Published online Jul 7, 2005. doi: 10.3748/wjg.v11.i25.3962
Revised: September 15, 2004
Accepted: September 19, 2004
Published online: July 7, 2005
AIM: To study the expression of interferon-alpha/beta (IFN-α/β) receptor protein in liver of patients with hepatitis C virus (HCV)-related chronic liver disease and its clinical significance.
METHODS: A total of 181 patients with HCV-related chronic liver disease included 56 with HCV-related liver cirrhosis (LC) and 125 with chronic hepatitis C (CHC). CHC patients were treated with five megaunits of interferon-α1b six times weekly for the first 2 weeks and then every other day for 22 wk. The patients were divided into interferon (IFN) treatment-responsive and non-responsive groups, but 36 patients lost follow-up shortly after receiving the treatment. The expression of IFN-α/β receptor (IFN-α/βR) protein in liver of all patients was determined with immunofluorescence.
RESULTS: In liver of patients with HCV-related chronic liver disease, the expression of IFN-α/βR protein in liver cell membrane was stronger than that in cytoplasm and more obvious in the surroundings of portal vein than in the surroundings of central vein. Moreover, it was poorly distributed in hepatic lobules. The weak positive, positive and strong positive expression of IFN-α/βR were 40% (50/125), 28% (35/125), 32% (40/125), respectively in CHC group, and 91.1% (51/56), 5.35% (3/56), and 3.56% (2/56), respectively in LC group. The positive and strong positive rates were higher in CHC group than in LC group (P < 0.01). In IFN treatment responsive group, 27.8% (10/36) showed weak positive expression; 72.2% (26/36) showed positive or strong positive expression. In the non-responsive group, 71.7% (38/53) showed weak positive expression; 28.3% (15/53) showed positive or strong positive expression. The expression of IFN-α/βR protein in liver was more obvious in IFN treatment responsive group than in non-responsive group.
CONCLUSION: Expression of IFN-α/βR protein in liver of patients with HCV-related chronic liver disease is likely involved in the response to IFN treatment.
- Citation: Meng XW, Chi BR, Chen LG, Zhang LL, Zhuang Y, Huang HY, Sun X. Expression of interferon-alpha/beta receptor protein in liver of patients with hepatitis C virus-related chronic liver disease. World J Gastroenterol 2005; 11(25): 3962-3965
- URL: https://www.wjgnet.com/1007-9327/full/v11/i25/3962.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i25.3962
Interferon (IFN) is effective in the treatment of chronic hepatitis C. It results in viral eradication and normalization of liver function in about 35%-40% of the patients[1-4]. The anti-virus mechanism of IFN is to transmit the signal to nuclei and to activate 2’-5’ adenylic acid synthase and protein kinase after IFN molecule combines with IFN receptor, which then blocks the translations of virus protein and RNA. IFN receptor (IFN-R) is the initial protein for the chain reaction of IFN[5]. Human interferon receptors are divided into type I IFN-R which can combine with IFN-α/β, and type II IFN-R which has specific sensitivity to IFN-γ. Furthermore, it has been proved that type II IFN-R is also sensitive to IFN-α/β to some extent[6], meanwhile IFN-β and subtypes of IFN-α have high sensitivity to IFN-R[7].
In this study, we determined the expression of IFN-R protein in liver of patients with HCV-related chronic liver disease and its clinical significance.
A total of 181 patients were enrolled in this study, including 125 patients with chronic hepatitis C and 56 patients with HCV-related liver cirrhosis. All the patients were seropositive for HCV-RNA and underwent liver biopsy. None of the patient was infected with other hepatitis viruses. All the patients with chronic hepatitis C received IFN treatment. However, only 89 patients were evaluated for treatment response since 36 patients lost follow-up shortly after receiving the treatment. According to the response to IFN treatment, we studied the expression of IFN-α/βR protein in liver of 89 patients with chronic hepatitis C at least six months after IFN treatment.
IFN treatment was standardized as follows. Five megaunits of IFN-α1b was administrated to 89 patients with chronic hepatitis C by intramuscular injection six times weekly for the first 2 weeks and then every other day for 22 wk. The total dosage of IFN was 470 MU[8]. The study followed the ethical guidelines of the Declaration of Helsinki and was approved by the institutional ethics committee. Info-rmed consent was obtained from all patients before IFN treatment.
According to the response to IFN, 89 patients with chronic hepatitis C who were seropositive for HCV-RNA and received IFN treatment were divided into responder group (36) and non-responder group (53). Responders were defined as patients who were seronegative for HCV-RNA with their serum alanine aminotransferase (ALT) decreased to the normal range for at least 6 mo after IFN treatment. The other patients were non-responders.
Liver biopsy specimens were defined by Knodell’s pathologic classification. The degree of liver fibrosis was determined according to the criteria for staging fibrosis (F0 to F4) , including F0 (no fibrosis), F1(portal area fibrosis), F2 (bridging fibrosis), F3(bridging fibrosis with lobule deformation), F4 (cirrhosis). Inflammation activities were scored as follows: 1-3 points (mild hepatitis), 4-8 points (moderate hepatitis), 9-12 points (severe hepatitis).
Making specimens with immunofluorescence technique IFN-α/βR in the liver was immunostained with an indirect immunofluorescence technique. The liver biopsy specimens were sampled during laparoscopy, fixed with fixatives, and frozen to make histological sections. The sections were incubated with non-marked antibody for 45 min at 37°C, washed thrice in 0.15 mol/L pH7.6 phosphate buffered saline (PBS) for 3 min and once in 0.01 mol/L pH 7.6 PBS for 1 min, then incubated with anti-idiotype antibody (AId) for 30-45 min at 37°C and washed with PBS. The sections were dehydrated at room temperature and mounted in glycerol. We cloned the gene coding for the outside-membrane of IFN-α/βR in Daudi’s cells and immunized white rabbits with protein produced by the gene-transfected E.coli to get the first antibody (Amersham, Buckinghamshire, UK). The AId (Amersham, Buckinghamshire, UK) was an anti-rabbit-IgG class antibody labeled with fluorochrome. Negative control was serum of rabbits.
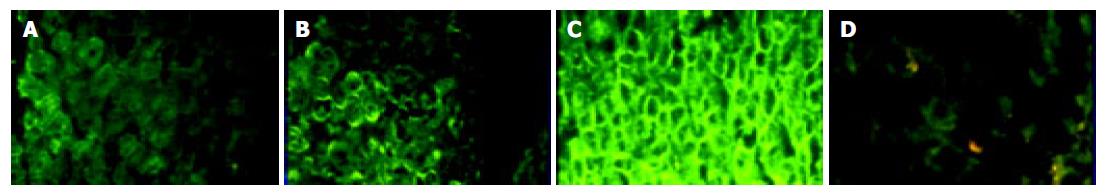
Intensity of fluorescence was standardized as follows (Figure 1): weak positive (+), positive (++) and strong positive (+++). If a minority of hepatocytes in the portal area were stained, it was defined as weak positive (+). The strong positive (+++) means almost all hepatocytes in the hepatic lobule were stained, and hepatocytes between the two grades were defined as positive (++).
Statistical significance of difference was determined by χ2-test.
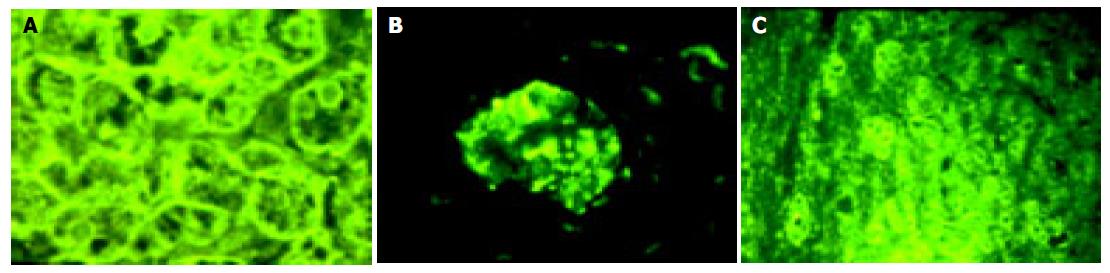
IFN-α/βR protein was expressed in all the samples of HCV-related chronic liver disease, however the degree of expression varied. Expression of IFN-α/βR protein in cell membrane was stronger than that in cytoplasm (Figure 2A).The expression of protein in the surroundings of the portal vein was stronger than that in the surroundings of the central vein. The protein was poorly distributed in hepatic lobules (Figure 2B). Almost all the IFN-α/βR proteins expressing cells were hepatic parenchymal cells. However, IFN-α/βR proteins were also expressed in part of interlobular cholangioepithelia (Figure 2C).
Among the 125 patients with chronic hepatitis C, 40% (50/125) showed weak positive (+), 28% (35/125) showed positive (++), 32% (40/125 ) showed strong positive (+++) expression of IFN-α/βR protein. Among the 56 patients with HCV-related liver cirrhosis, 91.1% (51/56) showed weak positive (+), 5.35% (3/56) showed positive (++), 3.56% (2/56) showed strong positive (+++) expression of IFN-α/βR protein. The total frequency of positive and strong positive expression in chronic hepatitis C patients was much higher than that in patients with HCV-related liver cirrhosis (P < 0.01 Table 1).
Six months after IFN treatment, the responders and non-responders were 36 and 53 respectively, and 27.8% (10/36) of the responders showed weak positive (+), and 72.2% (26/36) showed positive (++) or strong positive (+++) expression of the IFNα/βR protein; 71.7% (38/53) of non-responders showed weak positive (+), and 28.3% (15/53) showed positive (++) or strong positive (+++) expression of the IFN-α/βR protein. After the treatment, the total frequency of positive (++) and strong positive (+++) expression of the IFN-α/βR protein in responder group was much higher (P < 0.05) than that in non-responder group (Table 2).
Isimura[9] has proved that IFN R protein exists in liver by ELISA and reported that the expression degree of IFN-αR protein in cytoplasm is different. By immunohistochemical method and competitive polymerase chain reaction assay, Fujiwara et al[9] found that determination of the expression of IFN-α/βR protein is more useful than that of its mRNA. In this study, we found that expression of IFN-α/βR protein in cell membrane was stronger than that in cytoplasm by immunofluorescence assay. IFN-α/βR protein is a membrane receptor just like other cytoplasm receptors. Moreover, this study also found that the expression of IFN-α/βR protein in the surroundings of the portal vein was stronger than that in the central vein, and the protein was poorly distributed in hepatic lobules. This may be attributed to the following aspects. The blood stream in surrounding of the portal vein is more affluent, providing a good nutritional condition for virus infection which then induces the expression of IFN-α/βR. The expression intensity of IFN-α/βR protein varies in different chronic hepatic diseases. It is weaker in patients with HCV-related liver cirrhosis than in patients with chronic C hepatitis. The reason may be that the bloodstream in patients with cirrhosis is not well-distributed and the blood supply becomes deficient due to various kinds of fibrosclerosis in hepatic lobules, and liver function and liver cell membrane are damaged because of hepatic cellular inflammation. Because of these factors, liver could not provide a good condition for the expression of IFN-α/βR protein in patients with cirrhosis. Defective expression of IFN-α/βR protein can reduce the intake of IFN, resulting in increased reproduction of virus and activity of hepatitis.
This study demonstrated that expression of IFN-α/βR protein had a close correlation with hepatic fibrosis. The majority of responders with chronic hepatitis C had a very strong expression of IFN-α/βR protein prior to the treatment, which coincides with other researches[10-12]. The reasons are as follows. The infection of hepatitis virus C can induce expression of protein effectively[13] , since some patients with HCV-related chronic liver disease become very sensitive to IFN treatment. Expression of IFN-α/βR protein in liver in responsive cases is much stronger than that in non-responsive cases. That is to say, non-responsive reaction to the treatment may be caused by the defective expression of protein in patients with chronic hepatitis C. Although expression of IFN-α/βR protein in the responder group is stronger than that in non-responder group, the expression also exists in some non-responsive cases. On the contrary, expression of IFN-α/βR protein in some responsive cases is as weak as that in non-responsive cases. These inconsistencies are attributed to the following aspects. IFN-α/βR protein cannot represent all IFN R proteins involved in the anti-virus mechanism in chronic hepatitis C. The protein we studied is IFN-α/βR protein which could respond to IFN-α and IFN-β[7]. The protein plays a key role, in the anti-virus mechanism against HCV-related chronic disease. The combining activity of R proteins maybe changed in the responsive cases when IFN-α/βR protein is expressed. Among the responsive and non-responsive cases, some patients with severe chronic hepatitis C and cirrhosis were not sensitive to IFN treatment, and defective expression of IFN-α/βR protein in these patients was a major cause. It coincides with other studies[14,15]. In conclusion, expression of IFN-α/βR protein in liver of patients with HCV-related chronic disease is likely involved in response to IFN treatment, the expression of IFN-α/βR protein may be useful in predicting IFN therapeutic effect, and defective expression of IFN-α/βR protein in liver may result in resistance to IFN treatment in patients with HCV-related chronic liver disease.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Hagiwara H, Hayashi N, Mita E, Takehara T, Kasahara A, Fusamoto H, Kamada T. Quantitative analysis of hepatitis C virus RNA in serum during interferon alfa therapy. Gastroenterology. 1993;104:877-883. [PubMed] [Cited in This Article: ] |
| 2. | Kanai K, Kato M, Okamoto H. HCV genotypes in chronic hepatits C and response to interferon. Lancet. 1992;339:1543. [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 211] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Tsubota A, Chayama K, Ikeda K, Yasuji A, Koida I, Saitoh S, Hashimoto M, Iwasaki S, Kobayashi M, Hiromitsu K. Factors predictive of response to interferon-alpha therapy in hepatitis C virus infection. Hepatology. 1994;19:1088-1094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 253] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Poynard T, Leroy V, Cohard M, Thevenot T, Mathurin P, Opolon P, Zarski JP. Meta-analysis of interferon randomized trials in the treatment of viral hepatitis C: effects of dose and duration. Hepatology. 1996;24:778-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 421] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 5. | Müller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918-1921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1978] [Cited by in F6Publishing: 1940] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 6. | Novick D, Cohen B, Rubinstein M. The human interferon alpha/beta receptor: characterization and molecular cloning. Cell. 1994;77:391-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 509] [Cited by in F6Publishing: 507] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 7. | Colamonici OR, Domanski P. Identification of a novel subunit of the type I interferon receptor localized to human chromosome 21. J Biol Chem. 1993;268:10895-10899. [PubMed] [Cited in This Article: ] |
| 8. | Colamonici OR; Isimura. The significance of interferon in liver as an impact factor for hepatitis C therapy. Hepatology. 1997;38:292-299. [Cited in This Article: ] |
| 9. | Fujiwara D, Hino K, Yamaguchi Y, Ren F, Satoh Y, Korenaga M, Okuda M, Okita K. Hepatic expression of type I interferon receptor for predicting response to interferon therapy in chronic hepatitis C patients: a comparison of immunohistochemical method vs. competitive polymerase chain reaction assay. Hepatol Res. 2003;25:377-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Morita K, Tanaka K, Saito S, Kitamura T, Kondo M, Sakaguchi T, Morimoto M, Sekihara H. Expression of interferon receptor genes (IFNAR1 and IFNAR2 mRNA) in the liver may predict outcome after interferon therapy in patients with chronic genotype 2a or 2b hepatitis C virus infection. J Clin Gastroenterol. 1998;26:135-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Mizukoshi E, Kaneko S, Yanagi M, Ohno H, Kaji K, Terasaki S, Shimoda A, Matsushita E, Kobayashi K. Expression of interferon alpha/beta receptor in the liver of chronic hepatitis C patients. J Med Virol. 1998;56:217-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 12. | Fujiwara D, Hino K, Yamaguchi Y, Kubo Y, Yamashita S, Uchida K, Konishi T, Nakamura H, Korenaga M, Okuda M. Type I interferon receptor and response to interferon therapy in chronic hepatitis C patients: a prospective study. J Viral Hepat. 2004;11:136-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Yamaguchi Y, Hino K, Fujiwara D, Ren F, Katoh Y, Satoh Y, Okita K. Expression of type I interferon receptor in liver and peripheral blood mononuclear cells in chronic hepatitis C patients. Dig Dis Sci. 2002;47:1611-1617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Affabris E, Romeo G, Belardelli F, Jemma C, Mechti N, Gresser I, Rossi GB. 2-5A synthetase activity does not increase in interferon-resistant Friend leukemia cell variants treated with alpha/beta interferon despite the presence of high-affinity interferon receptor sites. Virology. 1983;125:508-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Roffi L, Colloredo G, Antonelli G, Beliati G, Panizzuti F, Piperno A, Pozzi M, Ravizza D, Angeli G, Diaazani F. Breakthrough during recombinant interferon alpha therapy in patients with chronic hepatitis C virus infection: prevalence, etiology, and management. Hepatology. 1995;21:645-649. [DOI] [Cited in This Article: ] |