Published online Nov 14, 2005. doi: 10.3748/wjg.v11.i42.6624
Revised: July 16, 2004
Accepted: July 19, 2004
Published online: November 14, 2005
AIM: To observe the imbalance between T helper cell Th1 and Th2 cytokines in several chronic hepatitis disease at different stages of disease progression.
METHODS: We measured the cytokine levels of Th1 (IL-2 and IL-2R), Th2 (IL-10) and the pro-inflammatory cytokines (IL-6 and IL-6R and TNF and TNF-RI and II) by the ELISA technique in the sera of 33 hepatocellular carcinoma (HCC) patients and 20 chronic liver disease (CLD) patients. In addition, 20 asymptomatic hepatitis C virus carriers and 20 healthy subjects negative for hepatitis C virus(HCV) markers served as controls.
RESULTS: Anti-HCV antibodies were found to be positive in 94% of HCC cases and 75% of CLD cases. On the other hand, HCV viremia was detected using RT-PCR in 67% of HCC cases and 65% of CLD cases. HBsAg was positive in 9% of HCC cases and 30% of CLD cases. Also bilharzial-Ab was positive in 55% of HCC cases, 65% of CLD cases and in 70% of asymptomatic carriers (ASC). HCC patients had significantly higher values of IL-2R, TNF-RII (P<0.001), and TNF-RI (P>0.05), but lower TNFα (P<0.001) and IL-6 (P = 0.032) in comparison to ASC. But, in comparison to non-cancer controls, HCC patients had higher values of IL-2R, IL-6R, TNF-RI and TNF-RII, but lower TNF-α (P<0.001). CLD patients had higher IL-2R, TNF-RI, and TNF-RII (P<0.001) than ASC. But, in comparison to non-cancer controls, CLD patients had higher values of IL-2R, TNF-RI and TNF-RII, but lower TNF-α (P<0.001). IL-10 was higher (though not significantly) in HCC and CLD patients than in symptomatic carriers and non-cancer controls.
CONCLUSION: Liver disease progression from CLD to HCC due to HCV genotype-4 infection is associated with an imbalance between Th1 and Th2 cytokines. IL-2R, TNF-RI, and TNF-RII could be used as potential markers.
- Citation: Zekri ARN, Ashour MSED, Hassan A, El-Din HMA, El-Shehaby AM, Abu-Shady MA. Cytokine profile in Egyptian hepatitis C virus genotype-4 in relation to liver disease progression. World J Gastroenterol 2005; 11(42): 6624-6630
- URL: https://www.wjgnet.com/1007-9327/full/v11/i42/6624.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i42.6624
Hepatitis C virus(HCV) is a common cause of hepa-tocellular injury that is associated with complex and vigorous immunologic mechanisms. Both humoral and cell-mediated immune responses participate in the host defense against HCV infection, but it is increasingly recognized that cellmediated response to the cytokine system plays a role in the im-munopathogenesis of chronic hepatitis C[1].
Cytokines constitute a complex network of molecules involved in the regulation of the inflammatory response and the homeostasis of organ functions. Moreover, cytokines coordinate physiologic and pathologic processes going on in the liver, such as liver growth and regeneration, inflammatory processes including viral liver disease, liver fibrosis and cirrhosis. Liver growth and regeneration are regulated by several cytokines. The cell-mediated immune response plays a central role in hepatocellular necrosis and in the immunopathogenic mechanisms involved in viral clearance and persistence in liver disease of viral etiology[2].
T lymphocytes and immunoregulatory cytokines are of critical importance in the host defense against HCV infection. T-helper type 1 (Th1) cytokines (interleukin-2 [IL-2], interferon-γ [IFN-γ]) are required for host anti-viral responses, while T-helper type 2 (Th2) cytokines (IL-4, IL-10) can inhibit the development of these effectors[3]. It has been demonstrated that pro-inflammatory IL-6 is able to influence hepatocarcinoma progression in patients with liver cancer[4]. Tumor necrosis factor (TNF) plays a role in the pathogenesis of chronic hepatitis C[5]. Also, chronic HCV infection is associated with an increase in the levels of soluble TNF receptors I and II[6].
Screening high-risk populations with ultrasonography and serum αFP levels produces diagnosis of only 40-60% of patients with hepatocellular carcinoma (HCC) at a stage where the tumor can be resected or treated with curative intent[7].
In this study, we aimed to characterize serum cytokine levels of IL-2 and its receptor (IL-2R), IL-6 and its re-ceptor (IL-6R), IL-10, TNF-α and its soluble receptors (TNF-RI and TNF-RII) by enzyme immunosorbent assay in HCC patients, chronic liver disease (CLD) patients, and HCV asymptomatic carriers (ASC), to figure out the possible imbalance between Th1 and Th2-like cytokines and their possible relation to hepatocarcinogenesis, as well as to evaluate the clinical significance of these cytokines in different stages of HCV infection, and their possible use as markers of disease progression, since there are no reliable markers for disease progression from CLD to HCC.
This study was conducted on 33 histologically proven HCC and 20 CLD patients (i.e., chronic active hepatitis with or without cirrhosis). All patients were presented before treatment to the specialized liver clinic of the National Cancer Institute (NCI), Cairo University, between April 2000 and June 2001. The study also included 40 control subjects: 20 asymptomatic carriers of HCV infection (ASC) as positive controls (positive for both HCV-AB and HCV RT-PCR), and 20 subjects without infection with HCV (NC) as negative controls (negative for HCV by both anti-HCV-Ab and HCV RT-PCR). The criteria for inclusion in the study groups were as follows: (a) ASC group: persistently normal alanine aminotransferase (ALT) values for 6 mo and no detectable liver changes by sonography except for a bright fatty liver, which is common in the Egyptian population. (b) CLD group: (1) persistent increase of the ALT values more than three times the normal value for atleast 6 mo; (2) exclusion of other causes of CLD such as alcoholism or hepatotoxic drugs; (3) histopathological examination of core needle biopsies. Accordingly, patients were classified into 8 mild, 7 moderate, and 5 severe cases of CLD. (c) HCC group: HCC neoplastic cells were identified histopathologically in H&E-stained sections of a core needle biopsy. Cases were classified into G1 (8 cases), G2 (22 cases) and G3 (3 cases). A detailed history and physical examination of the patients were carried out with special emphasis on history of bilharzias, prior parenteral therapy, infective hepatitis and jaundice or other signs of liver cell failure. Complete clinical examination, which includes the manifestations of hepatitis and liver cell failure such as jaundice, hepatomegaly, tenderness in the right hypochondrium, ascites, spleenomegaly, lower limb edema as well as abdominal ultrasonography was also done side by side with routine laboratory investigations including complete blood picture, liver and kidney function tests.
Sera collected from 5 mL of coagulated blood was aliquoted and stored at -80 °C until use. All the sera of patients and controls were tested for HCV antibody and HBsAg by the third-generation ELISA using kits from Innogenetics (Belgium) and the Equipar (Saronno, Italy). They were also tested for antibodies of Schistosomal infestation by quantitative indirect hemagglutination kits from Fumouze Laboratories (Paris, France). All tests were done according to the manufacturer’s instructions.
Nucleic acid extraction was done by QIAGEN viral RNA Mini-extraction kit (QIAGEN) using 140 μL of patient serum according to the manufacturer’s procedure.
RT and PCR were done as previously described by Zekri et al[8]. After completion of the amplification reaction, 10 μL of each PCR reaction product was analyzed by electrophoresis through an agarose 1.2% gel stained by ethidium bromide in Tris-acetate-EDTA buffer (pH 8.0) and DNA was transferred from the gel onto a nitrocellulose filter with alkaline buffer (4 N NaOH). The transferred DNA was cross-linked by incubation for 2-3 h at 80 °C and the blot was then hybridized with an internal probe[8].
The line immuno-probe assay was used to determine the HCV genotype as described previously[9] using INNO-LiPA II and III provided by Innogenetics (Belgium).
The following cytokines were assayed for all study groups using quantitative ELISA plate method: IL-2 (Quantikine, R&D Systems, Inc., Minneapolis, USA), soluble IL-2 receptor (sIL-2R) (Diaclone Research, France), IL-10 (Quantikine R&D Systems, Inc., Minneapolis, USA), IL-6 (Accucyte, Cytimmune Sciences Inc., MD, USA), soluble IL-6 receptor (sIL-6R) (Diaclone Research, France), tumor necrosis factor alpha (TNF-α) (Accucyte, Cytimmune Sciences Inc., MD, USA), as well as their soluble receptors (sTNF-RI, sTNF-RII; Immunotech, France). We considered the cut-off values for the studied cytokines as mean+2SD of the negative controls.
Liver core needle biopsies (atleast 10 mm long) from CAH and HCC patients who participated in the study were examined by two independent pathologists. Biopsy specimens were assessed for fibrosis (score 0-4) and activity (score 0-18) according to the scoring system of Knodell. Chronic hepatitis C was defined as mild, if the total score was 6, moderate, if the score was between 6 and 9, and severe, if the score was 9.
SPSS package (version 10) was used. Mean and standard deviation were estimates of quantitative data. Non-parametric t test (Mann-Whitney test) or non-parametric ANOVA (Kruskal-Wallis test) was used to compare means of more than two independent groups. Fisher’s exact and chi-square tests were used to validate the hypothesis of proportional independency. Correlation analysis was used to detect the association between quantitative data.
The clinical characteristics of the studied groups are shown in Table 1. HCC patients had significantly higher cirrhosis, irregular surface of liver, jaundice, serum AST and HCV-Ab positivity than in CLD cases (0.05, 0.007, 0.002, 0.006, and 0.05 respectively) and only HCC patients had significantly lower HBsAg than CLD patients. All the studied cases showed HCV genotype-4 by INNO-LiPA.
| Variables | HCC (n = 33) | CLD (n = 20) | ASC (n = 20) | NC (n = 20) | P |
| Age (mean±SD) | 55.3±10.1 | 63.3±8.3 | 34±7.7 | 31.7±11.8 | |
| Gender (M/F) | 27/6 | 14/6 | 14/6 | 12/8 | |
| Liver state: | |||||
| Cirrhosis | 30 (91%) | 14 (70%) | 0 | 0 | 0.05 |
| Irregular surface | 30 (91%) | 12 (60%) | 0 | 0 | 0.007 |
| Ascites | 13 (39.4%) | 11 (55%) | 0 | 0 | 0.27 |
| Jaundice | 27 (81.8%) | 8 (40%) | 0 | 0 | 0.002 |
| LL edema1 | 19 (57.6%) | 11 (55%) | 0 | 0 | 0.85 |
| Megaly | 33 (100%) | 19 (95%) | 0 | 0 | 0.38 |
| Spleenomegaly | 19 (57.6%) | 13 (65%) | 0 | 0 | 0.59 |
| Schistosomal-Ab | 18 (54.5%) | 13 (65%) | 14 (70%) | 1 (5%) | 0.45 |
| HCV-Ab | 31 (93.9%) | 15 (75%) | 20 (100%) | 1 (5%) | 0.05 |
| HBsAg | 3 (9%) | 6 (30%) | 4 (20%) | 0 | 0.05 |
| HCV-RNA | 22 (66.7%) | 14 (70%) | 20 (100%) | 0 | 0.8 |
| Liver function tests | |||||
| ALT (mean±SD) | 67.2±43.3 | 48±33.1 | 27.2±(7.0) | 25.3±(4.2) | 0.06 |
| AST (mean±SD) | 126.4±57.4 | 83.6±54.1 | 26.1±(5.0) | 19.1±(5.0) | 0.006 |
| Alk ph (mean±SD) | 260.4±193.4 | 221±126.7 | 70±26.1 | 65±24.2 | 0.414 |
| Bil (mean±SD) | 2.28±2.1 | 2.02±1.9 | 1.0±0.2 | 0.9±0.1 | 0.34 |
No significant difference was found in the history of bilharziasis, prior to the parenteral anti-bilharzial therapy, smoking, diabetes and hypertension in relation to the presence of HCV-Ab was found in HCC and CLD patients. HCV viremia by RT-PCR was positive in 22 of 31 (71%) HCV-Ab positive HCC cases, and in 11 of 15 (73%) HCV-Ab positive CLD cases.
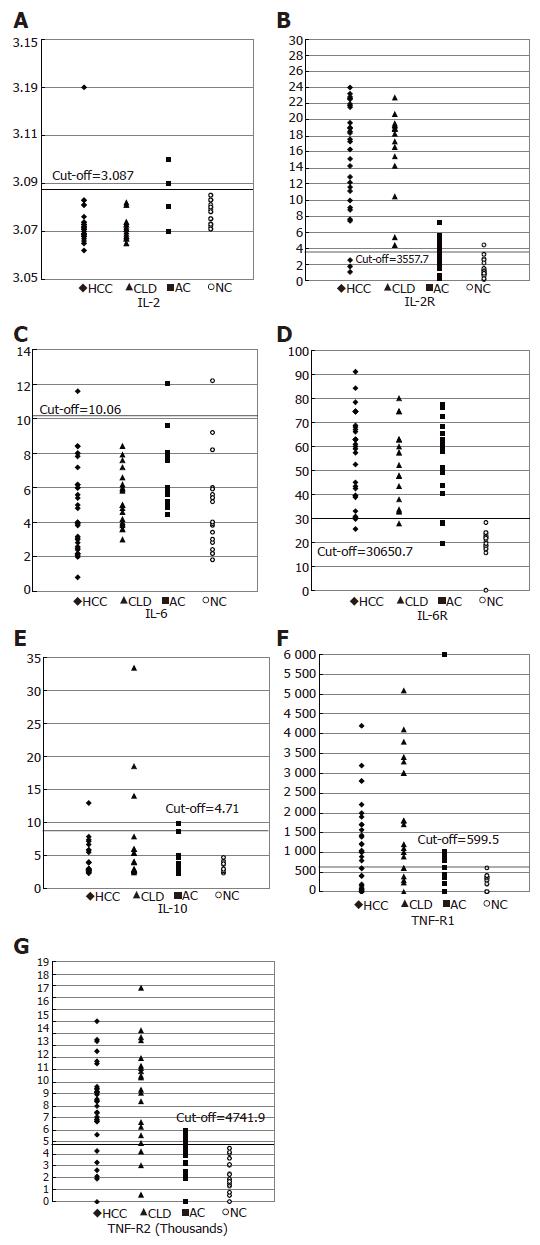
Scatter diagram representing the values of IL-2, IL-2R, IL-6, IL-6R, IL-10, TNF-α and their soluble receptors TNF-RI and TNF-RII in HCC, CLD cases, ASC and normal controls around the cut-off value are shown in Figures 1A-G.
Concentrations of IL-2R, IL-6R and IL-10 were higher in HCC patients than in other groups (16.7±5.4 ng/mL, 56.5±17.97 ng/mL, 9±26.07 pg/mL respectively). On the other hand, the mean concentrations of TNF-RI and TNF-RII were higher (1.87±1.5 and 9.160±0.4 ng/mL) in CLD patients than in other groups. Schistosomal-Ab and TNF-α were higher (1205±120 and 811±5.8 ng/mL) in asymptomatic HCV carriers than in other groups. IL-6R was significantly higher in HCC, CLD and ASC than NC group and there is a consistent increase in the IL-10 level with the disease progression from NC to HCC (Table 2).
| Cytokine | HCC (n = 33) | CLD(n = 20) | ASC(n = 20) | NC(n = 20) | P |
| IL-2 | 3.07±0.01 | 3.07±0.005 | 3.07±0.009 | 3.08±0.004 | >0.05 |
| Cut-off=3.087 pg/mL | |||||
| Number of positive cases | 1 (3%) | 0 | 6 (30%) | 0 | |
| IL-2R | 16.67±5.44 | 16.28±5.28 | 3.42±1.85 | 1.39±1.09 | <0.001 |
| Cut-off=3.5 ng/mL | |||||
| Number of positive cases | 30 (91%) | 16 (80%) | 9 (45%) | 1 (5%) | |
| IL-6 | 4.71±2.49 | 5.2±1.68 | 6.4±1.9 | 4.82±2.62 | 0.032 |
| Cut-off=10.06 ng/mL | |||||
| Number of positive cases | 1 (3%) | 0 | 1 (5%) | 1 (5%) | |
| IL-6R | 56.48±17.97 | 54.47±16.72 | 54.13±16.35 | 19.62±5.52 | <0.001 |
| Cut-off=30.65 ng/mL | |||||
| Number of positive cases | 29 (88%) | 19 (95%) | 17 (85%) | 0 | |
| IL-10 | 9±26.07 | 6.59±7.57 | 3.96±2.24 | 3.13±0.79 | >0.05 |
| Cut-off=4.71 pg/mL | |||||
| Number of positive cases | 9 (31%) | 8 (40%) | 3 (15%) | 0 | |
| TNF-α | 4.77±3.2 | 5.53±1.9 | 811±5.8 | 58±196 | <0.001 |
| Cut-off=17.2 ng/mL | |||||
| Number of positive cases | 0 | 0 | 20 (100%) | 1 (5%) | |
| TNF-α RI | 1.27±0.98 | 1.87±1.50 | 0.56±0.23 | 0.29±0.16 | <0.001 |
| Cut-off=0.599 ng/mL | |||||
| Number of positive cases | 24 (72%) | 16 (80%) | 7 (35%) | 1 (5%) | |
| TNF-α RII | 7.89±3.57 | 9.16±4.26 | 3.42±1.72 | 2.17±1.29 | <0.001 |
| Cut-off=4.74 ng/mL | |||||
| Number of positive cases | 25 (81%) | 17 (85%) | 4 (20%) | 0 |
In HCC group, positive HCV-RT-PCR cases had higher values of IL-2R, IL-6, IL-10 and TNF-RII (P = 0.689, P = 0.925, P = 0.636 and P = 0.05 respectively) than non-viremic cases, whereas positive HCV-RT-PCR in CLD cases had higher values of IL-6R, IL-10 and TNF-α (P = 0.28, P = 0.08 and P = 0.966) (results not shown).
No significant difference was noticed in any of the clinical characteristics of the patients with cytokine values above the cut-off, when compared to those below the cut-off in both HCC and CLD patients (results not shown). Scatter diagram representing the values of IL-1, IL-2R, IL-6, IL-6R, IL-10, TNF-RI and TNF-RII in HCC, CLD cases, ASC and non-cancer controls (NC) around the cut-off value are shown in Figures 1A-G. Mean serum cytokine levels in the different study groups are shown in Table 2. Regarding IL-2, no difference in its level was observed among the four groups. However, HCC patients had significantly higher values of IL-2R, TNFRII (P<0.001), and TNF RI (P>0.05), but lower TNF-α (P<0.001) and IL-6 (P = 0.032) in comparison to ASC. But, in comparison to non-cancer controls, HCC patients had higher values of IL-2R, IL-6R, TNF-RI and TNF-RII, but less TNF-α (P<0.001). CLD patients had higher IL-2R, TNF-RI and TNF-RII (P<0.001) than in ASC. But, in comparison to non-cancer controls, CLD patients had higher IL-2R, TNF-RI and TNF-RII, but lower TNF-α (P<0.001). IL-10 was higher (though not significantly) in HCC and CLD patients than in symptomatic carriers and non-cancer controls. No significant difference was noticed in any of the clinical characteristics of patients with cytokines values above the cut-off, when compared with those below the cut-off in both HCC and CLD patients.
The most sensitive cytokines as markers for disease progression in HCV-infected patients were IL-2R (91% of HCC and 80% of CLD patients had values above the cut-off), IL-6R (88%, 95%, and 85% of HCC, CLD and ASC respectively, had values above the cut-off) and also TNF-RII (81% of HCC and 85% of CLD cases had values above the cut-off).
It has been reported that hepatotropic viruses HBV, HDV, HCV are associated with HCC, and more than 80% of HCCs that occur worldwide are thought to be associated with chronic viral hepatitis[10]. In our series, the prevalence of anti-HCV antibodies by the third-generation ELISA was 93.9% in HCC patients. HCV-AB was positive in 86.5% of 37 HCC patients in our previous study[11]. However, this ratio is higher than those reported by other researchers[12,13]. The present study showed that the prevalence of anti-HCV antibodies was 75% in CLD patients, which is also higher than those reported by other researchers[14,15].
The HCV viremia in our HCV-serpositive HCC cases was also higher than those reported by other researchers[11,16,17]. This discrepancy might be explained by the fluctuations of the amount of the viruses in the serum[18]; or the discrepancy of RT-PCR methods used by the different series[8].
In our study, HBsAg was found in 9% of HCC cases, which is similar to that in our previous studies[11,12]. HBsAg was found in 30% of our CLD cases, which is higher than that reported by Angelico et al[14]. In the present study, history of bilharziasis and prior anti-bilharzial parenteral therapy was found in 51.6% and 41.9% of HCC patients and in 73.3% and 60% of CLD patients respectively, indicating that history of parenteral anti-schistosomal therapy is a major risk factor for transmitting HCV infection.
Anti-bilharzial antibodies were found in 55% of HCC patients in our study, which is higher than that reported in our previous studies[11]. This high incidence could be attributed to the fact that most of our HCC patients (66.7%) came from rural areas. Rural populations in both Egypt and other African areas have a higher incidence of viral hepatitis. In the rural areas, many individuals may become infected due to tattooing[19].
A characteristic feature of HCV infection is a high frequency of persistence and progression to CLD. Persistent infection upsets the balance between immunostimulatory and inhibitory cytokines, which can prolong inflammation and lead to necrosis, fibrosis, and CLD[1]. Elevated concentrations of cytokines also represent a characteristic feature of CLD, regardless of underlying etiology, which may represent a consequence of liver dysfunction instead of inflammatory disorder[20].
Missale et al[2] and Cacciarelli et al[3] found that serum IL-2 is significantly elevated in HCV chronically HCV-infected patients. However, Simsek and Kadayifci[21] found that serum IL-2 has no significant change in the same group of patients. In contrast, our study showed no significant change in the levels of IL-2 among the different groups. The high level of sIL-2R might explain the apparently normal level of IL-2 in our patients as low IL-2 level could therefore be due to soluble receptor binding to IL-2. This theory is also supported by Sismek and Kadayifci[21]. On the other hand, Izzo et al[22] found that serum levels of sIL-2R correlate with the histological severity of liver damage in patients with chronic HCV infection and may be used as a marker in patients at high risk of getting HCC and the highest levels of soluble IL-2R occur in patients with HCC[23]. We could not, however, correlate serum levels of sIL-2R with the different clinical and biochemical findings in HCC in this study.
Thus, sIL-2R may play a role in the pathogenesis of chronic active hepatitis and HCC, and could be considered as a good marker for disease progress in chronic HCV-infected patients. IL-2R was above normal in 91% of HCC and in 80% of CLD cases in our study.
Interleukin-6, a multifunctional cytokine produced by a variety of cells, plays a central role in regulating the immune system, hematopoiesis, and acute phase reaction. It interacts with a receptor complex consisting of a specific ligand-binding protein (IL-6R, gp80) and a signal transduction protein (gp130)[24,25]. Serum IL-6 levels are higher in patients with chronic HCV infection in comparison to healthy adults[26,27]. In contrast, our results showed that IL-6 was slightly higher only in asy-mptomatic HCV carriers than in non-cancer controls, but apparently normal in both HCC and CLD patients, which is in accordance with that reported by Tovey et al[28]. Altered IL-6 gene expression is a characteristic feature of advanced stage of severe liver disease. McGuinness et al[29] also found that IL-6 mRNA is down-regulated in chronic HCV-infected patients.
On the other hand, Steffen et al[30] revealed that IL-6 is inversely correlated with sIL-6R, as IL-6 may play a role in the decrease of sIL-6R either by the direct inhibitory effect on the expression of the IL-6R gene or by the formation of IL-6R/sIL-6R complexes followed by their internalization in target cells. This might partially explain the apparently normal levels of IL-6 in our HCC and CLD patients by the increase in their sIL-6R levels.
The pro-inflammatory cytokine tumor necrosis factor-α plays an important role in the pathophysiology of liver disease. Specific antagonists of this cytokine have been found in recent years. TNF soluble receptors p55 and p57 derived from the cell surface are naturally occurring substances that inhibit the biological effects of tumor necrosis factor.
The striking elevation of pro-inflammatory cytokine TNF-α in asymptomatic HCV carriers may reflect both insufficiency of HCV elimination and a failure to control the cytokine cascade. Our result is opposite to those reported by Goyal A[20] and Toyoda et al[31]. This could be attributed also to the difference in the epitopes of the ELISA system used by the different groups or to the difference in genotypes. All our cases showed HCV genotype-4.
The promoter in the IL-6 gene has been shown to contain regulatory regions to which DNA-binding proteins can bind. These DNA-binding proteins, induced by IL-1 and TNF-α, stimulate transcription of the IL-6 gene. Thus, as IL-1 and TNF-α levels increase, production of IL-6 also increases[32]. Our result is in accordance with the above findings, and the decrease in TNF-α is associated with a decrease in IL-6 in HCC and CLD cases.
In our study, HCC cases had significantly higher values of TNF-RII and TNF-RII (P<0.001) than ASC and NC subjects. Thus, the rise in concentrations of TNF receptors I and II in our patients suggests that HCV-related liver disease involves immunological mechanisms including activation of the TNF system and may reflect the degree of inflammation and development of HCC. These results are in accordance with other studies[33,34]. Tai et al[5] also showed that sTNF-RI levels correlate with liver inflammation in all patients, whereas this correlation cannot be found with sTNF-RII, IL-2, IL-10 and TNF-α.
Accordingly, we can use TNF-RII as a marker in HCV-infected cases at high risk of getting CLD and HCC.
Delpuech et al[35] showed that even if the infection of H9 T cell line with HCV does not result in any viral progeny, HCV induces the activation of IL-10 secretion, which supports the role of IL-10 in HCV pathogenesis. Cacciarelli et al[3] and Kakumu et al[33] found that serum IL-10 levels are significantly higher in all CLD groups than in controls, indicating that IL-10 reflects the degree of inflammation in the liver and may be related to the development of HCC. In our series, IL-10 levels were increased (though not significantly) in HCC, CLD in comparison to non-cancer controls. The positive HCV-viremic CLD cases also had higher values of IL-10 (P = 0.08) than non-viremic cases. In addition to the production at the site of inflammatory changes with activated infiltrating mononuclear cells in the liver, the high serum IL-10 levels in patients with HCC presumably also result from the secretion of IL-10 by tumor cells. Its secretion by human hepatocellular tumors has been observed previously[36]. Thus, a high IL-10 level is suggested to contribute to a relative state of immuno-suppression, and in patients with HCC, may help the tumor cells escape host immune surveillance and potentiate tumor cells to metastasize[37].
It has been postulated that an imbalance between Th1 and Th2 cytokine production is implicated in disease progression or inability to clear infections. It was reported that HCV-infected patients who develop chronicity have a predominant Th2 response, but a weak Th1 response, suggesting that this immune response imbalance can result from HCV interaction with dendritic cell functions[38]. These results agree with ours and support the notion that Th-lymphocyte polarization may play an important pathophysiologic role in influencing the outcome of HCV infection. All these immunological findings are mostly due to HCV infection rather than schistosomal infection, because patients with no schistosomal antibody had the same elevation of the same cytokines, late Schistosoma mansoni cases showed a suppressed cell-mediated immunity and a significant depletion of T-helper/inducer subset[39].
In conclusion, the most sensitive cytokines as markers for disease progression in HCV-infected patients are IL-2R, IL-6R and TNF-RII. Accordingly, we may use serum IL-2R, IL-6R, TNF-RII as markers in HCV-infected cases at high risk of getting CLD and HCC. Thus, disease progression due to HCV infection is associated with decrease of circulating Th1 cytokines (IL-2) and increase of Th2 cytokine (IL-10). Persistent infection upsets the balance between immunostimulatory and inhibitory cytokines, which can prolong inflammation and lead to necrosis, fibrosis, and CLD.
Science Editor Wang XL and Ma JY Language Editor Elsevier HK
| 1. | Jacobson Brown PM, Neuman MG. Immunopathogenesis of hepatitis C viral infection: Th1/Th2 responses and the role of cytokines. Clin Biochem. 2001;34:167-171. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Missale G, Ferrari C, Fiaccadori F. Cytokine mediators in acute inflammation and chronic course of viral hepatitis. Ann Ital Med Int. 1995;10:14-18. [PubMed] [Cited in This Article: ] |
| 3. | Cacciarelli TV, Martinez OM, Gish RG, Villanueva JC, Krams SM. Immunoregulatory cytokines in chronic hepatitis C virus infection: pre- and posttreatment with interferon alfa. Hepatology. 1996;24:6-9. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Malaguarnera M, Trovato BA, Laurino A, Di Fazio I, Romeo MA, Motta M. Interleukin-6 in hepatitis C cirrhosis. Panminerva Med. 1996;38:207-210. [PubMed] [Cited in This Article: ] |
| 5. | Tai DI, Tsai SL, Chen TC, Lo SK, Chang YH, Liaw YF. Modulation of tumor necrosis factor receptors 1 and 2 in chronic hepatitis B and C: the differences and implications in pathogenesis. J Biomed Sci. 2001;8:321-327. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Realdon S, Pontisso P, Adami F, Trentin L, Noventa F, Ferrari A, Migliorato I, Gatta A, Alberti A. High levels of soluble tumor necrosis factor superfamily receptors in patients with hepatitis C virus infection and lymphoproliferative disorders. J Hepatol. 2001;34:723-729. [DOI] [Cited in This Article: ] |
| 7. | Curely SA, Levin B, Rish TA. Liver and Bile ducts. In: Clinical oncology. Abdeloff, MD, Armitage, JO, Lichter, AS and Niederhuber, J E (eds.), Churchill livingstone, USA 1995; 1305-1372. [Cited in This Article: ] |
| 8. | Zekri AR, Bahnassy AA, Ramadan AS, El-Bassuoni M, Badran A, Madwar MA. Hepatitis C virus genotyping versus serotyping in Egyptian patients. Infection. 2001;29:24-26. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Zekri AR, Bahnassy AA, Shaarawy SM, Mansour OA, Maduar MA, Khaled HM, El-Ahmadi O. Hepatitis C virus genotyping in relation to neu-oncoprotein overexpression and the development of hepatocellular carcinoma. J Med Microbiol. 2000;49:89-95. [PubMed] [Cited in This Article: ] |
| 10. | Brooks GF, Butel JS, Morse SA. Hepatitis viruses. In: Medical microbiology. Brooks, GF, Butel, JS, and Morse, S A (eds.). Twenty-second edition, Lange medical books. McGraw-Hill, USA 2001; . [Cited in This Article: ] |
| 11. | Zekri AR, Sedkey L, el-Din HM, Abdel-Aziz AO, Viazov S. The pattern of transmission transfusion virus infection in Egyptian patients. Int J Infect Dis. 2002;6:329-331. [PubMed] [Cited in This Article: ] |
| 12. | Hassan MM, Zaghloul AS, El-Serag HB, Soliman O, Patt YZ, Chappell CL, Beasley RP, Hwang LY. The role of hepatitis C in hepatocellular carcinoma: a case control study among Egyptian patients. J Clin Gastroenterol. 2001;33:123-126. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Waked IA, Saleh SM, Moustafa MS, Raouf AA, Thomas DL, Strickland GT. High prevalence of hepatitis C in Egyptian patients with chronic liver disease. Gut. 1995;37:105-107. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Angelico M, Renganathan E, Gandin C, Fathy M, Profili MC, Refai W, De Santis A, Nagi A, Amin G, Capocaccia L. Chronic liver disease in the Alexandria governorate, Egypt: contribution of schistosomiasis and hepatitis virus infections. J Hepatol. 1997;26:236-243. [DOI] [Cited in This Article: ] |
| 15. | El-Medany OM, El-Din Abdel Wahab KS, Abu Shady EA, Gad El-Hak N. Chronic liver disease and hepatitis C virus in Egyptian patients. Hepatogastroenterology. 1999;46:1895-1903. [PubMed] [Cited in This Article: ] |
| 16. | Mabrouk GM. Prevalence of hepatitis C infection and schistosomiasis in Egyptian patients with hepatocellular carcinoma. Dis Markers. 1997;13:177-182. [PubMed] [Cited in This Article: ] |
| 17. | Yates SC, Hafez M, Beld M, Lukashov VV, Hassan Z, Carboni G, Khaled H, McMorrow M, Attia M, Goudsmit J. Hepatocellular carcinoma in Egyptians with and without a history of hepatitis B virus infection: association with hepatitis C virus (HCV) infection but not with (HCV) RNA level. Am J Trop Med Hyg. 1999;60:714-720. [PubMed] [Cited in This Article: ] |
| 18. | Bréchot C. Polymerase chain reaction for the diagnosis of hepatitis B and C viral hepatitis. J Hepatol. 1993;17 Suppl 3:S35-S41. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Attia MA. Prevalence of hepatitis B and C in Egypt and Africa. In: Therapies for viral hepatitis. Schinazi R F, Sommadossi J P and Thomas H C (eds.), International Medical Press. London, UK 1998; 15-24. [Cited in This Article: ] |
| 20. | Goyal A, Kazim SN, Sakhuja P, Malhotra V, Arora N, Sarin SK. Association of TNF-beta polymorphism with disease severity among patients infected with hepatitis C virus. J Med Virol. 2004;72:60-65. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Simsek H, Kadayifci A. Serum interleukin 2 and soluble interleukin 2 receptor in chronic active hepatitis C: effect of interferon therapy. J Int Med Res. 1996;24:239-245. [PubMed] [Cited in This Article: ] |
| 22. | Izzo F, Curley S, Maio P, Leonardi E, Imparato L, Giglio S, Cremona F, Castello G. Correlation of soluble interleukin-2 receptor levels with severity of chronic hepatitis C virus liver injury and development of hepatocellular cancer. Surgery. 1996;120:100-105. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Izzo F, Cremona F, Delrio P, Leonardi E, Castello G, Pignata S, Daniele B, Curley SA. Soluble interleukin-2 receptor levels in hepatocellular cancer: a more sensitive marker than alfa fetoprotein. Ann Surg Oncol. 1999;6:178-185. [DOI] [Cited in This Article: ] |
| 24. | Blum AM, Metwali A, Elliott D, Li J, Sandor M, Weinstock JV. IL-6-deficient mice form granulomas in murine schistosomiasis that exhibit an altered B cell response. Cell Immunol. 1998;188:64-72. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Giannitrapani L, Cervello M, Soresi M, Notarbartolo M, La Rosa M, Virruso L, D'Alessandro N, Montalto G. Circulating IL-6 and sIL-6R in patients with hepatocellular carcinoma. Ann N Y Acad Sci. 2002;963:46-52. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Malaguarnera M, Di Fazio I, Romeo MA, Restuccia S, Laurino A, Trovato BA. Elevation of interleukin 6 levels in patients with chronic hepatitis due to hepatitis C virus. J Gastroenterol. 1997;32:211-215. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Oyanagi Y, Takahashi T, Matsui S, Takahashi S, Boku S, Takahashi K, Furukawa K, Arai F, Asakura H. Enhanced expression of interleukin-6 in chronic hepatitis C. Liver. 1999;19:464-472. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Tovey MG, Gugenheim J, Guymarho J, Blanchard B, Vanden Broecke C, Gresser I, Bismuth H, Reynes M. Genes for interleukin-1, interleukin-6, and tumor necrosis factor are expressed at markedly reduced levels in the livers of patients with severe liver disease. Autoimmunity. 1991;10:297-310. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | McGuinness PH, Painter D, Davies S, McCaughan GW. Increases in intrahepatic CD68 positive cells, MAC387 positive cells, and proinflammatory cytokines (particularly interleukin 18) in chronic hepatitis C infection. Gut. 2000;46:260-269. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Steffen M, Pichlmeier U, Zander A. Inverse correlation of interleukin-6 with soluble interleukin-6 receptor after transplantation of bone marrow or peripheral blood stem cells. Bone Marrow Transplant. 1997;20:715-720. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Toyoda M, Kakizaki S, Horiguchi N, Sato K, Takayama H, Takagi H, Nagamine T, Mori M. Role of serum soluble Fas/soluble Fas ligand and TNF-alpha on response to interferon-alpha therapy in chronic hepatitis C. Liver. 2000;20:305-311. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1-78. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Kakumu S, Okumura A, Ishikawa T, Yano M, Enomoto A, Nishimura H, Yoshioka K, Yoshika Y. Serum levels of IL-10, IL-15 and soluble tumour necrosis factor-alpha (TNF-alpha) receptors in type C chronic liver disease. Clin Exp Immunol. 1997;109:458-463. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Zylberberg H, Rimaniol AC, Pol S, Masson A, De Groote D, Berthelot P, Bach JF, Bréchot C, Zavala F. Soluble tumor necrosis factor receptors in chronic hepatitis C: a correlation with histological fibrosis and activity. J Hepatol. 1999;30:185-191. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Delpuech O, Buffello-Le Guillou DB, Rubinstein E, Féray C, Petit MA. The hepatitis C virus (HCV) induces a long-term increase in interleukin-10 production by human CD4+ T cells (H9). Eur Cytokine Netw. 2001;12:69-77. [PubMed] [Cited in This Article: ] |
| 36. | Matsuguchi T, Okamura S, Kawasaki C, Niho Y. Production of interleukin 6 from human liver cell lines: production of interleukin 6 is not concurrent with the production of alpha-fetoprotein. Cancer Res. 1990;50:7457-7459. [PubMed] [Cited in This Article: ] |
| 37. | Chau GY, Wu CW, Lui WY, Chang TJ, Kao HL, Wu LH, King KL, Loong CC, Hsia CY, Chi CW. Serum interleukin-10 but not interleukin-6 is related to clinical outcome in patients with resectable hepatocellular carcinoma. Ann Surg. 2000;231:552-558. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Stoll-Keller F, Schvoerer E, Thumann C, Navas MC, Aubertin AM. Immunomodulating effect of HCV during the development of chronic hepatitis C: toward new therapeutic approaches. Bull Acad Natl Med. 2003;187:1147-1160; discussion 1147-1160;. [PubMed] [Cited in This Article: ] |
| 39. | Elrefaei M, El-Sheikh N, Kamal K, Cao H. HCV-specific CD27- CD28- memory T cells are depleted in hepatitis C virus and Schistosoma mansoni co-infection. Immunology. 2003;110:513-518. [PubMed] [DOI] [Cited in This Article: ] |