Published online Nov 14, 2005. doi: 10.3748/wjg.v11.i42.6707
Revised: April 26, 2005
Accepted: April 30, 2005
Published online: November 14, 2005
AIM: To determine the in vivo and in vivo effects of cysteamine (CS) on expression and activity of H+-K+-ATPase of gastric mucosal cells in weaning piglets.
METHODS: Eighteen litters of newborn Xinhuai piglets were employed in the in vivo experiment and allocated to control and treatment groups. From 12 d of age (D12), piglets in control group were fed basal diet, while the treatment group received basal diet supplemented with 120 mg/kg CS. Piglets were weaned on D35 in both groups. Six piglets from each group (n = 6) were slaughtered on D28 (one week before weaning), D35 (weaning), D36.5, D38, D42, and D45 (36 h, 72 h, one week and 10 d after weaning), respectively. Semi-quantitative RT-PCR was performed to determine the levels of H+-K+-ATPase mRNA in gastric mucosa. H+-K+-ATPase activity in gastric mucosa homogenate was also determined. Gastric mucosal epithelial cells from piglets through primary cultures were used to further elucidate the effect of CS on expression and activity of H+-K+-ATPase in vivo. Cells were treated for 20 h with 0.001, 0.01, and 0.1 mg/mL of CS (n = 4), respectively. The mRNA expression of H+-K+-ATPase and somatostatin (SS) as well as the H+-K+-ATPase activity were determined.
RESULTS: in vivo, both mRNA expression and activity of H+-K+-ATPase in gastric mucosa of control group exhibited a trend to increase from D28 to D45, reaching a peak on D45, but did not show significant age differences. Furthermore, neither the mRNA expression nor the activity of H+-K+-ATPase was affected significantly by weaning. CS increased the mRNA expression of H+-K+-ATPase by 73%, 53%, 30% and 39% on D28 (P = 0.014), D35 (P = 0.017), D42 (P = 0.013) and D45 (P = 0.046), respectively. In accordance with the mRNA expression, H+-K+-ATPase activities were significantly higher in treatment group than in control group on D35 (P = 0.043) and D45 (P = 0.040). In vivo, CS exhibited a dose-dependent effect on mRNA expression and activity of H+-K+-ATPase. Both H+-K+-ATPase mRNA expression and activity in gastric mucosal epithelial cells were significantly elevated after 20 h of exposure to the moderate (H+-K+-ATPase expression: P=0.03; H+-K+-ATPase activity: P = 0.014) and high concentrations (H+-K+-ATPase expression: P=0.017; H+-K+-ATPase activity: P = 0.022) of CS. Significant increases in SS mRNA expression were observed to accompany the elevation of H+-K+-ATPase expression and activity induced by the moderate (P = 0.024) and high concentrations (P = 0.022) of CS. Low concentration of CS exerted no effects either on expression and activity of H+-K+-ATPase or on SS mRNA expression in cultured gastric mucosal epithelial cells.
CONCLUSION: No significant changes are observed in mRNA expression and activity of H+-K+-ATPase in gastric mucosa of piglets around weaning from D28 to D45. CS increases expression and activity of gastric H+-K+-ATPase in vivo and in vivo. SS is involved in mediating the effect of CS on gastric H+-K+-ATPase expression and activity in weaning piglets.
- Citation: Shi ZM, Du GM, Wei XH, Zhang L, Chen J, Zhao RQ. Cysteamine increases expression and activity of H+-K+-ATPase of gastric mucosal cells in weaning piglets. World J Gastroenterol 2005; 11(42): 6707-6712
- URL: https://www.wjgnet.com/1007-9327/full/v11/i42/6707.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i42.6707
The proton pump, H+-K+-ATPase consisting of α- and β- subunits, is the molecular base of gastric acid production and the final common pathway mediating secretion of hydrochloric acid by gastric parietal cells. The enzyme, which is typically located in the parietal cells, mediates the electroneutral exchange of intracellular H+ and extracellular K+ to achieve acid secretion when parietal cells are under the stimulation of secretagogues[1]. In H+-K+-ATPase α- or β-subunit deficiency mice, achlorhydria and destruction of parietal cells have been observed[2,3]. The capability of gastric acid secretion is dependent on the gastric H+-K+-ATPase activity[4]. Therefore, H+-K+-ATPase activity can serve as an accurate indicator for evaluating the ability of gastric acid secretion from parietal cells.
Gastric acid secretion is regulated by stimulatory factors such as gastrin, histamine and acetylcholine, as well as inhibitory factors including somatostatin (SS). SS is a typical brain-gut-peptide releasing from D cells in the mucous membrane of stomach. Numerous publications reported that SS inhibits gastric acid secretion directly or indirectly by inhibiting the stimulatory effects of gastrin and histamine[5,6]. We found in our previous study that gastric expression of SS mRNA is upregulated in weaning piglets[7], and that gastric acid secretion is low in piglets[8]. Therefore, it is presumed that the increased inhibitory tone of SS is responsible for retarded gastric function development and insufficient gastric acid secretion which contribute, atleast partly, to diarrhea, poor growth and even death in newborn and early-weaning piglets.
Cysteamine (CS), which is able to deplete tissue SS, induces a profound loss of biological and immunological activities of SS both in vivo and in vitro[9,10]. CS is known to increase gastric acid secretion in rats, and is often used to produce the clinical model of gastric ulcer[11-13]. CS is approved to use as a feed additive in animal production to promote growth rate and improve feed efficiency[14]. However, CS application in pig production is mostly restricted to growing and fattening stages[15]. Up to now, the possible effect of CS on gastric acid secretion in weaning piglets remains unknown. Therefore, the present study was designed to examine the effect of CS on gastric acid secretion both in vivo and in vitro, the mRNA expression and activity of H+-K+-ATPase in gastric mucosa tissue and cultured mucosal epithelial cells were determined as response criteria. In addition, the change of SS mRNA expression in mucosal epithelial cells responding to CS exposure was also measured for elucidating the possible mechanisms underlying the CS action.
Eighteen litters of newborn piglets from the 2nd or 3rd farrowing Xinhuai sows were employed in the in vivo experiment and allocated to control and treatment groups. From 12 d of age (D12), piglets in control group were fed basal diet, while the treatment group received basal diet supplemented with 120 mg/kg CS. The diet was formulated according to the requirement of piglets and provided ad libitum. Piglets were weaned on D35 in both groups. Six piglets from each group were slaughtered on D28 (one week before weaning), D35 (weaning), D36.5, D38, D42 and D45 (36 h, 72 h, one week and 10 d after weaning), respectively. Samples of the gastric fundic mucosa were frozen in liquid nitrogen immediately and then stored at -70 ºC until RNA extraction.
For in vitro experiment, four piglets at the age of D28 were killed to collect gastric mucosa for primary cell culture. DMEM (high glucose) and HEPES were products of Gibco, Hyclone, respectively. Trypsin was bought from Sigma and fetal bovine serum was purchased from Hangzhou Sijiqing Company, China.
Cells were dispersed from freshly obtained gastric mucosa of piglets as described previously[16], with minor modifications. Briefly, the gastric mucosa was washed in D-Hank’s solution containing 400 U/mL penicillin, 400 μg/mL streptomycin and dipped in D-Hank’s solution for 30 min. Then the tissues were dispersed by trypsin (0.15 mg/mL) at 37 °C for 1 h, filtrated and centrifuged (1 000 r/min, 5 min). Viability of the cells exceeded 95% as judged by trypan blue exclusion. Then cells at the density of 1×106/mL were cultured (37 °C, 50 mL/L CO2) in a six-well plate containing DMEM (high glucose) with 10% fetal bovine serum, 15 mmol/L HEPES buffer, and 100 U/mL penicillin, 100 μg/mL streptomycin. After 24 h, the culture medium was refreshed by a new medium containing 0, 0.001, 0.01 and 0.1 mg/mL CS, respectively. The cells were continuously cultured for 20 h, and then collected for RNA extraction and H+-K+-ATPase activity determination.
The experiments were undertaken following the guidelines of the regional Animal Ethics Committee.
Total RNA was extracted from the tissue samples with the single-step method of RNA extraction by acid guan-idinium thiocyanate-phenol-chloroform[17]. Total RNA concentration was then quantified by measuring the absorbance at 260 nm in a photometer (Eppendorf Biophotometer). Ratios of absorption (260/280 nm) of all preparations were between 1.8 and 2.0. Aliquots of RNA samples were subjected to electrophoresis through a 1.4% agarose-formaldehyde gel to verify their integrity.
Two micrograms of total RNA was reverse transcribed by incubation at 42 °C for 1 h in a 25 µL mixture consisting of 10 U avian myeloblastosis virus reverse transcriptase, 10 U RNase inhibitor, 12 µmol/L random primers, 50 mmol/L Tris-HCl (pH 8.3), 10 mmol/L MgCl2, 50 mmol/L KCl, 10 mmol/L DDT, 0.5 mmol/L spermidine and 0.8 mmol/L each dNTP. The reaction was terminated by heating at 95 °C for 5 min and quickly cooling on ice.
The primers for H+-K+-ATPase were designed according to the cDNA sequence published on GenBank (M22724): 5’-gagaaccaccacctacaag-3’ as sense primer, and 5’-caacagcgaactccaag-3’ as anti-sense primer, the predicted PCR product being 362 bp in size. The SS primers were designed according to the coding region of porcine SS genomic DNA sequence (GenBank, U36385): sense, 5’-agctgctgtctgaacccaac-3’ and anti-sense, 5’-gaaattcttgcagccagctt-3’, the expected PCR product being 161 bp in size. The PCR primers were designed using Primer Premier 5.0 and synthesized by Haojia Biotech. Ltd, China. The Quantum RNA 18S Internal Standards kit (catalogue no. 1716, Ambion Inc., Austin, TX, USA), containing primers and competitors, was used to normalize variations in pipetting and amplification.
Different controls were set to monitor the possible contaminations of genomic DNA and environment DNA both at the stage of RT and RCR. The pooled samples made by mixing equal quantity of total RNA from all samples were used for optimizing the PCR condition and normalizing the intra-assay variations. PCR conditions were established as follows: for H+-K+-ATPase, the total volume of reaction was 25 µL, including 0.5 U Taq DNA polymerase (Promega, Shanghai), 5 mmol/L Tris-HCl (pH 9.0), 10 mmol/L NaCl, 0.1 mmol/L DDT, 0.01 mmol/L EDTA, 5% (w/v) glycerol, 0.1% (w/v) Triton X-100, 0.2 mmol/L each dNTP, 1.5 mmol/L MgCl2, 0.7 μmol/L specific primers, 1.0–2.6 µL 18S rRNA. The program is set as: denaturation at 94 °C for 5 min, 20 cycles (for in vivo samples), 24 cycles (for in vitro samples) at 94 °C for 30 s, at 52 °C for 30 s, at 72 °C for 60 s, and a final extension at 72 °C for 8 min; for SS, the reaction mix contained 0.5 U Taq DNA polymerase, 5 mmol/L Tris-HCl (pH 9.0), 10 mmol/L NaCl, 0.1 mmol/L DDT, 0.01 mmol/L EDTA, 5% (w/v) glycerol, 0.1% (w/v) Triton X-100, 0.2 mmol/L each dNTP, 1.5 mmol/L MgCl2, 1.6 μmol/L specific primers, 1.0-2.6 µL 18S rRNA. The program is set as: denaturation at 94 °C for 5 min, 26 cycles at 94 °C for 30 s, at 54 °C for 30 s, at 72 °C for 30 s, and a final extension at 72 °C for 8 min. All samples were included in the same run of PCR on GeneAmp PCR system 9600 (Perkin Elmer, USA) and repeated atleast thrice.
Twenty microliters of PCR products was analyzed by 2% agarose gel electrophoresis. The gels were stained with ethidium bromide and photographed with a digital camera. The net intensities of individual bands were measured using Kodak Digital Science 1D software (Eastman Kodak Company, Rochester, NY, USA). The ratio of band density for target genes to that for 18S rRNA was used to represent the abundance of H+-K+-ATPase and SS mRNA expression.
H+-K+-ATPase activity was measured according to the method described by Hervatin et al[18]. H+-K+-ATPase activity was evaluated as the amount of inorganic phosphate released from ATP by the method of Sanui[19]. The reaction was initiated at 37 °C by addition of 2 mmol/L ATP-Mg2+ salt as substrate and proceeded in a total volume of 1.0 mL containing 60 mmol/L Tris-1,4-piperzine-bis (ethanesulfonic acid) (pH 7.4), 0.1 mmol/L ouabain, 90 mmol/L sucrose, 100 μL sample and either 15 mmol/L KCl or 30 mmol/L sucrose. It was terminated after 10 min by addition of 1.5 mL of ice-cold 14% trichloroacetic acid. Ouabain was included to avoid Na+-K+-ATPase activity. The ATPase activity measured without K+ was taken as the basal Mg2+-ATPase activity. The difference between the activities measured with and without K+ was defined as H+-K+-ATPase activity. Bradford[20] assay was employed to determine the tissue protein content and the activity of H+-K+-ATPase was expressed as the amount of inorganic phosphate released per milligram of protein per hour (μmol Pi/mg prot/h).
All data were expressed as mean±SE. The data were analyzed by t-test for independent samples or ANOVA with Statistical Packages for the Social Sciences (2000). P<0.05 was considered statistically significant.
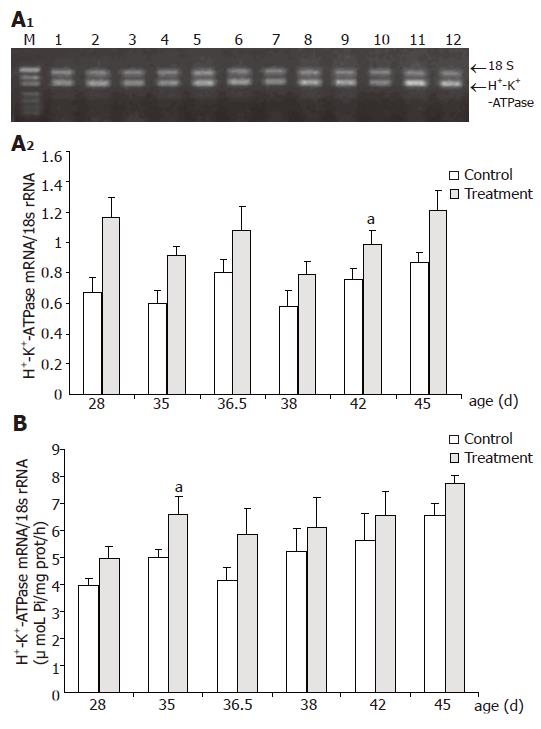
H+-K+-ATPase mRNA expression and H+-K+-ATPase activity in control group exhibited a trend to increase from D28 to D45, reaching a peak on D45, but did not show significant age differences (Figure 1). Furthermore, neither the mRNA expression nor the activity of H+-K+-ATPase was affected significantly by weaning.
As shown in Figure 1, CS increased the level of H+-K+-ATPase mRNA expression and the differences were significant on D28 (P = 0.014), D35 (P = 0.017), D42 (P = 0.013) and D45 (P = 0.046), respectively. Relative abundances of H+-K+-ATPase mRNA expression were significantly increased by 73%, 53%, 30%, and 39% in treatment group compared with control at the same age. CS supplementation increased markedly H+-K+-ATPase activity by 32.3% on D35 (P = 0.043) and 18.3% on D45 (P = 0.040), respectively, compared with that of the control counterparts.
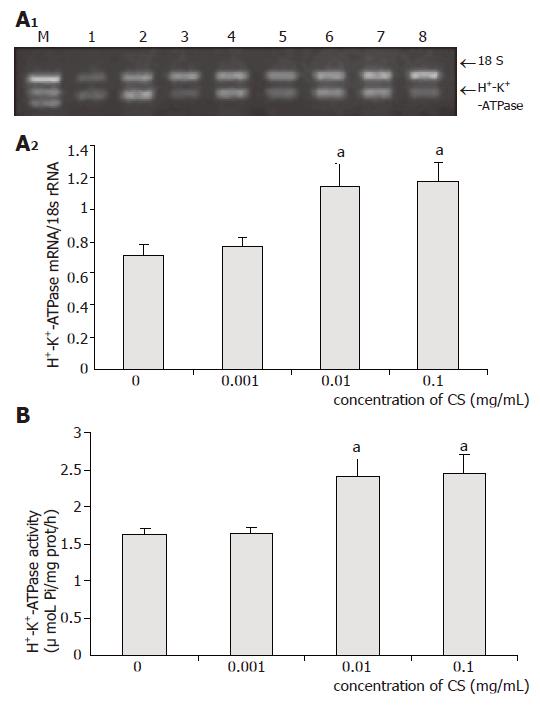
As shown in Figure 2, low concentration of CS exhibited no effects on both mRNA expression and activity of H+-K+-ATPase, while moderate and high concentrations of CS markedly increased H+-K+-ATPase mRNA expression by 61% (P = 0.03) and 65% (P = 0.014), and H+-K+-ATPase activity by 48% (P = 0.017) and 50% (P = 0.022), respectively.
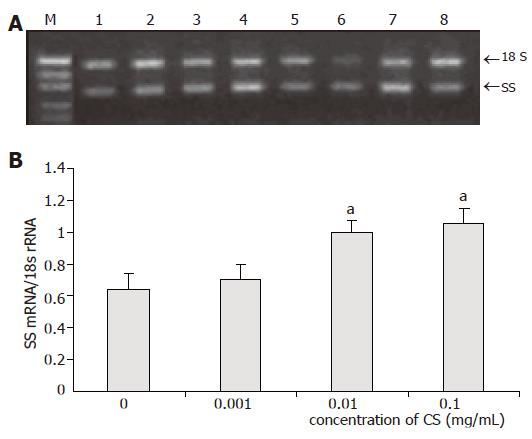
Low concentration of CS had no influence on SS mRNA expression, whereas SS mRNA expression was markedly increased by 56% (P = 0.024) in moderate concentrations of CS and 64% (P = 0.022) in high concentrations of CS (Figure 3).
The developmental pattern of H+-K+-ATPase was found to be in agreement with that of gastric acid secretion capacity, both at the level of mRNA expression and enzyme activity. Yang et al[21] reported that H+-K+-ATPase mRNA expression and activity in fundic gland keep increasing with age from gestational day 19.5 to week 18 of postnatal age in rats. The developmental pattern of human gastric H+-K+-ATPase from week 25 of gestation by Western blot analysis agrees with that of gastric pH recorded in preterm infants[22]. Furthermore, the H+-K+-ATPase mRNA content in rat fundus increases with age from one week to six weeks of age and the change parallels the developmental change of acid secretion capacity[23]. However, the developmental pattern of H+-K+-ATPase expression and activity in pigs has not been reported. In contrary to the reported data, the present study failed to show significant developmental change in both the expression and the activity of H+-K+-ATPase in piglets around weaning throughout the period of observation. It seems that the developmental pattern of H+-K+-ATPase is species-specific. In addition, it is documented that gastrin is involved in the regulation of H+-K+-ATPase expression. In ovine fetus, developmental pattern of gastrin mRNA agrees with that of H+-K+-ATPase expression[24]. Gastrin can stimulate H+-K+-ATPase expression[25] and improve its activity by increasing the intracellular Ca2+ concentration to initiate the phosphorization of H+-K+-ATPase in parietal cells[26]. In accordance with our findings, gastrin contents in gastric tissues are stable from 4 to 6 wk of age in piglets[27]. Since the period of observation was limited to less than 3 wk from D28 to D45 in the present study, the possibility that H+-K+-ATPase expression and activity subjected to change during growth in a longer term cannot be excluded.
Until now no report is available describing the effect of weaning stress on expression and activity of H+-K+-ATPase. Our results indicated that weaning stress exhibited no effect on expression and activity of H+-K+-ATPase, which might be attributed to the relatively mature age of weaning on D35. This explanation is supported by the study of Efird et al[28], who found that gastric acid secretion of piglets weaned after 35 d of age is not easily affected by weaning.
Our earlier publication reported that the inhibition of gastric acid secretion agrees with the upregulation of gastric SS expression in pre-weaning piglets[7]. Read et al[24] reported that the developmental pattern of gastric SS mRNA is on the contrary to the patterns of gastrin mRNA and H+-K+-ATPase mRNA in ovine. In vitro studies have provided further evidence that endogenous SS plays a role as a strong inhibitory factor in gastric acid secretion, since SS antiserum significantly increases gastric acid release from perfused stomach of sheep[29].
CS possesses the ability to deplete tissue SS. Szabo and Reichlin[9] found that, in rats, CS administration brings about a prompt depletion of radioimmunoassayable SS in plasma, stomach, duodenum, pancreas, and hypo-thalamus. Some researches showed that gastrin plays a significant role in CS-induced hypersecretion of gastric acid. Intravenous infusion of CS in the perfused rat stomach results in a significant increase in acid secretion, which is accompanied with a marked increase in plasma gastrin concentration. The injection of anti-gastrin rabbit serum completely blocks CS-induced acid increase, and infusion of a gastrin receptor antagonist also suppresses CS-induced increase in acid secretion[13]. Van de Brug et al[12] also found that intravenous bolus administration of CS induces increase in serum gastrin concentration and gastric acid outputs. However, there is no report concerning the effect of CS on expression and activity of H+-K+-ATPase. In the present study, we found that CS significantly increased both the expression and activity of H+-K+-ATPase in vivo. To further affirm the effect of CS on H+-K+-ATPase, we added CS to the culture medium of gastric mucosal epithelial cells in vitro. The results indicated that moderate and high concentrations of CS increased significantly both the expression and activity of H+-K+-ATPase, accompanied with a marked increase in the expression of SS. The upregulation of SS mRNA expression might be the consequence of SS depletion. The signal of SS depletion feeds back to the cells to boost SS synthesis in order to maintain homeostasis. Kanayama and Liddle[30] also found that the content of SS mRNA in duodenum is reduced after SS perfusion in rats. However, this upregulation might be temporal, since SS mRNA in stomach and brain significantly increases, then reduces after perfusion of CS in rats[31]. These authors presumed that SS mRNA upregulation results from SS depletion, and the subsequent SS mRNA downregulation is caused by direct effect of CS on SS expression.
In conclusion, the present experiments provide evidence that the mRNA expression and activity of H+-K+-ATPase in gastric mucosa remain relatively constant in piglets around weaning from D28 to D45. CS increases gastric expression and activity of H+-K+-ATPase both in vivo and in vitro. In addition, SS is involved in mediating the effect of CS on gastric H+-K+-ATPase expression and activity in weaning piglets, although the complex effect of CS on SS mRNA expression awaits further investigation.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Yao X, Forte JG. Cell biology of acid secretion by the parietal cell. Annu Rev Physiol. 2003;65:103-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 203] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | Spicer Z, Miller ML, Andringa A, Riddle TM, Duffy JJ, Doetschman T, Shull GE. Stomachs of mice lacking the gastric H,K-ATPase alpha -subunit have achlorhydria, abnormal parietal cells, and ciliated metaplasia. J Biol Chem. 2000;275:21555-21565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 145] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Scarff KL, Judd LM, Toh BH, Gleeson PA, Van Driel IR. Gastric H(+),K(+)-adenosine triphosphatase beta subunit is required for normal function, development, and membrane structure of mouse parietal cells. Gastroenterology. 1999;117:605-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Wallmark B, Larsson H, Humble L. The relationship between gastric acid secretion and gastric H+,K+-ATPase activity. J Biol Chem. 1985;260:13681-13684. [PubMed] [Cited in This Article: ] |
| 5. | Park J, Chiba T, Yamada T. Mechanisms for direct inhibition of canine gastric parietal cells by somatostatin. J Biol Chem. 1987;262:14190-14196. [PubMed] [Cited in This Article: ] |
| 6. | Zaki M, Harrington L, McCuen R, Coy DH, Arimura A, Schubert ML. Somatostatin receptor subtype 2 mediates inhibition of gastrin and histamine secretion from human, dog, and rat antrum. Gastroenterology. 1996;111:919-924. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Xia D, Zhao RQ, Wei XH, Xu QF, Chen J. Developmental patterns of GHr and SS mRNA expression in porcine gastric tissue. World J Gastroenterol. 2003;9:1058-1062. [PubMed] [Cited in This Article: ] |
| 8. | Xu RJ, Cranwell PD. Development of gastric acid secretion in pigs from birth to thirty six days of age: the response to pentagastrin. J Dev Physiol. 1990;13:315-326. [PubMed] [Cited in This Article: ] |
| 9. | Szabo S, Reichlin S. Somatostatin in rat tissues is depleted by cysteamine administration. Endocrinology. 1981;109:2255-2257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 180] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Widmann R, Sperk G. Cysteamine-induced decrease of somatostatin in rat brain synaptosomes in vitro. Endocrinology. 1987;121:1383-1389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Drago F, Montoneri C. Influence of growth hormone on cysteamine-induced gastro-duodenal lesions in rats: the involvement of somatostatin. Life Sci. 1997;61:21-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | van de Brug FJ, Jansen JB, Kuijpers IJ, Lamers CB. Contribution of gastrin to cysteamine-induced gastric acid secretion in rats. Life Sci. 1993;52:1861-1867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 13. | Shiratori K, Shimizu K, Ikeda M, Watanabe S, Hayashi N. Evidence for a significant role of gastrin in cysteamine-induced hypersecretion of gastric acid. J Clin Gastroenterol. 1997;25 Suppl 1:S84-S88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 14. | Wang C. Researches and applications of somatostatin and cysteamine. Shouyao Yu Siliao Tianjiaji. 2003;8:20-23. [Cited in This Article: ] |
| 15. | Chen AG, Wu LY, Hong QH. Effects of cysteamine on carcass characteristics of growing finishing pigs and approach to the mechanism. Zhongguo Xumu Zhazhi. 2004;40:11-13. [Cited in This Article: ] |
| 16. | Terano A, Ivey KJ, Stachura J, Sekhon S, Hosojima H, McKenzie WN, Krause WJ, Wyche JH. Cell culture of rat gastric fundic mucosa. Gastroenterology. 1982;83:1280-1291. [PubMed] [Cited in This Article: ] |
| 17. | Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40518] [Cited by in F6Publishing: 38838] [Article Influence: 1049.7] [Reference Citation Analysis (0)] |
| 18. | Hervatin F, Moreau E, Ducroc R, Garzon B, Avril P, Millet P, Geloso JP. Ontogeny of rat gastric H+-K+-ATPase activity. Am J Physiol. 1987;252:G28-G32. [PubMed] [Cited in This Article: ] |
| 19. | Sanui H. Measurement of inorganic orthophosphate in biological materials: extraction properties of butyl acetate. Anal Biochem. 1974;60:489-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 174] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173357] [Cited by in F6Publishing: 153329] [Article Influence: 3194.4] [Reference Citation Analysis (0)] |
| 21. | Yang DH, Tsuyama S, Murata F. The expression of gastric H+-K+-ATPase mRNA and protein in developing rat fundic gland. Histochem J. 2001;33:159-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Grahnquist L, Ruuska T, Finkel Y. Early development of human gastric H,K-adenosine triphosphatase. J Pediatr Gastroenterol Nutr. 2000;30:533-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Marino LR, Muglia BH, Yamada T. H(+)-K(+)-ATPase and carbonic anhydrase II gene expression in the developing rat fundus. Am J Physiol. 1990;259:G108-G115. [PubMed] [Cited in This Article: ] |
| 24. | Read MA, Chick P, Hardy KJ, Shulkes A. Ontogeny of gastrin, somatostatin, and the H+/K(+)-ATPase in the ovine fetus. Endocrinology. 1992;130:1688-1697. [PubMed] [Cited in This Article: ] |
| 25. | Campbell VW, Yamada T. Acid secretagogue-induced stimulation of gastric parietal cell gene expression. J Biol Chem. 1989;264:11381-11386. [PubMed] [Cited in This Article: ] |
| 26. | Geibel J, Abraham R, Modlin I, Sachs G. Gastrin-stimulated changes in Ca2+ concentration in parietal cells depends on adenosine 3',5'-cyclic monophosphate levels. Gastroenterology. 1995;109:1060-1067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Xu RJ, Cranwell PD. Gastrin in fetal and neonatal pigs. Comp Biochem Physiol B. 1991;98:615-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Efird RC, Armstrong WD, Herman DL. The development of digestive capacity in young pigs: effects of age and weaning system. J Anim Sci. 1982;55:1380-1387. [PubMed] [Cited in This Article: ] |
| 29. | Westbrook SL, McDowell GH, Hardy KJ, Shulkes A. Active immunization against somatostatin alters regulation of gastrin in response to gastric acid secretagogues. Am J Physiol. 1998;274:G751-G756. [PubMed] [Cited in This Article: ] |
| 30. | Kanayama S, Liddle RA. Somatostatin regulates duodenal cholecystokinin and somatostatin messenger RNA. Am J Physiol. 1990;258:G358-G364. [PubMed] [Cited in This Article: ] |
| 31. | Papachristou DN, Liu JL, Patel YC. Cysteamine-induced reduction in tissue somatostatin immunoreactivity is associated with alterations in somatostatin mRNA. Regul Pept. 1994;49:237-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |