Published online Aug 14, 2005. doi: 10.3748/wjg.v11.i30.4758
Revised: January 23, 2005
Accepted: January 26, 2005
Published online: August 14, 2005
AIM: NONI juice (Morinda citrifolia) is an increasingly popular wellness drink claimed to be beneficial for many illnesses. No overt toxicity has been reported to date. We present two cases of novel hepatotoxicity of NONI juice. Causality of liver injury by NONI juice was asses-sed. Routine laboratory tests and transjugular or percutaneous liver biopsy were performed. The first patient underwent successful liver transplantation while the second patient recovered spontaneously after cessation of NONI juice. A 29-year-old man with previous toxic hepatitis associated with small doses of paracetamol developed sub-acute hepatic failure following consumption of 1.5 L NONI juice over 3 wk necessitating urgent liver transplantation. A 62-year-old woman without evidence of previous liver disease developed an episode of self-limited acute hepatitis following consumption of 2 L NONI juice for over 3 mo. The most likely hepatotoxic components of Morinda citrifolia were anthraquinones. Physicians should be aware of potential hepatotoxicity of NONI juice.
- Citation: Stadlbauer V, Fickert P, Lackner C, Schmerlaib J, Krisper P, Trauner M, Stauber RE. Hepatotoxicity of NONI juice: Report of two cases. World J Gastroenterol 2005; 11(30): 4758-4760
- URL: https://www.wjgnet.com/1007-9327/full/v11/i30/4758.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i30.4758
Due to the known side effects of approved pharmaceuticals, patients often turn to alternative medicine which is considered “natural” and “healthy”. Herbal medicine is thus gaining popularity, but lack of knowledge of the mechanisms and side effects of these preparations as well of safety regulations for their preparation may have serious consequences[1]. Reports of hepatotoxicity associated with the use of herbal medicines are accumulating, ranging from mild elevations of liver enzymes to fulminant liver failure requiring liver transplantation[2]. Healthcare professionals should be made aware of the potential dangers of herbal preparations. We present two cases of acute drug-induced hepatitis associated with the consumption of NONI juice.
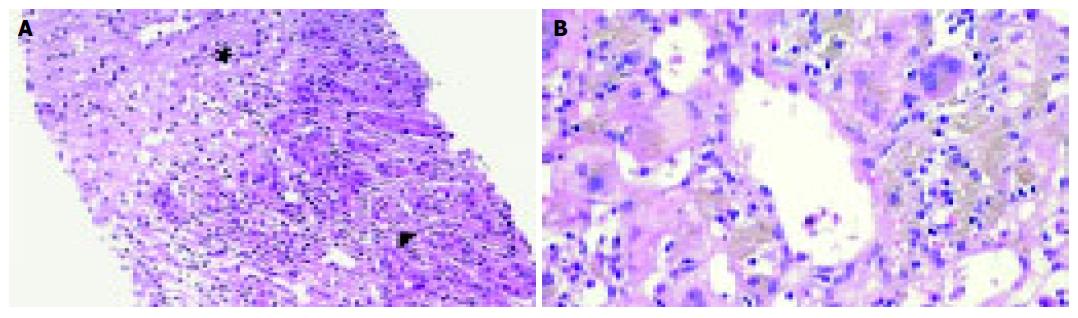
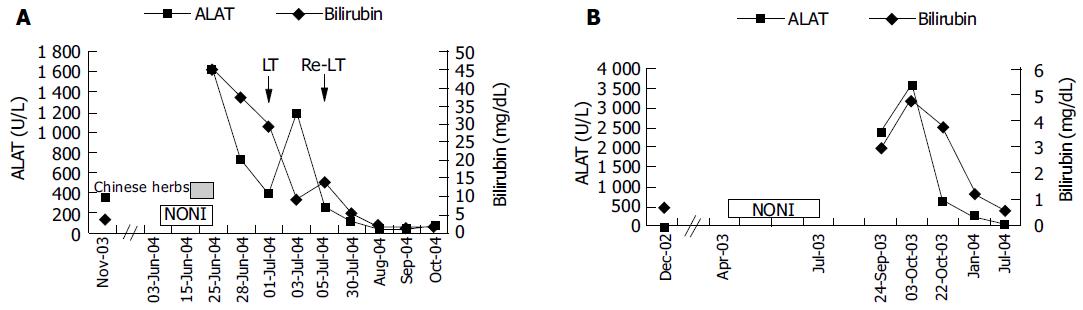
A 29-year-old male patient presented in March 2003 with acute hepatitis following treatment of an upper respiratory tract infection with paracetamol (4 g daily for 5 d followed by lower doses over 4 wk, cumulative dose of 40 g) associated with reduced food intake. Laboratory abnormalities included a bilirubin concentration of 26.3 mg/dL (normal: 0.1-1.2 mg/dL), ASAT 2 926 U/L (normal: < 35 U/L), ALAT 2 665 U/L (normal: < 45 U/L), γ-glutamyl transpeptidase 75 U/L (normal: < 55 U/L), and alkaline phosphatase 209 U/L (normal: 40-130 U/L). Besides, the patient had allergic asthma treated with inhalative β2-agonists and glucocorticoids and eosinophilia of up to 12% in his peripheral blood smear. During military service at the age of 18, liver function tests were normal except for bilirubin (1.8 mg/dL) attributed to Gilbert’s syndrome. Viral hepatitis, autoimmune hepatitis, hemochromatosis, α1-antitrypsin deficiency, Wilson’s disease as well as other infections (leptospirosis, toxoplasmosis, coxsackie, and brucellosis) were ruled out and his abnormal liver tests were interpreted as low-dose paracetamol toxicity facilitated by fasting. Three months later, aminotransferase and bilirubin levels improved (ASAT 222, ALAT 300 U/L, bilirubin 4.8 mg/dL) and the patient was lost to follow-up. From April 2003 until June 2004, he treated himself with various homeopathic preparations containing small amounts of ethanol (< 1 g/d). In June 2004 he was readmitted with acute liver failure. During the 3 wk prior to readmission, he ingested 1.5 L Tahitian NONI juice and for 9 d prior to admission, approximately 7 g/d of a Chinese herbal mix containing bupleuri, pinellia, scutellaria, codonopsis, glycyrrhizae, schizonepeta, and paeonia. He denied any other medication over the past year. Other etiologies for acute liver failure such as fulminant hepatitis A or B, acute Wilson’s disease, mushroom poisoning, and Budd-Chiari syndrome were ruled out. On re-admission, he showed grade 1 hepatic encephalopathy, ASAT 1 557, ALAT 1 626 U/L, bilirubin 45.3 mg/dL, INR 1.4 (normal: 0.85-1.15), albumin 3.3 g/dL (normal: 3.5-5.3 g/dL), and ammonia 90 μmol/L (normal: 15-55 μmol/L). A transjugular liver biopsy was performed and revealed acute hepatitis consistent with an idiosyncratic drug reaction (Figure 1A). Over the next 3 d, liver failure progressed rapidly. On d 4, the patient fulfilled King’s College criteria for emergency liver transplantation and was listed for urgent liver transplantation. During the waiting time, he underwent two consecutive sessions of extra-corporeal liver support using fractionated plasma separation, adsorption and dialysis (FPAD, PrometheusTM, Fresenius Medical Care, Bad Homburg, Germany) which resulted in temporary improvement of hepatic encephalopathy from grade 3 to grade 2 and a decrease of arterial ammonia levels from 144 to 97 μmol/L. On d 6, liver transplantation was performed. On d 11, hepatic artery thrombosis necessitated re-transplantation. Thereafter, the patient recovered completely and was discharged on d 30.
A 62-year-old woman was admitted in September 2003 with vomiting and diarrhea. On admission, routine laboratory tests revealed acute hepatitis (ASAT 1 415, ALAT 2 381 U/L, γ-glutamyl transpeptidase 241, alkaline phosphatase 292 U/L, bilirubin 2.9 mg/dL). In 1999, she was diagnosed with chronic B-cell leukemia which was treated with fludarabine in 2002 resulting in remission. Throughout that time, liver function tests were normal and the patient did not take any other regular medication. From April to July 2003, she ingested 2 L of Tahitian NONI juice. Viral hepatitis, auto-immune hepatitis, hemochromatosis, α1-antitrypsin deficiency, and Wilson’s disease were ruled out. There was no evidence of biliary obstruction or Budd-Chiari syndrome on ultrasound. The patient did not take any other potentially hepatotoxic drugs during that period. A percutaneous liver biopsy revealed acute hepatitis consistent with an idiosyncratic drug reaction (Figure 1B). Laboratory values peaked at ASAT 2 022, ALAT 3 570 U/L, and bilirubin 4.9 mg/dL and gradually improved over the next 30 d (ASAT 194, ALAT 645 U/L, and bilirubin 3.9 mg/dL). Nine months later, aminotransferases and bilirubin normalized and the patient remained asymptomatic.
We present two cases of toxic hepatitis associated with NONI juice (Morinda citrifolia) which represent the first reports of hepatotoxicity of this herbal preparation. To our knowledge, only one further case of NONI hepatotoxicity observed at the Medical University Innsbruck, Austria, has been published to date[3]. Case 1 developed fulminant liver failure requiring emergency liver transplantation while liver function was preserved in case 2 and the patient recovered spontaneously after cessation of NONI juice. The temporal relationship between NONI intake and liver dysfunction (Figures 2A and B) and extensive exclusion of alternative causes of acute hepatitis confirmed herbal hepatotoxicity in both cases. Causality of liver injury by NONI juice was assessed using the Council for International Organizations of Medical Sciences scale[4] and yielded a score of +5 (“possible”) in case 1 and a score of +7 (“probable”) in case 2. Histology revealed acute hepatitis with centrolobular necrosis consistent with acute drug-induced hepatotoxicity of idiosyncratic type in both cases (Figures 1A and B). However, we could not exclude concomitant pre-existing liver damage by paracetamol and/ or an additional hepatotoxic effect of components of the Chinese herbal mix in case 1.
NONI juice is prepared from the fruit of Morinda citrifolia, a Polynesian plant with a long history of medicinal applications[5]. Since 1996, various preparations of NONI juice have been sold as wellness drinks. Short-term safety studies in rats have not yielded overt toxicity[5]. One case of hyperkalemia due to self medication with NONI juice is reported in a patient with renal failure[6]. So far, no detailed pharmacological studies were reported on the putative active ingredients of the NONI plant, which include scopoletin, including scopoletin, potassium, ascorbic acid, terpenoids, alkaloids, anthraquinones, β-sitosterol, carotene, vitamin A, flavone glycosides, linoleic acid, alizarin, amino acids, acubin, L-asperuloside, caproic acid, caprylic acid, ursolic acid, rutin, and several volatile components such as acids (mainly octanoic acid and hexanoic acid), alcohols, esters, ketones, and lactones[5,7]. The most likely hepatotoxic components are anthraquinones such as nordamnacanthal, moridone, and rubiadin. Anthraquinones in other herbal remedies have been reported to cause hepatotoxicity[8-12]. A possible mechanism of hepatotoxicity of anthraquinones has been described for rhein (4,5-dihydroxyanthraquinone-2-carboxylic acid) which produces oxygen-derived free radicals by redox cycling resulting in depletion of intracellular reduced glutathione, decreased mitochondrial membrane potential, initiation of lipid peroxidation, and cell death[13]. Further studies are required to evaluate the exact mechanism of toxicity. Physicians should be aware of potentially serious hepatotoxicity of NONI juice.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Boullata JI, Nace AM. Safety issues with herbal medicine. Pharmacotherapy. 2000;20:257-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Stedman C. Herbal hepatotoxicity. Semin Liver Dis. 2002;22:195-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 158] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Millonig G, Stadlmann S, Vogel W. Herbal hepatotoxicity: acute hepatitis caused by a Noni preparation (Morinda citrifolia). Eur J Gastroenterol Hepatol. 2005;17:445-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323-1330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1015] [Cited by in F6Publishing: 1004] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 5. | Wang MY, West BJ, Jensen CJ, Nowicki D, Su C, Palu AK, Anderson G. Morinda citrifolia (Noni): a literature review and recent advances in Noni research. Acta Pharmacol Sin. 2002;23:1127-1141. [PubMed] [Cited in This Article: ] |
| 6. | Mueller BA, Scott MK, Sowinski KM, Prag KA. Noni juice (Morinda citrifolia): hidden potential for hyperkalemia? Am J Kidney Dis. 2000;35:310-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Farine JP, Legal L, Moreteau B, Le Quere JL. Volatile compo-nents of ripe fruits of morinda citrifolia and their effects on drosophila. Phytochemistry. 1996;41:433-438. [DOI] [Cited in This Article: ] |
| 8. | Li FK, Lai CK, Poon WT, Chan AY, Chan KW, Tse KC, Chan TM, Lai KN. Aggravation of non-steroidal anti-inflammatory drug-induced hepatitis and acute renal failure by slimming drug containing anthraquinones. Nephrol Dial Transplant. 2004;19:1916-1917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Park GJ, Mann SP, Ngu MC. Acute hepatitis induced by Shou-Wu-Pian, a herbal product derived from Polygonum multiflorum. J Gastroenterol Hepatol. 2001;16:115-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Nadir A, Reddy D, Van Thiel DH. Cascara sagrada-induced intrahepatic cholestasis causing portal hypertension: case report and review of herbal hepatotoxicity. Am J Gastroenterol. 2000;95:3634-3637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Beuers U, Spengler U, Pape GR. Hepatitis after chronic abuse of senna. Lancet. 1991;337:372-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 92] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Itoh S, Marutani K, Nishijima T, Matsuo S, Itabashi M. Liver injuries induced by herbal medicine, syo-saiko-to (xiao-chai-hu-tang). Dig Dis Sci. 1995;40:1845-1848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 99] [Article Influence: 3.4] [Reference Citation Analysis (5)] |
| 13. | Bironaite D, Ollinger K. The hepatotoxicity of rhein involves impairment of mitochondrial functions. Chem Biol Interact. 1997;103:35-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |