Published online Jul 7, 2005. doi: 10.3748/wjg.v11.i25.3823
Revised: December 23, 2004
Accepted: December 26, 2004
Published online: July 7, 2005
AIM: The aim of the present study is to use immunohisto-chemical methods to investigate the clinical implications of tumor markers in esophageal squamous cell carcinoma and evaluate their impact on prognosis.
METHODS: From November 1990 to December 1996, 47 patients were treated with preoperative radiation followed by radical esophagectomy. All patients were confirmed pathologically as suffering from squamous cell carcinoma. Immunohistochemical stain was done for PCNA, cyclinD1 protein expression and DNA content analyzed by image cytometry. Kaplan-Meier method for single prognostic factor and log-rank test was used to test the significant difference. Cox stepwise regression model and prognosis index model were used for survival analysis with multiple prognostic factors.
RESULTS: Radio-pathological change, T stage and N stage, as the traditional prognostic factors had statistical difference in 3-, 5- and 10-year survival rates. While, tumor cell proliferating marked PCNA, cyclinD1 and DNA content served as independent prognostic factors of esophageal carcinoma. There was definitely an identity between the single and multiple factor analyses. PI was more accurate to evaluate the prognosis of esophageal carcinoma.
CONCLUSION: It is possible that tumor cell proliferating marked PCNA, cyclinD1 and DNA content would become the endpoints for evaluating the prognosis of esophageal carcinoma.
- Citation: Zhu SC, Li R, Wang YX, Feng W, Li J, Qiu R. Impact of simultaneous assay, the PCNA, cyclinD1, and DNA content with specimens before and after preoperative radiotherapy on prognosis of esophageal cancer-possible incorporation into clinical TNM staging system. World J Gastroenterol 2005; 11(25): 3823-3829
- URL: https://www.wjgnet.com/1007-9327/full/v11/i25/3823.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i25.3823
Esophageal cancer, the sixth most common cancer worldwide, is one of the most interesting cancers in terms of its geographic distribution, variety of environmental factors including tobacco, alcohol, nutritional deficiencies and nitrosamines[1,2]. The ratio of the incidence rates between high-risk and low-risk areas can be as great as 500:1, e.g., Cixian, in Hebei Province of northern China, has received worldwide attention over the past decades because of its high esophageal cancer incidence. Results from earlier studies show that malignant transformation of human esophageal mucosa is a multistage process. An early indicator of abnormality in persons predisposed to squamous cell carcinoma (SCC) is the proliferation of esophageal epithelial cells, morphologically manifested as basal-cell hyperplasia (BCH) and dysphasia (DYS). However, key molecular changes in the esophageal carcinogenesis still remain to be characterized[3].
Furthermore, genetic factors, such as the activation of oncogene and the inactivation of tumor suppressor genes, are believed to play important roles. Earlier studies have shown the PCNA (proliferating cell nuclear antigen) is a 36-ku, 261-amino acid non-histone polypeptide that is localized in the nucleus and is associated with cell proliferation with function as an auxiliary protein to DNA polymerase-delta. PCNA appears to be necessary for DNA replication and elevated level of this protein at the G1/S phase transition are present in cells undergoing division[4,5].
Recent evidence has suggested that cyclinD1 gene (also referred to as prad1) amplification might also be involved in the development of esophageal carcinoma. The cyclinD1 gene has been mapped to the chromosome 11q13 locus close to the int2 and hst1 genes. The complex of cyclinD1 protein and cyclin-dependent protein kinases (cdks) may govern key transitions in the cell cycle. Zhang[6] found that cyclinD1 gene was amplified and its protein expression increased in only one-third of the esophageal SCC. However, the knowledge of the role of cyclinD1 in esophageal carcinogenesis is not yet definite in detail, since the level of cyclinD1 protein in different stages of carcinogenesis of esophageal carcinoma has not been determined[6].
In the current study, it is possible to use only cancer cells for analyzing the DNA patterns and ploidy in cytophotometry. Most investigators have shown in clinical esophageal SCC, high-ploidy tumors grow more rapidly than low-ploidy ones, and the duration from curative esophagectomy to recurrence decreases in proportion to the degree of DNA aneuploidy[7].
Thus, despite aggressive therapy for esophageal cancer, the outcome is generally poor with a large number of patients developing rapid recurrence even after curative operation[8,9]. If the prospect of prognosis and metastasis could be predicted preoperatively, it would be appropriate to select the most adequate treatment for individual cancer patient. Thus multi-disciplinary treatment including chemotherapy and irradiation would be reserved for patients predicted to have a poor prognosis while a less aggressive approach could be undertaken for those with better prognosis. Though most of the previous lectures showing DNA ploidy, AgNoRs and amplification of some oncogenes had been investigated to determine their prognostic value in esophageal cancer, the indication by PCNA, cyclinD1 protein expression combined with DNA ploidy have not been reported. It is expected to be of interest to evaluate them together as predictors of the malignant potential. In this report, we examined the cyclinD1, PCNA protein and DNA ploidy of esophageal carcinoma in 47 paired specimens from endoscopic biopsy and surgical resection tissue, with biologic features and prognostic value was also discussed.
There are 47 patients (median age 62 years, range 33-74 years, 28 males and 19 females) with SCC of esophagus treated from November 1990 to December 1993 by preoperative radiotherapy followed by curative esophagectomy at the Fourth Hospital, Hebei Medical University, in northern China. All patients had been examined by gastrointestinal endoscopy, barium swallow, chest X-ray and liver ultrasound scans. Patients with stage IV disease shown by computed tomography were excluded. Pathological evaluation done according to the criteria established by the Chinese Society for Esophageal Disease (Henan,1987), showed all patients were suffering from SCC. We had obtained two sets of specimens from each patient: endoscopic biopsy before preoperative radiotherapy and the surgical specimens of the same patient after esophagectomy of which the tumor markers in all 47 paired specimens were investigated. The maximum follow-up was 144 mo with a median of 56 mo.
All the 47 patients received preoperative radiotherapy, the external beam radiation was given by a 10-MV X-ray (Linear Accelerator, SIEMENS, PRIMUS) using the conventional fractionated irradiation. The irradiated volume included the gross tumor with a safety margin of 5.0 cm both proximally and distally. Treatment was given through two opposing fields (anterior and posterior); 2.0 Gy was delivered daily for 5d a wk to a total dose of 30-40 Gy in 15-20 fractions over 3-4 wk.
Transthoracic esophagectomy, the most common approach through a right or left thoracotomy incision depending on the preference of the surgeon and the location of the tumor was performed. Tumor located in the lower third of the esophagus usually was approached through a left thoracotomy. A left sixth interspace incision provides excellent exposure of the lower mediastinum. Gastrointestinal reconstruction was subsequently achieved by prepared esophageal substitute (usually the stomach), by passing of a prepared esophageal substitute, usually the stomach underneath the aortic arch and suture to the esophageal stump. Definite esophagectomy was undertaken 3 wk after radiotherapy.
Biopsy and surgical specimens were fixed in 40 g/L formaldehyde and embedded in paraffin wax. Five-micron sections cut from each specimen, dewaxed in xylene, rehydrated through grading concentrations of ethanol were immersed in 3% hydrogen peroxide to block the endogenous peroxidase and washed in phosphate buffered saline. For cyclinD1 and PCNA immunohistochemical stain, tissue sections were heated in 10 mmol/L sodium citrate (pH 6.0) in a microwave oven for 10 min to expose the antigens, and, then, treated with normal goat serum (10%) before staining to reduce nonspecific antibody binding. Tissue sections were incubated at 4 °C overnight for cyclinD1. CyclinD1 and PCNA stain with the following antibodies: mouse monoclonal anti-cyclinD1 antibody (Novocastra Laboratories Ltd., UK) at 1:20 dilution and mouse monoclonal anti-PCNA antibody (PC-10, Dakopath,Glostrup, Denmark) at 1:100 dilution. The sections were then washed and incubated with biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) at room temperature for 30 min. After washing, the sections were incubated with avidin-biotin-peroxidase complex at room temperature for 30 min with the vectastain Elite ABC kit (Vector Laboratories Burlingame, CA). Color developed with 3,3-diaminobenzidine as the substrate. Sections were counter-stained with Harris acid hematoxylin to demonstrate the specificity of the immunostain and the primary antibodies were replaced with similar protein concentrations of normal rabbit IgG.
The PCNA, cyclinD1 protein expression of the endoscopic biopsy and surgical specimens were compared by two histopathologists by the double-blind arrangement in assessing the tumor response to radiotherapy. For cyclinD1 and PCNA, 1 000 tumor cells in five high-power fields were counted respectively. PCNA index was the percentage of nuclei stained positive. PCNA index greater than 25% was taken as PCNA positive. PCNA index lower than 25% was taken as PCNA-negative. For cyclinD1, positive cell nucleus not found was taken as negative. When the percentage of positive cell nucleus was greater than 30%, it was taken as strongly positive. Between the two levels, it was positive. Therefore three grades were ascertained for cyclinD1 expression[5,9,10].
Cellular DNA analysis was performed on paraffin sections cut 100 μm thick. The sections were stained with Feulgen stain and examined using a microspectrophotometer by two-wavelength method. Data processing was carried out with a personal computer (HP-85, Hewlett-Packard, Palo Alto, CA, USA). In each section, the mean DNA value of 20 stromal lymphocytes was used as a control of the normal diploid complement (2C). The relative DNA content as compared with the 2C value was determined in 100 tumor cells in each lesion as previously described[11,12]. The DNA distribution patterns were then classified into four types, according to the degree of the peak and dispersion on the DNA histogram, as follows: Type I, a prominent peak in the 2C region with a dispersion to the 4C region, Type II, a relative high peak in the 2C-3C regions with a dispersion limited to the 6C region, Type III, a low peak beyond the 3C region with less than 20% of the cells beyond the 6C region and Type IV, multiple peaks with a broad dispersion and more than 20% of cells beyond the 6C region.
PCNA, cyclinD1 protein expression index and DNA distribution pattern were analyzed and regarded in the clinico-pathological features as determined by the χ2 test. Difference between the cumulative survival rates of patient groups were calculated by log-rank test for comparison of Kaplan– Meier survival curves. The significance of various parameters on survival were analyzed by multivariate Cox regression model. A P value of 0.05 or less was considered statistically significant (statistical software SPSS 10.0 for Windows).
All the patients had received preoperative radiotherapy followed by definite surgical resection. The complete response rate as shown by pathology was 29.8% (14/47) for the entire group treated by preoperative radiotherapy and 5-year survival rate was 71.4% (10/14) for 14 patients with complete response by pathology. The overall survival rates of all these patients in the 3, 5 and 10-years were 54.3%, 46.2% and 38.8%, respectively.
PCNA stain was almost entirely confined to the cell nuclei and showed diffuse and granular pattern or a mixture of both. While cyclinD1 stain was present in the cytoplasm around the cell nuclei in most parts, and a few parts in the tumor cell nuclei. At the same time, PCNA stain was observed in the basal layer of stratified squamous epithelium in normal tissue. In this study, all cyclinD1 present both in the stained nuclei and the cytoplasm around them were considered as protein expression positive carcinoma cells. We took both Types I and II DNA content as the low ploidy pattern and Types III and IV as the high ploidy pattern.
Kaplan-Meier method was used to analyze the single factors, including age, sex, irradiation dose, location of disease, length, and radiation response observed by pathology, T stage, N stage, PCNA index, cyclinD1 protein expression and DNA content pattern, e.g. Types I and II or Types III and IV influenced the prognosis of esophageal carcinoma obviously. The P value as checked by the log-rank test ≥ 0.05 was taken as significant. Only the four preoperative radiotherapy factors: radiation changes observed by pathology, T stage, N stage and cyclinD1 protein expression before radiation in the endoscopic biopsies; those observed in the surgery rescected specimens showing, PCNA index, cyclinD1expression level, DNA content pattern, revealed significant difference (P < 0.05, Table 1), whereas age, sex, total tumor dose, location and length of disease possessed no value in prognosis.
| Factors | Patient number | Median (mo) | Mean (mo) | 3 yr (%) | 5 yr (%) | 10 yr (%) | Log-rank | P |
| Sex | ||||||||
| Male | 28 | 48 | 76 | 60.7 | 49.7 | 38.2 | ||
| Female | 19 | 27 | 62 | 44.9 | 45.1 | 38.1 | 0.6705 | 0.2513 |
| Age (yr) | ||||||||
| 50 | 26 | 31 | 66.6 | 50.4 | 49.7 | 36.8 | ||
| > 50 | 21 | 50 | 74.2 | 57.7 | 45.2 | 39.6 | 0.2317 | 0.4084 |
| Total dose | ||||||||
| 35 Gy | 23 | 53 | 78.4 | 56.5 | 56.5 | 46.3 | ||
| > 35 Gy | 24 | 35.5 | 63.5 | 52.6 | 39.6 | 30.3 | 0.647 | 0.2588 |
| Site of lesion | ||||||||
| Upper-thoracic | 10 | 69.5 | 81.5 | 70 | 70 | 46.7 | ||
| Middle-thoracic | 37 | 31 | 67.9 | 50.2 | 41.7 | 35.4 | 0.8864 | 0.1877 |
| Tumor length | ||||||||
| 7.0 cm | 39 | 48 | 77.1 | 58.1 | 50.1 | 41.4 | ||
| > 7.0 cm | 8 | 22.5 | 40 | 37.5 | 37.5 | 18.8 | 0.9653 | 0.1672 |
| Pathological change | ||||||||
| after radiation | ||||||||
| Grade III | 24 | 93 | 95 | 70.8 | 62.3 | 57.6 | ||
| Grade II | 15 | 26 | 49.3 | 40 | 40 | 16 | ||
| Grade I | 8 | 13 | 38.6 | 31.1 | 15.6 | 15.6 | 5.145 | 0.0764 |
| T stage | ||||||||
| T1 | 11 | 135 | 112.9 | 90.9 | 81.8 | 61.9 | ||
| T2 | 19 | 48 | 77.5 | 57.9 | 46.9 | 41 | ||
| T3 | 17 | 19 | 36.1 | 26 | 26 | 17.3 | 8.98 | 0.0113 |
| N stage | ||||||||
| N0 | 39 | 60 | 81.7 | 61.5 | 53.7 | 42.6 | ||
| N1 | 8 | 12 | 17.6 | 16.7 | 16.7 | 0 | 2.417 | 0.0078 |
| Pre-radiotherapy PCNA | ||||||||
| Low expression | 21 | 35.5 | 67.4 | 50 | 42 | 32.9 | ||
| High expression | 26 | 48 | 75.1 | 60.4 | 55.2 | 44.2 | 0.888 | 0.1873 |
| Pre-radiotherapy cyclinD1 | ||||||||
| Negative | 21 | 135 | 106.3 | 80.9 | 71.4 | 61.6 | ||
| Positive | 20 | 19 | 46.2 | 32.5 | 32.5 | 24.4 | ||
| Strong positive | 6 | 24 | 28.7 | 33.3 | 16.7 | 0 | 10.399 | 0.0055 |
| Pre-radiotherapy | ||||||||
| DNA content | ||||||||
| I+II Type | 10 | 98.5 | 88.9 | 60 | 60 | 50 | ||
| III+IV Type | 37 | 40 | 65.9 | 53 | 44.3 | 34.3 | 0.5158 | 0.3032 |
| Post-radiotherapy PCNA | ||||||||
| Low expression | 17 | 135 | 107.4 | 81.5 | 73.9 | 61.8 | ||
| High expression | 30 | 18 | 21.4 | 16.1 | 9.8 | 0 | 4.1124 | 2E-05 |
| Post-radiotherapy cyclinD1 | ||||||||
| Negative | 17 | 77 | 94.1 | 76.5 | 70.4 | 49.8 | ||
| Positive | 22 | 30 | 64.4 | 53.1 | 43.1 | 37.8 | ||
| Strongly positive | 8 | 21 | 39 | 12.5 | 12.5 | 12.5 | 7.9215 | 0.0191 |
| Pre-radiotherapy | ||||||||
| DNA content | ||||||||
| I+II Type | 23 | 135 | 110.7 | 87 | 78.3 | 60.2 | ||
| III+IV III+IV Type | 24 | 20 | 32.5 | 22 | 16.6 | 16.6 | 3.7074 | 0.0001 |
T stage, N stage, pathological changes after radiotherapy, pre-radiotherapy cyclinD1 protein expression and post-radiotherapy PCNA index, cyclinD1 protein expression and DNA content pattern with Cox hazard risk regression model were analyzed. The most significant factors were the post-radiotherapy PCNA index and DNA content pattern (specimens from surgical specimens). Furthermore, each factor by single parameter analysis gave the most marked prognostic difference, while multivariation considered by Cox model, clearly affected the prognosis of esophageal cancer patients (Table 2).
| Factors | Group | Group value | β value | SD | P | Risk |
| Pathological radiation change’s | III/II/I Grades | 0/1/2 | 0.6405 | 0.2451 | 0.0090 | 0.1582 |
| Surgical specimens T stage | T1/T2/T3 | 0/1/2 | 0.7678 | 0.2737 | 0.0050 | 0.1744 |
| Surgical specimens N stage | N0/N1 | 0/1 | 1.2957 | 0.4845 | 0.0075 | 0.1634 |
| Pre-radiotherapy cyclinD | N/P/SP | 0/1/2 | 0.8434 | 0.2564 | 0.0010 | 0.2139 |
| Post-radiotherapy cyclinD1 | N/P/SP | 0/1/2 | 0.6538 | 0.2643 | 0.0134 | 0.1462 |
| Post-radiotherapy PCNA | Low/high expression | 0/1 | 2.0965 | 0.4720 | 0.0000 | 0.3032 |
| Post-radiotherapy DNA | I+II Type/III+IV Type | 0/1 | 1.5593 | 0.4287 | 0.0003 | 0.2413 |
According to the formulation of prognosis index (PI) model from statistic software, it was defined as 0 which had the least influence on prognosis. Number 2 had the greatest effect on prognosis .When the number was 1, the prognosis was between 0 and 2. For example, T stage divided by T1, T2, T3 would show difference on patients survival and we gave them the number 0, 1, 2 respectively as shown in Table 2. The group’s weight (0, 1, 2 or 0, 1) times the coefficient of Cox regression model (β value). Then by adding each group’s data results would give each patient’s PI value, e.g., different factors (T stage, N stage, PCNA, cyclinD1 expression, etc.) at the same weight number. For example, all number 0 patients, or number 1 patients, or number 2 patients would give the PI (prognosis index) value for each patient (Table 3).
| Factors | Group | Group value (1) | β (2) | prognosis index (PI)1 |
| Pathological radiation change’s | III/II/I Grades | 0/1/2 | 0.6405 | (1)x(2) |
| Surgical specimens T stage | T1/T2/T3 | 0/1/2 | 0.7678 | (1)x(2) |
| Surgical specimens N stage | N0/N1 | 0/1 | 1.2957 | (1)x(2) |
| Pre-radiotherapy cyclinD | N/P/SP | 0/1/2 | 0.8434 | (1)x(2) |
| Post-radiotherapy cyclinD1 | N/P/SP | 0/1/2 | 0.6538 | (1)x(2) |
| Post-radiotherapy PCNA | low/high expression | 0/1 | 2.0965 | (1)x(2) |
| Post-radiotherapy DNA | I+II Type/III+IV Type | 0/1 | 1.5593 | (1)x(2) |
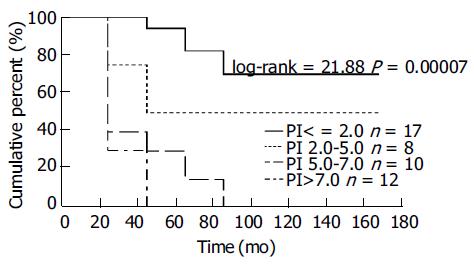
According to the distribution of PI values in all patients, the four subgroups were divided according to different PI values. PI values lower than 2.0, between 2.0 and 5.0, between 5.0 and 7.0 or higher than 7.0 may go stepwise further with the median survival terms and 3-, 5-, 10-year survival rates of the four subgroup patients would reveal significant differences (Table 4, Figure 1). It was multiple variety combined analysis, which showed a clear difference in survival among the four subgroups.
| Prognosis indexes | Number patients | Median month | 3 yr | 5 yr | 10 yr |
| < 2.0 | 17 | 146 | 100 | 88.2 | 69.7 |
| 2.0-5.0 | 8 | 93.5 | 50 | 50 | 50 |
| 5.0-7.0 | 10 | 20 | 30 | 30 | 0 |
| > 7.0 | 12 | 13 | 9.6 | 0 | 0 |
Cancer of the esophagus is the most common cancer in northern China, for which current consensus of treatment varies from surgery, radiotherapy, chemotherapy, and endoscopic laser surgery or their combination. The outcome, however, remains dismal because most patients are seen with advanced disease on diagnosis[13-15]. Thus, what the majority of patients need is a multimodality program. Yet how to know which patients really need which modality is still an open focus. Nevertheless, traditional prognostic factors: T stage, N stage are basically the most important. Yet up to now, their application has merely been used by the surgically treated patients. For conservative treatment, besides the TNM classification system, additional variables such as biological staging for the intrinsic malignant potential of the tumor, are to be useful to determine the treatment and long-term survival[16].
Recently, with rapid advance in biological and genetic studies, more and more tumor markers are being correlated with tumor characteristics[8]. The ability to predict, on the basis of immunocytochemical assessment of pretreatment biopsy, which patients are likely to respond to chemoradiotherapy would be a major advance in the management of cancer patients. Thus, it is possible to provide the most adequate protocol for different patients by combining traditional factors and the markers of tumor cells[16].
Several studies[8,9,15] have shown that high PCNA expression in esophageal tumor is associated with a worse prognosis in surgical patients and greater likelihood of recurrence. However, such a report on PCNA, and cyclinD1 protein expression examined simultaneously in biopsy and resected specimens of esophageal carcinoma has never been seen. Nor possible correlation between PCNA, cyclinD1 protein expression and the traditional prognostic factors (T stage, N stage) to chemoradiotherapy has never been investigated. Immunohistochemical detection of PCNA being simple and useful for the study of cell kinetics, allows the retrospective evaluation of the proliferating state of the tumor as it can be used in conventionally fixed materials. Many authors[17,18] showed the higher PCNA index patients usually gave the worse prognosis with local recurrence. In the past years, it has been proved that the combination of PCNA index with the AgNOR count appears to be an effective means of identifying patients with poor prognosis. For patients with a PCNA index of ≥ 44 and AgNOR count of ≥ 6, multidisciplinary treatment should be recommended in an attempt to prolong survival. However, in this study, PCNA index as checked by endoscopic biopsies have not shown such influence on survival[9].
Furthermore, we have found PCNA protein expression to be significantly high in surgically resected specimens with PCNA index 63.8% (30/47) compared to the endoscopic biopsy before radiation 55.3% (26/47). At the same time, patients with high PCNA index in the surgically resected specimens had the worse prognosis. PCNA protein expression examined after radiotherapy made a significant difference to esophageal cancer prognosis. The repopulation of tumor cells after radiation may exist which results in local recurrence of esophageal carcinoma after radiotherapy.
The most important advantage of PCNA, cyclinD1 immunohistochemical assay is that the analysis changes are restricted to the tumor cells. In contrast, flow cytometry assay, all cells in the tissue block are measured and in DNA diploid cases, this means that SPF reflects the sum of cell proliferation that estimates the malignant and benign (stromal and inflammatory cells) components[9].
Most studies[6,19] reported patients with cyclinD1 amplification and protein expressions had a poor outcome and a higher incidence of distant metastasis than those in amplification-negative or protein expression-negative groups. The surgical survival rates and disease-free survival in the high cyclinD1 expression group were significantly shorter than in the cyclinD1 low or non-expression group. CyclinD1 amplification and protein expression in patients with SCC of the esophagus would mean a grave biological malignancy. Naiton et al[20] reported 55 esophageal cancer patients with a ratio of cyclinD1 protein-positive expression of 38% gave a 5-year survival rate of 7%, as compared with the 5-year survival rate of 59% in patients who showed cyclinD1 protein negative expression (P < 0.01). In 1998, Ishikawa[21] studied cyclinD1 protein expression in esophageal SCC and observed similar results. Our checked results showing higher cyclinD1 protein expression both in endoscopic biopsy and surgical resected specimens also gave bad prognostic results (Table 1).
In our previous study, the clinical characteristics of patients with SCC of the esophagus, such as outcome and distant organ metastases, were found to be closely correlated with cyclinD1 amplication and protein expression in the tumor tissue. It was observed that parameters were independent from clinicopathological factors for the outcome and metastasis. It was the second highest partial regression coefficient after the pN factor in the life table with Cox proportional hazard model, and the probability rate was significant[19,21]. In this study, the same results were obtained. From Table 2, it is apparent that both the cyclinD1 protein expression in biopsy and surgical specimens had a significant effect on the prognosis of SCC of the esophagus. Specially, cyclinD1 protein-positive expression in endoscopic biopsy was the third highest partial regression coefficient after the PCNA index and DNA content in the resected specimens in multivariate analysis using the Cox proportional hazard model. We speculate that the cyclinD1 protein prognostic value maybe relatively high as the most useful factor for predicting the outcome in SCC of the esophagus in the previous study, e.g., pN factor and pT. According to the results of both univariate and multivariate analyses, the fact that patients with cyclinD1 protein-positive had a poor outcome in every group implies this agent is a useful prognostic indicator in esophageal carcinoma. Hence, it may very well supplement the TNM classification for predicting clinical characteristics and outcome, especially for patients who are destined to undergo non-operative treatment. For these patients, TNM classification could hardly be of any assistance to offer an endpoint and select more appropriate protocol for patients with SCC of the esophagus. By checking cyclinD1 protein expression level, the ultimate combined TNM classification could thus be perfected.
The essence of cell division really is the DNA replication and changes of DNA distribution pattern which predict tumor cell proliferation kinetics. Here cytophotometric technique is to analyze the DNA content pattern both in endoscopic biopsy and resected specimens. The most important advantage of DNA content checked by cytophotometric method is that the analysis is restricted to the tumor cells, as compared to flow cytometry, by which all cells in the tumor tissue are measured .It reflects the sum of cell proliferation which estimates both malignant and benign (stromal cell and inflammatory cell) components[9]. In clinical esophageal carcinoma, high-ploidy tumors grow more rapidly than low-ploidy ones and the duration from curative esophagectomy to recurrence decreases in proportion to the degree of DNA aneuploidy, a view believed by many authors.
During early years, some authors[9,11,22] reported the relationship between DNA content of a variety of human cancer cells and their malignant potential as measured by cytophotometric analysis, most of who reported that such measurement are useful in elucidating the pattern of tumor progression or the prognosis with most of the results being negative between DNA content and long-term survival. For example, Miun et al[22] reported 78 esophageal cancer patients who gave 5-year survival rate of 14% and 57% in high DNA content and low DNA content groups with statistical significance in the difference (P = 0.03). Matsuura[11] found that not one patient survived over 5 years in DNA triploids or tetraploidy, but the 5-year survival rate was 70% in DNA diploidy patients. Our study shows no influence on survival or prognosis by biopsy DNA content measurement. The measured results may have been inaccurate due to the very small size of biopsy specimens. From Tables 1 and 2, the surgically resected specimens gave DNA content measurement showing significant correlation between the results and the prognosis of SCC of esophagus as assessed by univariate and multivariate analyses. This result conforms well to most of the studies reported so far[9,11].
Through combination of the traditional prognostic factors and tumor cell markers, the radiation pathological changes, T stage, N stage, cyclinD1, PCNA protein expression and DNA content as analyzed by Cox proportional hazard model, it is found that patients with grade III pathological changes, T1 stage, N0 stage, cyclinD1 protein-negative, lower PCNA index and DNA content pattern Types I and II, give higher long-term survival than the control groups.
With the total patients divided into four subgroups according to the formulation of prognosis index model, prognosis is observed to be worse with higher prognosis index, giving significant difference in spite of the few patients allotted. This result clearly demonstrates the evaluation of SCC of esophagus by prognosis index model with multivariate factors is dependable and accurate.
The authors wish to thank Ms Laura Kresty for her expert technical assistance and N Sugimoto (Sanwa Kagakau Co., Ltd, Nagoya, Japan) for his support in the statistical analysis.
Co-first-author: Ren Li
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Castellsagué X, Muñoz N, De Stefani E, Victora CG, Castelletto R, Rolón PA, Quintana MJ. Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int J Cancer. 1999;82:657-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 2. | Zhao WX, Shi TX, Gao XP, Zhang HX, Li SL. Association of p53, PCNA expression and trace element content in esophageal mucosa. Ai Zheng. 2002;21:757-760. [PubMed] [Cited in This Article: ] |
| 3. | Dong Wang L, Bin Yue W, Zhou Y, Wei Feng C, Liu B, Zhou Q, Ying Jia Y, Zheng S, Gao SS, Ji Xie X. Endoscopic screening and determination of p53 and proliferating cell nuclear antigen in esophageal multistage carcinogenesis: a comparative study between high- and low-risk populations in Henan, northern China. Dis Esophagus. 2002;15:80-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Gelb AB, Kamel OW, LeBrun DP, Warnke RA. Estimation of tumor growth fractions in archival formalin-fixed, paraffin-embedded tissues using two anti-PCNA/Cyclin monoclonal antibodies. Factors affecting reactivity. Am J Pathol. 1992;141:1453-1458. [PubMed] [Cited in This Article: ] |
| 5. | Siitonen SM, Kallioniemi OP, Isola JJ. Proliferating cell nuclear antigen immunohistochemistry using monoclonal antibody 19A2 and a new antigen retrieval technique has prognostic impact in archival paraffin-embedded node-negative breast cancer. Am J Pathol. 1993;142:1081-1089. [PubMed] [Cited in This Article: ] |
| 6. | Zhang J, Li Y, Wang R, Wen D, Sarbia M, Kuang G, Wu M, Wei L, He M, Zhang L. Association of cyclin D1 (G870A) polymorphism with susceptibility to esophageal and gastric cardiac carcinoma in a northern Chinese population. Int J Cancer. 2003;105:281-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Morita M, Kuwano H, Tsutsui S, Ohno S, Matsuda H, Sugimachi K. Cytophotometric DNA content and argyrophilic nucleolar organiser regions of oesophageal carcinoma. Br J Cancer. 1993;67:480-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Ueno H, Hirai T, Nishimoto N, Hihara J, Inoue H, Yoshida K, Yamashita Y, Toge T, Tsubota N. Prediction of lymph node metastasis by p53, p21(Waf1), and PCNA expression in esophageal cancer patients. J Exp Clin Cancer Res. 2003;22:239-245. [PubMed] [Cited in This Article: ] |
| 9. | Kuwano H, Sumiyoshi K, Nozoe T, Yasuda M, Watanabe M, Sugimachi K. The prognostic significance of the cytophotometric DNA content and its relationship with the argyrophilic nucleolar organizer regions (AgNOR) and proliferating cell nuclear antigen (PCNA) in oesophageal cancer. Eur J Surg Oncol. 1995;21:368-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Hagiwara N, Tajiri T, Tajiri T, Miyashita M, Sasajima K, Makino H, Matsutani T, Tsuchiya Y, Takubo K, Yamashita K. Biological behavior of mucoepidermoid carcinoma of the esophagus. J Nippon Med Sch. 2003;70:401-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Ma J, Nicholas HA, Lin SX, Patel N, Mai HG, Hong MH, Lu TX, Cui NJ, Min HQ. Prognostic significance of DNA ploidy and proliferative indices in patients with nasopharyngeal carcinoma. Ai Zheng. 2002;21:644-650. [PubMed] [Cited in This Article: ] |
| 12. | Korenaga D, Okamura T, Saito A, Baba H, Sugimachi K. DNA ploidy is closely linked to tumor invasion, lymph node metastasis, and prognosis in clinical gastric cancer. Cancer. 1988;62:309-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Michel P, Magois K, Robert V, Chiron A, Lepessot F, Bodenant C, Roque I, Seng SK, Frebourg T, Paillot B. Prognostic value of TP53 transcriptional activity on p21 and bax in patients with esophageal squamous cell carcinomas treated by definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2002;54:379-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Wang LS, Chow KC, Chi KH, Liu CC, Li WY, Chiu JH, Huang MH. Prognosis of esophageal squamous cell carcinoma: analysis of clinicopathological and biological factors. Am J Gastroenterol. 1999;94:1933-1940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Yasunaga M, Tabira Y, Nakano K, Iida S, Ichimaru N, Nagamoto N, Sakaguchi T. Accelerated growth signals and low tumor-infiltrating lymphocyte levels predict poor outcome in T4 esophageal squamous cell carcinoma. Ann Thorac Surg. 2000;70:1634-1640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Horii N, Nishimura Y, Okuno Y, Kanamori S, Hiraoka M, Shimada Y, Imamura M. Impact of neoadjuvant chemotherapy on Ki-67 and PCNA labeling indices for esophageal squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2001;49:527-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Dabrowski A, Szumiło J, Brajerski G, Wallner G. Proliferating nuclear antigen (PCNA) as a prognostic factor of squamous cell carcinoma of the oesophagus. Ann Univ Mariae Curie Sklodowska Med. 2001;56:59-67. [PubMed] [Cited in This Article: ] |
| 18. | Shiozaki H, Doki Y, Kawanishi K, Shamma A, Yano M, Inoue M, Monden M. Clinical application of malignancy potential grading as a prognostic factor of human esophageal cancers. Surgery. 2000;127:552-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Shamma A, Doki Y, Shiozaki H, Tsujinaka T, Yamamoto M, Inoue M, Yano M, Monden M. Cyclin D1 overexpression in esophageal dysplasia: a possible biomarker for carcinogenesis of esophageal squamous cell carcinoma. Int J Oncol. 2000;16:261-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Naitoh H, Shibata J, Kawaguchi A, Kodama M, Hattori T. Overexpression and localization of cyclin D1 mRNA and antigen in esophageal cancer. Am J Pathol. 1995;146:1161-1169. [PubMed] [Cited in This Article: ] |
| 21. | Ishikawa T, Furihata M, Ohtsuki Y, Murakami H, Inoue A, Ogoshi S. Cyclin D1 overexpression related to retinoblastoma protein expression as a prognostic marker in human oesophageal squamous cell carcinoma. Br J Cancer. 1998;77:92-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Minu AR, Endo M, Sunagawa M. Role of DNA ploidy patterns in esophageal squamous cell carcinoma. An ultraviolet microspectrophotometric study. Cancer. 1994;74:578-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |