Published online Nov 21, 2005. doi: 10.3748/wjg.v11.i43.6765
Revised: May 21, 2005
Accepted: May 24, 2005
Published online: November 21, 2005
AIM: To examine surgical specimens of pancreas with either chronic pancreatitis or pancreatic cancer in order to study whether ductal hyperplasia and dysplasia in pancreas represent precursor lesions for pancreatic cancer.
METHODS: We examined expression of Ki-67, CEA, p53, and K-ras, in the surgical specimens of pancreas with adenocarcinomas (n = 11) and chronic pancreatitis (n = 12). Cellular proliferation was assessed by Ki-67 proliferation index using the proliferation marker Ki-67. In specimens with pancreas cancer, we divided pancreas epithelium into normal (n = 7), ductal hyperplasia (n = 3), dysplasia (n = 4), and cancerous lesion (n = 11) after hematoxylin and eosin staining, Ki-67, and CEA immunohistochemical staining. In cases with chronic pancreatitis, the specimen was pathologically examined as in cases with pancreas cancer, and they were also determined as normal (n = 10), ductal hyperplasia (n = 4), or dysplasia (n = 5). p53 and K-ras expression were also studied by immunohistochemical staining.
RESULTS: In pancreatic cancer, the Ki-67 index was 3.73±3.58 in normal site, 6.62±4.39 in ductal hyperplasia, 13.47±4.02 in dysplasia and 37.03±10.05 in cancer tissue, respectively. Overall, p53 was positive in normal ducts, ductal hyperplasia, dysplasia, and carcinoma cells in 0 of 14 (0%), 0 of 7 (0%), 7 of 9 (78%), and 10 of 11 (91%), respectively, and K-ras was positive in 0 of 8 (0%), 1 of 3 (33%), 4 of 6 (67%), 4 of 5 (80%), respectively.
CONCLUSION: Our results favorably support the hypothesis that ductal hyperplasia and dysplasia of the pancreas might be precursor lesions for pancreas cancer. Further evaluation of oncogenes by the molecular study is needed.
-
Citation: Jeong S, Lee DH, Lee JI, Lee JW, Kwon KS, Kim PS, Kim HG, Shin YW, Kim YS, Kim YB. Expression of Ki-67, p53, and K-
ras in chronic pancreatitis and pancreatic ductal adenocarcinoma. World J Gastroenterol 2005; 11(43): 6765-6769 - URL: https://www.wjgnet.com/1007-9327/full/v11/i43/6765.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i43.6765
The pancreatic cancer has the poorest prognosis among various cancers with a 5-year survival rate of 3%, making it the fourth most common cause of cancer-related mortality rate[1-3]. Although the surgical resection offers the only chance for cure, only 10-30% of patients have resectable tumor[4]. Since early diagnosis followed by complete resection is the only way to the complete recovery, understanding the pathogenesis of pancreatic cancer seems to be of great value.
The ductal hyperplasia of pancreas is defined by abnormally increased number of epithelial cells of pancreatic duct. It may be detected in both normal and chronically inflamed pancreatic tissues, and its incidence is generally increased with age[5]. However, the patients with chronic pancreatitis and ductal hyperplasia have been reported to be associated with the development of pancreas cancer[5-8]. In addition, there has been an investigation suggesting three times higher incidence of papillary hyperplasia of pancreatic duct epithelium in patients with pancreatic cancer compared with the control group[6]. There is also a report that 41% of patients with pancreatic cancer showed hyperplasia of pancreatic duct epithelium when 9% of the control group have that pathological finding[7].
In an effort to search for the pathogenesis of pancreatic cancer, mutations of K-ras, p53, and overexpression of HER-2/neu were found in human pancreatic ductal adenocarcinoma[9].
Currently, the hypothesis of multistep carcinogenesis that states cumulative genetic mutations in the normal pancreas might have progressed into pancreatic cancer is mostly accepted. However, detailed explanation for each step of carcinogenesis needs further investigation. In the current study, we performed histological examination of pancreas and detected normal epithelium and that with ductal hyperplasia, dysplasia, or cancerous lesions. Then we immunohistochemically determined the expression of Ki-67, K-ras, and p53 to identify if ductal hyperplasia and dysplasia of pancreas might be precancerous lesion.
The study was performed in 11 patients with pancreatic ductal adenocarcinoma and 12 patients with chronic pancreatitis, the total 23 patients went through surgical pancreatic resection at Inha University Hospital. The mean age of patients was 67 years (range 40-74 years) for pancreatic cancer and 61 years (range 37-67 years) for chronic pancreatitis. Male to female ratio was 6:5 for pancreatic cancer and 10:2 for chronic pancreatitis.
The degree of pancreatic ductal lesion was classified morphologically according to Cubilla and Fitzgerald[6], and the pancreatic carcinoma according to Kloppel[10]. Most pancreatic duct epitheliums are composed of cuboidal and low columnar cells, their cytoplasm filled with mucous components. They are surrounded by loose fibrous connective tissue. Hyperplasia of pancreatic duct epithelium is divided into either flat or papillary type. However, recent studies show that there is no difference in genetic mutations between these two forms of hyperplasia[9]. Therefore, we considered these two different forms of hyperplasia in the same tissue in this study. Dysplasia was classified according to flattening, papillary hyperplasia of ductal epithelium, increased nucleus/cytoplasm ratio as the epithelium changes into atypical form, loss of nuclear polarity, morphologic form, and aggregation. A little evidence of cellular division was also considered as dysplasia. The degree of hyperplasia was evaluated objectively by counting the numbers of hyperplasia using the method of Ki-67 (Immunotec, France) immunohistochemical staining. To see whether dysplasia develops or not, we performed CEA immunohistochemical staining in the area of hyperplasia stained with Ki-67 using double staining method and classified them into different categories, so that we can minimize the errors arising from differential classification of pancreatic tissue.
After 10% of surgically resected pancreatic tissue was fixed in neutral formalin, paraffin-embedded tissue was sliced to the thickness of 4 µm continuously. One slice was stained with hematoxylin-eosin (H&E), and the others were stuck to the slide with poly-L-lysine for immunohistochemical staining. After all the slices went through autoclave (120 °C, 15 lb) for 15 min to expose the antigens fixed by formalin, we treated with H2O2 for 30 min to eliminate intrinsic peroxidase. For immunohistochemical staining, CEA monoclonal antibody (Ab) (DAKO, Carpenteria, CA, USA) and Ki-67 monoclonal Ab (Immunotech, Marseille, Cedex, France) were diluted to 1:150 and 1:100, respectively, then incubated at room temperature for 3 h. Labeled streptavidin biotin kit (LSAB kit, DAKO) and 3’,3-diaminobenzidine (DAB) were used for staining. Also p53 monoclonal Ab (Novocastra, Newcastle, UK) was diluted to 1:150 and incubated at 4 °C for one day. Then alkaline phosphatase, anti-alkaline phosphatase kit (APAAP kit, DAKO) was used for staining and fast red (DAKO) for expression. For K-ras staining, p21ras monoclonal Ab (Oncogene Research Products, Calbiochem, Germany) was diluted to 1:50. The serially sliced tissues were examined morphologically at the same area using H&E staining for histologic diagnosis, and then were compared with the results of immunohistochemical staining in response to CEA, p53, K-ras, and Ki-67. The following Ki-67 proliferation index was expressed as the percent of Ki-67-positive cells of 1 000 pancreatic duct epithelial cells. The double staining was performed using Ki-67 and CEA monoclonal Ab to ascertain the presence of dysplasia. Dysplasia was classified using CEA+p53 and CEA+Ki-67 staining.
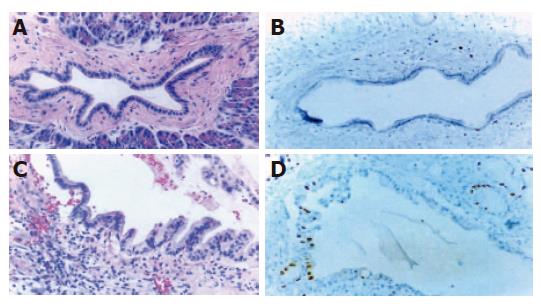
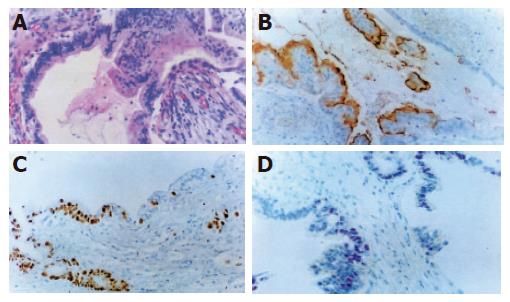
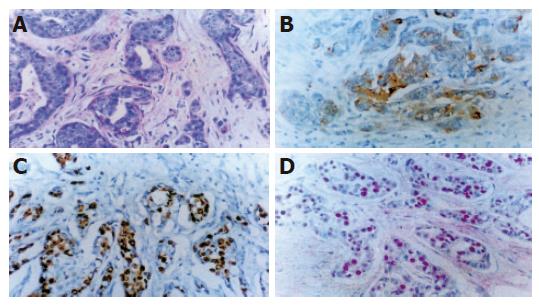
Hematoxylin and eosin staining Normal pancreatic duct epithelium is composed of mainly cuboidal and low columnar cells and surrounded by fibrous connective tissues (Figure 1A). We detected basally located nuclei, aggregation and intraductal papillary proliferation of pancreatic duct epithelial cells in ductal hyperplasia (Figure 1C). Dysplasia of the pancreatic duct showed an increased nuclear to cytoplasm ratio and loss of nuclear polarity (Figure 2A). The tissue of pancreatic duct showed numerous cell divisions, an increased nuclear-to-cytoplasm ratio, and loss of cellular polarity (Figure 3A).
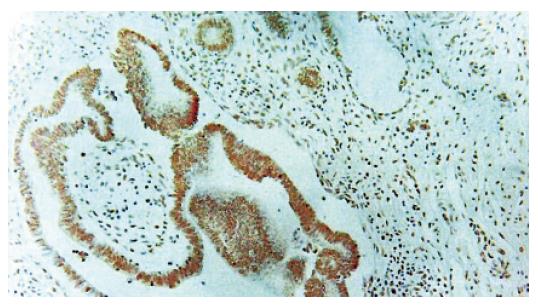
Immunohistochemical staining Normal pancreatic duct showed negative reaction to Ki-67, CEA, p53, K-ras staining (Figure 1B), whereas in hyperplasia, the nuclei were stained by Ki-67 (Figure 1D), cellular membrane by CEA and cytoplasm by K-ras, respectively. In dysplasia, the nuclei were stained by Ki-67, p53, and cell membrane by CEA respectively (Figures 2B-D). Focal, weak positive reaction to CEA was also detected in the cytoplasm. In pancreatic cancer, the nuclei were stained by Ki-67 and p53, whereas both cell membrane and cytoplasm showed strong positive reaction to CEA (Figures 3B-D). K-ras showed negative reaction to K-ras (Table 1, Figure 4).
| Case of studyn (%) | Ki-67 index | p53n (%) | K-rasn (%) | |
| Overall Normal Hyperplasia Dysplasia Carcinoma Pancreatic cancer Normal Hyperplasia Dysplasia Carcinoma Chronic pancreatitis Normal Hyperplasia1 Dysplasia | 17/23 (74) 7/16 (44) 9/16 (56) 11/23 (48) 7/11 (64) 3/7 (43) 4/7 (57) 11/11 (100) 10/13 (77) 4/9 (44) 5/9 (56) | 2.35±2.79 9.07±5.39 10.79±5.51 37.03±10.05 3.73±3.58 6.62±4.39 13.47±4.02 37.03±10.05 2.35±2.79 7.66±5.58 8.64±5.51 | 0/14 (0) 0/7 (0) 7/9 (78) 10/11 (91) 0/6 (0) 0/3 (0) 4/4 (100) 10/11 (91) 0/8 (0) 0/4 (0) 3/5 (60) | 0/8 (0) 1/3 (33) 4/6 (67) 4/5 (80) 0/5 (0) 1/3 (33) 2/3 (67) 4/5 (80) 0/3 (0) 2/3 (67) |
Pancreatic cancer tissue Ki-67 proliferation index of pancreatic cancer tissue based on the histologic classification was 3.73±3.58, 6.62±4.39, 13.47±4.02, and 37.03±10.05 in normal, ductal hyperplasia, dysplasia, and cancer site respectively.
Chronic pancreatitis tissue Ki-67 proliferation index based on the histologic classification in chronic pancreatitis tissue was 2.35±2.79, 7.66±5.78, and 8.64±5.51 in normal, ductal hyperplasia, and dysplasia, respectively.
Results based on random histologic classification Ki-67 proliferation index based on random histologic classification was 2.35±2.79, 9.07±5.39, 10.79±5.51, and 37.03±10.05 in normal, hyperplasia, dysplasia, and cancer area, respectively.
In all 11 pancreatic cancer tissues, the expression of p53 protein was observed in all dysplasia (4/4), but not in all normal sites. The expression of p53 protein was also observed in 91% of cancer area that involved lesions. In 12 samples of all chronic pancreatitis tissues, the expression of p53 protein was negative in both normal and hyperplasia sites, whereas positive in 60% of dysplasia site. Overall, the expression of p53 protein was negative in all normal sites and pancreatic epithelial hyperplasia, whereas positive in 78% of dysplasia and 91% of cancer sites.
The positive reaction to K-ras protein seen in cytoplasm was observed in 33% of hyperplasia sites and 67% of dysplasia sites, but not in normal tissue in the pancreatic cancer patients. The positive staining to K-ras was in 80% of cancer tissue. The character and intensity in staining was not proportional to the differentiation of cancer. In chronic pancreatitis, K-ras was expressed in 67% of dysplasia, but not in normal sites. Overall, K-ras was expressed in 33% of pancreatic duct hyperplasia, 67% of dysplasia and 80% of cancerous sites, but no expression of K-ras could be detected in any normal sites.
It is generally accepted that three to seven accumulated mutations might be required for a normal cell to transform into a neoplastic cell[11]. A famous example of multistep development of malignancy has been reported in colorectal carcinoma[12], and similar multiple mutational steps seem to be associated in the development of pancreatic cancer[13]. Chronic pancreatitis is accepted as a precancerous lesion of pancreatic cancer, although a detailed mechanism regarding this is yet to be delineated. Several possible mechanisms have been proposed. There has been a study suggesting that ductal hyperplasia might play an important role[6,8]. Unfortunately, this hypothesis is hard to be tested since ductal hyperplasia could not be objectively determined on general means of pathological examination. Moreover, morphologically and functionally different kinds of pancreatic tissue tend to be mixed together which makes statistical analysis difficult. All these problems are partly due to the ambiguous pathologic terms and definitions; for example, the pancreatic duct hyperplasia, dysplasia, atypical ductal hyperplasia, intraductal tumor, carcinoma in situ, adenomatous hyperplasia, and atypical adenomatous hyperplasia[7,14].
In the present study, we only used the term, hyperplasia and dysplasia, since there are similar lesions, morphologically and cytologically, in other parts of the gastrointestinal tract. In order to objectively determine pancreatic duct hyperplasia, we used Ki-67 proliferation index. In evaluation of atypical hyperplasia, we used CEA immunohistochemical study.
The p53 gene is a tumor suppressor gene that involves regulation of the cell cycle and its mutation is most common in human cancers[15]. Mutated p53 in tumor cell accumulates nuclear p53 proteins and this overexpression is used as a marker for immunohistochemical staining[15,18]. It is reported that mutation of p53 has been reported to be detected in at least 50-70% of pancreatic adenocarcinoma[15-17]. In this study, p53 overexpression was most frequently shown in the cancerous lesion, rarely in the dysplastic cells and not at all in hyperplastic and normal cells. Therefore, it seems that the overexpression of p53 is implied to be the last phenomenon in the tumorigenesis. The fact that overexpression of p53 is shown more frequently in cancer tissues, regardless of the cell differentiation, suggests that it would be a useful marker in detecting activated dysplasia from well-differentiated cancer.
P21K-ras protein, synthesized by K-ras gene, accumulates in the cytoplasm[12], and found to be strongly stained in cytoplasm of most of the cancer cells[19]. The pancreas cancer originated from the exocrine gland showed 75-95% incidence of K-ras activation[20-24]. High incidence of K-ras mutation in pancreatic cancer implicates that this mutation might be a basic step of carcinogenesis. However, the earliest pancreatic lesion in which K-ras mutation can be found is not clearly known. As for the reports on the detection of K-ras mutation in the hyperplastic pancreatic duct of chronic pancreatitis patients, there have been some discrepancies. The current study immunohistochemically examined oncogene protein expressions in various pancreatic lesions. Although the presence of mutated gene needs to be confirmed at the molecular level, we found the possible continuity between pancreas diseases through protein expressions of oncogene. K-ras was strongly and frequently expressed in dysplastic and cancerous tissues compared with that of hyperplastic tissue. These results indicate that the overexpression of K-ras is associated with the pancreatic duct dysplasia in the carcinogenesis. Results in chronic pancreatitis were also similar to pancreatic cancer, suggesting that ductal dysplasia may be the precancerous lesion.
In our study, we found the possibility of the existence of stepwise mutation in the progression of advanced cancer from normal pancreatic cell. First, in the pancreatic cancer tissues, we found tissues presumed to be precancerous lesion, histologically different from normal epithelial cells. Second, this premalignant lesion was found more frequently in the tissues of pancreatic cancer compared with that of other disease states. Third, in the dysplastic and cancer tissues, the excessive expression of oncogene product was shown more frequently than in normal tissues. Fourth, K-ras protein overexpression developed in earlier stage of carcinogenesis, while the overexpression of p53 protein mostly occurred in advanced steps such as dysplasia or cancer. Finally, in chronic pancreatitis, some hyperplastic and dysplastic tissues showed the overexpression of oncogenic proteins similar to pancreatic cancer, although at low percentage. Therefore, chronic pancreatitis patients with protein overexpression of oncogene would have higher risk of cancer. Consequently, these results suggest that there is a correlation between the protein overexpression by genetic alteration and histological changes. Although we investigated mutation of only two important genes using low-sensitive immunohistochemical staining method, we believe that this study would be useful in the future researches on the pathogenesis of pancreatic cancer.
Sciencel Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Warshaw AL, Fernández-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1056] [Cited by in F6Publishing: 1002] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 2. | Boyle P, Hsieh CC, Maisonneuve P, La Vecchia C, Macfarlane GJ, Walker AM, Trichopoulos D. Epidemiology of pancreas cancer (1988). Int J Pancreatol. 1989;5:327-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 83] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Wingo PA, Tong T, Bolden S. Cancer statistics, 1995. CA Cancer J Clin. 1995;45:8-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 996] [Cited by in F6Publishing: 921] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 4. | Crist DW, Cameron JL. The current status of the Whipple operation for periampullary carcinoma. Adv Surg. 1992;25:21-49. [PubMed] [Cited in This Article: ] |
| 5. | Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP, Andrén-Sandberg A, Domellöf L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433-1437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1255] [Cited by in F6Publishing: 1106] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 6. | Cubilla AL, Fitzgerald PJ. Morphological lesions associated with human primary invasive nonendocrine pancreas cancer. Cancer Res. 1976;36:2690-2698. [PubMed] [Cited in This Article: ] |
| 7. | Kozuka S, Sassa R, Taki T, Masamoto K, Nagasawa S, Saga S, Hasegawa K, Takeuchi M. Relation of pancreatic duct hyperplasia to carcinoma. Cancer. 1979;43:1418-1428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 8. | SOMMERS SC, MURPHY SA, WARREN S. Pancreatic duct hyperplasia and cancer. Gastroenterology. 1954;27:629-640. [PubMed] [Cited in This Article: ] |
| 9. | Apple SK, Hecht JR, Lewin DN, Jahromi SA, Grody WW, Nieberg RK. Immunohistochemical evaluation of K-ras, p53, and HER-2/neu expression in hyperplastic, dysplastic, and carcinomatous lesions of the pancreas: evidence for multistep carcinogenesis. Hum Pathol. 1999;30:123-129 DOI : 10.1016/S0046-8177(99)90265-4. [Cited in This Article: ] |
| 10. | Kloppel G. Pancreatic Pathology. Edinburg, Scotland: Churchill Livingstone 1984; 79-113. [Cited in This Article: ] |
| 11. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8087] [Cited by in F6Publishing: 7708] [Article Influence: 226.7] [Reference Citation Analysis (1)] |
| 12. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4616] [Cited by in F6Publishing: 4368] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 13. | Chen J, Baithun SI, Ramsay MA. Histogenesis of pancreatic carcinomas: a study based on 248 cases. J Pathol. 1985;146:65-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Kloppel G. The pancreas: biology, pathobiology, and disease. 2nd ed. New York: Raven Press 1993; 871-898. [Cited in This Article: ] |
| 15. | Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855-4878. [PubMed] [Cited in This Article: ] |
| 16. | DiGiuseppe JA, Hruban RH, Goodman SN, Polak M, van den Berg FM, Allison DC, Cameron JL, Offerhaus GJ. Overexpression of p53 protein in adenocarcinoma of the pancreas. Am J Clin Pathol. 1994;101:684-688. [PubMed] [Cited in This Article: ] |
| 17. | Pellegata NS, Sessa F, Renault B, Bonato M, Leone BE, Solcia E, Ranzani GN. K-ras and p53 gene mutations in pancreatic cancer: ductal and nonductal tumors progress through different genetic lesions. Cancer Res. 1994;54:1556-1560. [PubMed] [Cited in This Article: ] |
| 18. | Yokoyama M, Yamanaka Y, Friess H, Buchler M, Korc M. p53 expression in human pancreatic cancer correlates with enhanced biological aggressiveness. Anticancer Res. 1994;14:2477-2483. [PubMed] [Cited in This Article: ] |
| 19. | Sakorafas GH, Lazaris A, Tsiotou AG, Koullias G, Glinatsis MT, Golematis BC. Oncogenes in cancer of the pancreas. Eur J Surg Oncol. 1995;21:251-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Berrozpe G, Schaeffer J, Peinado MA, Real FX, Perucho M. Comparative analysis of mutations in the p53 and K-ras genes in pancreatic cancer. Int J Cancer. 1994;58:185-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 175] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1497] [Cited by in F6Publishing: 1605] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 22. | Motojima K, Tsunoda T, Kanematsu T, Nagata Y, Urano T, Shiku H. Distinguishing pancreatic carcinoma from other periampullary carcinomas by analysis of mutations in the Kirsten-ras oncogene. Ann Surg. 1991;214:657-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 80] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Motojima K, Urano T, Nagata Y, Shiku H, Tsurifune T, Kanematsu T. Detection of point mutations in the Kirsten-ras oncogene provides evidence for the multicentricity of pancreatic carcinoma. Ann Surg. 1993;217:138-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 84] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Tabata T, Fujimori T, Maeda S, Yamamoto M, Saitoh Y. The role of Ras mutation in pancreatic cancer, precancerous lesions, and chronic pancreatitis. Int J Pancreatol. 1993;14:237-244. [PubMed] [Cited in This Article: ] |