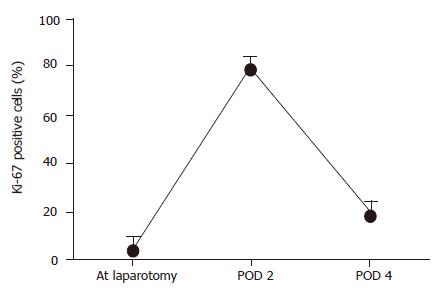
The graft weight in the recipients who survived for more than 4 d (n = 8) increased to 87.3% of the recipient native liver. Ki-67 labeling index before and after transplantation is shown in Figure 7. The Ki-67 labeling index showed only 1.0% of hepatocytes at laparotomy. However, it increased abruptly after transplantation, reaching a peak value of approximately 60% at POD 2, and decreasing to 30% on POD 4.
There were no significant differences in ALT, AST or T-Bil until POD 1 after reperfusion in either group (Table3). ALT and AST increased after reperfusion, taking a peak value on POD 2 and POD 1 and then decreased. However, T-Bil increased continuously until POD 4. AT-III decreased after reperfusion in both groups. At 6 h after reperfusion and on POD 1, AT-III in the group with PCS was significantly higher than that in the group without PCS (P<0.05).
In clinical liver surgery, an extended hepatectomy of 80% or 85% of the whole liver can be tolerable in patients with a normal liver[16]. In previous reports, 70-75% hepatectomy in animals represents a model of critical residual liver volume and is used to study the mechanism of liver regeneration[17-19]. This means that 25-30% of the liver can sustain hepatic function. However, this cannot be translated directly into a minimum graft volume in small-for-size liver transplantation, in which grafts are subjected to cold and warm ischemia and subsequent reperfusion injury. This is why the minimum graft volume for successful liver transplantation is presumably higher than the residual liver volume in extended hepatectomy. Currently, experience with living-related and split-liver transplantation has demonstrated that the size of the graft required for a successful liver transplantation is at least 40% of the recipient’s native liver volume and more than 0.8% of the recipient body weight[6,20]. This is associated with lack of portosystemic collateral circulation, which lessens the influence of a high portal flow on the grafts and prevents the development of severe portal hypertension[21]. The precise mechanism of graft injury in a small-for-size liver transplantation remains still unknown. But there is a case report of hemorrhagic necrosis secondary to excessive portal flow in an adult LDLT[22]. An adult LDLT with small-for-size graft has been successfully performed using a mesocaval shunt to avoid graft failure caused by portal hyperperfusion[23]. From these reports, we hypothesized that excessive portal flow could attribute to postoperative liver dysfunction caused by sinusoidal microcirculatory injury after small-for-size liver transplantation. In our experiment, we investigated the effect of PCS on excessive portal flow for the prevention of sinusoidal microcirculatory injury in extremely small-for-size liver transplantation in pigs, focusing on the prevention of primary graft dysfunction. We chose the pig model because the anatomy, metabolism, and physiology of the liver are similar to those in human beings. As a result, we clarified that PCS could prevent portal hypertension and excessive portal inflow in small-for-size liver transplantation.
The sinusoids are the principal vessels involved in the transvascular exchange between blood and the parenchymal cells and play an important role in hepatic microcirculation. In an experimental transplantation model using a small-for-size graft weighing less than 30% of the native liver, Asakura et al[14] and Man et al[24] demonstrated that portal hypertension is a determinant factor for the injury of sinusoidal endothelial cells and hepatic parenchyma. The portal flow through the reduced microvascular bed of the small-for-size graft after reperfusion is likely to induce injury of sinusoidal endothelial cells and activation of Kupffer’s cells, which is similar to those after extended hepatectomy in rats reported by Panis et al[19].
In this study, TLBF after reperfusion increased approximately twice in comparison to the flow at laparotomy in the group without PCS. The portal flow increased predominantly and HAF reduced. Histological findings clearly indicated disruption of the sinusoidal lining and disturbance of the sinusoidal microcirculation. Furthermore, the graft became swollen after reperfusion, progressing into severe bowel congestion and then blood oozing. These phenomena were associated with a poor survival rate in the group without PCS. On the other hand, in the group with PCS, graft inflow was modified by PCS which permitted a significantly lower portal flow and a higher HAF. The pathological findings of microcirculatory disturbance after reperfusion recognized in the group without PCS were not observed in the group with PCS. The results of our study strongly suggest that an excessive portal flow after reperfusion in small-for-size liver transplantation is one of the major factors that cause sinusoidal microcirculatory injury and result in graft failure.
With regard to the graft regeneration in the group with PCS, though the TLBF was low, the weight of the graft and Ki-67 index increased remarkably in the pigs that survived for more than 4 d. The decreased HAF subsequent to the excessive portal flow observed after reperfusion in the group without PCS was also believed to contribute to the poor regeneration of residual liver and the poor outcome. Sato et al[25] reported that portal hypertension is a trigger of liver regeneration following partial hepatectomy. But surplus portal hypertension induces liver dysfunction[25]. Results of our study support the hypothesis that portal flow controls liver regeneration in small-for-size liver transplantation and maintains liver function.
In conclusion, an extreme small-for-size graft weighing less than 25% of the native liver can be successfully transplanted with a PCS in pigs. Portal hyperperfusion in small-for-size liver transplantation appears to play a major role in aggravating microcirculatory injury of the graft and attenuation of portal hyperperfusion by PCS minimizes the injury and improves the survival. This study provides helpful information for transplant surgeons regarding the novel therapeutic strategies for the rescue of small-for-size liver grafts.
Co-correspondent: Hong-Sheng Wang
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Strong RW, Lynch SV, Ong TH, Matsunami H, Koido Y, Balderson GA. Successful liver transplantation from a living donor to her son. N Engl J Med. 1990;322:1505-1507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 604] [Cited by in F6Publishing: 594] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 2. | Hashikura Y, Makuuchi M, Kawasaki S, Matsunami H, Ikegami T, Nakazawa Y, Kiyosawa K, Ichida T. Successful living-related partial liver transplantation to an adult patient. Lancet. 1994;343:1233-1234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 310] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Testa G, Malago M, Broelsch CE. Living-donor liver transplantation in adults. Langenbecks Arch Surg. 1999;384:536-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Ito T, Kiuchi T, Yamamoto H, Oike F, Ogura Y, Fujimoto Y, Hirohashi K, Tanaka AK. Changes in portal venous pressure in the early phase after living donor liver transplantation: pathogenesis and clinical implications. Transplantation. 2003;75:1313-1317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 200] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | García-Valdecasas JC, Fuster J, Charco R, Bombuy E, Fondevila C, Navasa M, Rodríguez-Laiz G, Ferrer J, Amador MA, Llovet JM. Adult living donor liver transplantation. Analysis of the first 30 cases. Gastroenterol Hepatol. 2003;26:525-530. [PubMed] [Cited in This Article: ] |
| 6. | Kawasaki S, Makuuchi M, Matsunami H, Hashikura Y, Ikegami T, Nakazawa Y, Chisuwa H, Terada M, Miyagawa S. Living related liver transplantation in adults. Ann Surg. 1998;227:269-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 335] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 7. | Fan ST, Lo CM, Liu CL. Technical refinement in adult-to-adult living donor liver transplantation using right lobe graft. Ann Surg. 2000;231:126-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 172] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Sugawara Y, Makuuchi M. Small-for-size graft problems in adult-to-adult living-donor liver transplantation. Transplantation. 2003;75:S20-S22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Emond JC, Renz JF, Ferrell LD, Rosenthal P, Lim RC, Roberts JP, Lake JR, Ascher NL. Functional analysis of grafts from living donors. Implications for the treatment of older recipients. Ann Surg. 1996;224:544-52; discussion 552-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 288] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Nishizaki T, Ikegami T, Hiroshige S, Hashimoto K, Uchiyama H, Yoshizumi T, Kishikawa K, Shimada M, Sugimachi K. Small graft for living donor liver transplantation. Ann Surg. 2001;233:575-580. [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 162] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Kiuchi T, Kasahara M, Uryuhara K, Inomata Y, Uemoto S, Asonuma K, Egawa H, Fujita S, Hayashi M, Tanaka K. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999;67:321-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 741] [Cited by in F6Publishing: 700] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 12. | Lo CM, Fan ST, Chan JK, Wei W, Lo RJ, Lai CL. Minimum graft volume for successful adult-to-adult living donor liver transplantation for fulminant hepatic failure. Transplantation. 1996;62:696-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 129] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Kelly DM, Demetris AJ, Fung JJ, Marcos A, Zhu Y, Subbotin V, Yin L, Totsuka E, Ishii T, Lee MC. Porcine partial liver transplantation: a novel model of the "small-for-size" liver graft. Liver Transpl. 2004;10:253-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Asakura T, Ohkohchi N, Orii T, Koyamada N, Tsukamoto S, Sato M, Enomoto Y, Usuda M, Satomi S. Portal vein pressure is the key for successful liver transplantation of an extremely small graft in the pig model. Transpl Int. 2003;16:376-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Yanaga K, Kishikawa K, Suehiro T, Nishizaki T, Shimada M, Itasaka H, Nomoto K, Kakizoe S, Sugimachi K. Partial hepatic grafting: porcine study on critical volume reduction. Surgery. 1995;118:486-492. [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Starzl TE, Putnam CW, Groth CG, Corman JL, Taubman J. Alopecia, ascites, and incomplete regeneration after 85 to 90 per cent liver resection. Am J Surg. 1975;129:587-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Kahn D, Hickman R, Terblanche J, von Sommoggy S. Partial hepatectomy and liver regeneration in pigs--the response to different resection sizes. J Surg Res. 1988;45:176-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Nagao M, Isaji S, Iwata M, Kawarada Y. The remnant liver dysfunction after 84% hepatectomy in dogs. Hepatogastroenterology. 2000;47:1564-1569. [PubMed] [Cited in This Article: ] |
| 19. | Panis Y, McMullan DM, Emond JC. Progressive necrosis after hepatectomy and the pathophysiology of liver failure after massive resection. Surgery. 1997;121:142-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 154] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | García-Valdecasas JC, Fuster J, Charco R, Bombuy E, Fondevila C, Ferrer J, Ayuso C, Taura P. Changes in portal vein flow after adult living-donor liver transplantation: does it influence postoperative liver function? Liver Transpl. 2003;9:564-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Smyrniotis V, Kostopanagiotou G, Kondi A, Gamaletsos E, Theodoraki K, Kehagias D, Mystakidou K, Contis J. Hemodynamic interaction between portal vein and hepatic artery flow in small-for-size split liver transplantation. Transpl Int. 2002;15:355-360. [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Ayata G, Pomfret E, Pomposelli JJ, Gordon FD, Lewis WD, Jenkins RL, Khettry U. Adult-to-adult live donor liver transplantation: a short-term clinicopathologic study. Hum Pathol. 2001;32:814-822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Boillot O, Delafosse B, Méchet I, Boucaud C, Pouyet M. Small-for-size partial liver graft in an adult recipient; a new transplant technique. Lancet. 2002;359:406-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 182] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Man K, Lo CM, Ng IO, Wong YC, Qin LF, Fan ST, Wong J. Liver transplantation in rats using small-for-size grafts: a study of hemodynamic and morphological changes. Arch Surg. 2001;136:280-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 160] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Sato Y, Koyama S, Tsukada K, Hatakeyama K. Acute portal hypertension reflecting shear stress as a trigger of liver regeneration following partial hepatectomy. Surg Today. 1997;27:518-526. [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 107] [Article Influence: 4.0] [Reference Citation Analysis (0)] |