Published online May 28, 2005. doi: 10.3748/wjg.v11.i20.3151
Revised: June 30, 2004
Accepted: August 31, 2004
Published online: May 28, 2005
We report the case of a patient affected by an extra-nodal non-Hodgkin lymphoma presenting as a unique, large retroperitoneal mass with an unusual clinical presentation mimicking gastric peptic or neoplastic disease. The patient was successfully treated with a first generation therapy, CHOP modified regimen (cyclophosphamide 600 mg/m2 intravenously on d 1, epirubicin 55 mg/m2 intravenously on d 1, vincristine 1.2 mg/m2 intravenously on d 1, prednisone 60 mg/m2 on d 1-5), and a complete response was achieved. The (18)F-fluorodeoxyglucose positron emission tomography was used to assess the therapy outcome. A brief review of literature is provided.
- Citation: Fulignati C, Pantaleo P, Cipriani G, Turrini M, Nicastro R, Mazzanti R, Neri B. An uncommon clinical presentation of retroperitoneal non-Hodgkin lymphoma successfully treated with chemotherapy: A case report. World J Gastroenterol 2005; 11(20): 3151-3155
- URL: https://www.wjgnet.com/1007-9327/full/v11/i20/3151.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i20.3151
Non-Hodgkin lymphoma (NHL) causes many deaths worldwide, and its incidence is increasing[1]. It represents a heterogeneous group of neoplasms originating from lymphocytes[1-3]. In Western countries, 80% of NHL consists of a neoplastic growth originating from B lymphocytes[4]. The etiology of this disease is still partially unclear[1]. Some infectious agents have been associated more or less strictly with the development of a NHL as i.e., the Epstein-Barr virus, the human herpesvirus 8, and the human T-cell lymphotropic virus type I[1]. Also the Helicobacter pylori (H pylori) chronic infection has been strictly implicated in the pathogenesis of gastric lymphomas[5]. Several other environmental factors have been linked with the increasing incidence of NHL with conflicting evidence[6].
Also the disease classification is still complex and debated. The older classification systems, such as the Kiel classification and the Working Formulation, were based on NHL morphology[7]. Only recently, a new system was developed by the International Lymphoma Study Group, the Revised European and American Classification of Lymphoid Neoplasms that incorporate all available information to define the disease such as clinical features, morphology, immunophenotype as well as genetic features[8]. The stage of the disease is classified according to the Ann Arbor staging system[6].
Nearly 70% of lymphomas present a generally multi-localized, single or multiple lymphoadenomegaly without pain, with involvement in almost 30-40% of latero-cervical lymphnodes. In 30% of patients an extranodal localization is reported in Waldeyer’s ring, in the stomach and generally in the gastro-intestinal tract. The retro-peritoneal localization is extremely rare and its diagnosis is often difficult. It often requires a time consuming and costly diagnostic workup.
Indolent NHL is generally considered incurable. Several regimens have been commonly used; however, treatment has never been shown to extend overall survival[9].
The poly-chemotherapy with CHOP regimen has been so far considered as the standard first-line treatment for advanced large B-cell lymphoma but upcoming therapies seem to promise a relevant improvement in patients’ outcomes[10].
Several efforts have been made also to enhance the pre- and post-treatment disease assessment. This evaluation relies mainly on a CT scan performed after three or four conventional cycles of treatment[11,12]. The current evidence for the ability of (18)F-fluorodeoxyglucose positron emission tomography (FDG-PET) to detect disease not suspected from conventional staging is still equivocal, although an increased number of sites of disease are reported to be found in many patients by the means of FDG-PET but whether this is an independent prognostic factor remains to be seen[13,14].
The role of FDG-PET in NHL follow-up has not been widely investigated but there is some evidence that an early negative FDG-PET scan after completion of treatment is a strong predictor for patients who will be cured, and in this group of patients the follow-up could be minimized. Therapy also, in the case of aggressive disease, could be tailored[15].
In this article we report the case of an uncommon clinical presentation of a large B-cell lymphoma in a male patient treated by a standard CHOP regimen in which a complete remission was achieved. Besides the uncommon presentation, this article emphasizes the role and the good sensitivity of FDG-PET in assessing response to therapy.
At the beginning of May 2002, a 56-year-old man, a hard smoker (30 packs/year) with hypertension, referred to the outpatient section of the Day-Hospital Unit of the Centre of Experimental and Clinical Medicine (Department of Internal Medicine, University of Florence) with recurrent epigastric burning pain following meals. Anorexia and asthenia associated with a marked loss of weight (a 7 kg reduction in 6 wk) were also present. The patient was referred to us by the general practitioner since he suspected from the symptom, the presence of a gastric neoplasia. In the past clinical history of the patient, no relevant disease was present and he underwent surgery at the age of 51 because of renal gallstones. No familiar history of gastric or esophageal cancer was reported.
Physical examination was normal and an accurate cardiological examination and electrocardiogram ruled out any cardiological origin of the pain. No alteration was found in the standard hematochemical determinations, in the hemachrome and in the differential blood count.
He firstly underwent an ultrasound (US) abdominal tomography on May 29, 2002 that did not show any relevant finding.
An esophago-gastro-duodenoscopy (EGDS) was performed just few days later, on June 4, 2002, and a biopsy during the procedure was performed in a erythematous area of the gastric antrum. No evidence of esophagitis was present. The histopathological examination performed on the gastric mucosal samples showed the presence of a superficial gastritis. Both the histopathological examination and urea breath test for H pylori were positive for the presence of this bacterium and the specific therapy with antibiotics and a proton pump inhibitor was immediately begun (amoxicillin 1000 mg along with clarithromicin 500 mg b.i.d. for a week and omeprazole 40 mg for the 1st wk and thereafter 20 mg for 3 wk once a day).
A further abdominal US examination performed by a different examiner from that of the first one did not reveal any pathological finding. However, despite the therapy, after 2 wk, the patient reported an increase of the epigastric pain and a posterior irradiation was associated in that occasion.
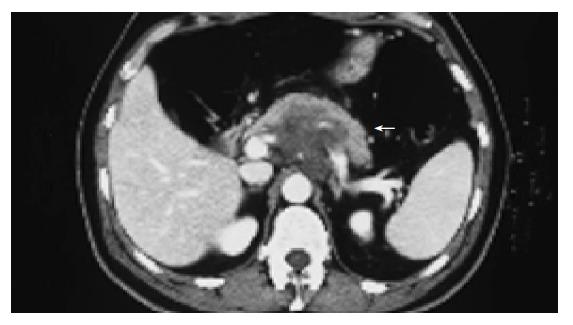
On July 19, 2002 an abdominal contrast-enhanced CT scan demonstrated the presence of a solid mass in the retroperitoneal space. Its size was 9.5 cm×6.5 cm×12 cm and was located superiorly between the left hepatic lobe and the stomach. The gastric corpus was displaced to the left, while the antro-pyloric region forward. No cleavage limits were observed at the contrast-enhanced CT scans between the mass, the kidneys and the pancreas. Some lymphadenopathies were present at pre- and para-aortic levels (Figure 1). Few days later the patient was referred to the Surgery Department because of a further increase of the pain. A port-a-cath catheter was placed in the right subclavian vein and an intravenous therapy for the pain with morphine chlorhydrate (up to 60 mg/d) and ketorolac salt of trometamol (40 mg/d) was started.
Since it did not appear to be possible to obtain a sample of the mass percutaneously both under US or CT guidance, the patient underwent an explorative laparotomy and a biopsy of the mass that appeared as a solid mass in the retroperitoneal space was taken. The histopathological examination revealed the presence of a B large-cell lymphoma. The immunohistochemical staining was positive for the following antigens: CD-45, CD-20, Bcl, Bcl-6, and MIB-1. Based on these data the disease was staged IV according to the Ann Arbor classification[5].
On August 29, 2002, the patient was eventually referred to our Day-Hospital Unit and was started on a treatment with CHOP modified regimen (cyclophosphamide 600 mg/m2 intravenously on d 1, epirubicin 55 mg/m2 intravenously on d 1, vincristine 1.2 mg/m2 intravenously on d 1, prednisone 60 mg/m2 on d 1-5). He was scheduled to receive seven administrations of this regimen starting from August 29, 2002 until March 10, 2003. The patient tolerated the therapy well. No major toxicity, as assessed by the CTC criteria[16], was observed during the whole period of the therapy administration.
The general condition improved just after the administration of the first course of treatment and continued to improve with the further courses of therapy. The Karnofsky performance status was 40% before treatment and improved to 100% after the fourth cycle. The pain was controlled during the treatment period with the administration of fentanyl via a transdermal device. The dosage was 75 μg/h at the beginning and it was reduced to 50 μg/h and finally to 25 μg/h during the second course of the treatment and completely discontinued after the third one. Anorexia and asthenia disappeared and the patient’s body mass index rose from 20 to 24 in about 6 mo.
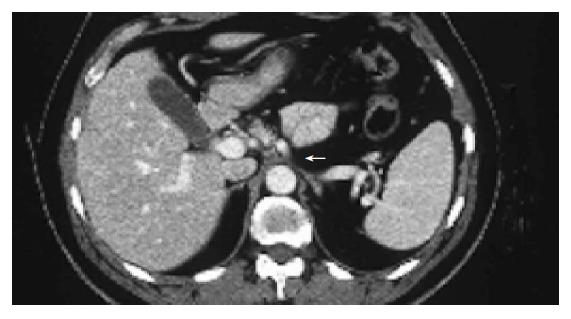
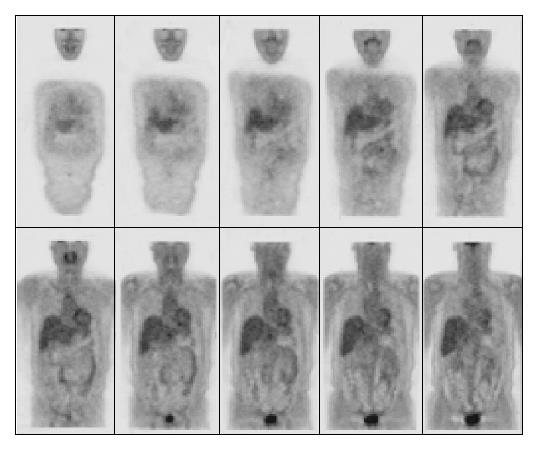
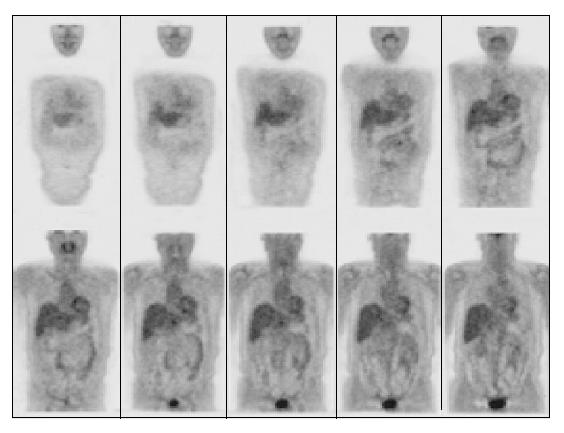
A contrast-enhanced CT scan of the abdomen was performed after the third treatment and it demonstrated a reduction of the mass with residual disease of about 1 cm localized at celiac trunk and it was considered as a partial response according to the SWOG criteria (Figure 2)[17]. The lymphoadenomegalies observed in the prior examination were still present. After the seventh course of treatment a total-body FDG-PET was performed and it did not show any pathological finding (Figure 3). However, 4 mo after the completion of the treatment (July 2003), a contrast-enhanced CT showed a minimal residual disease localized at the celiac tripod (Figure 4). Finally a further FDG-PET performed in October 2003 showed the complete remission of the disease (Figure 5).
Two months later the port-a-cath catheter was removed.
At the moment of writing this case report (April 4, 2004), the patient is alive and no evidence of a relapse of the disease is observed. His general condition is good and he entered the follow-up program.
A primary and unique retroperitoneal localization of NHL is quite rare and, according to our knowledge, only case that has been described yet[18], and a clinical presentation with an epigastric pain and ulcer-like dyspepsia mimicking a peptic disease is extremely rare. Abdominal NHL typically present themselves as a solid mass and they cause abdominal pain due to compression or infiltration of nerves and its pain is often referred as a diffuse pain[19]. Abdominal lymphomas often belong to the sclerosant variety of follicle centre lymphomas[19].
The symptoms presented in this case were very similar to that of esophagitis and gastric ulcer and the relationship with meals suggested to the general practitioner, the presence at least of a peptic-related disease or a gastric neoplasia since the patient had a previously known risk factor for gastric cancer (cigarette smoking) and the presence of asthenia, anorexia and the weight loss prompted the general practitioner to start a diagnostic workup oriented to gastric cancer. The negative result of the first abdominal US scan prompted us to perform a further US and a EGDS. This last examination was negative for gastric cancer and resulted only with the presence of a mild gastritis associated with H pylori infection. Even the second US resulted negative. Therefore, since the data obtained from the first investigations were in some way contradictory, further assessments were planned. Contrast-enhanced CT scans identified the mass, but no indication on the nature was obtained. A laparoscopic examination of the retroperitoneal space was thereafter planned and performed. So, the patient underwent several expensive investigations to achieve the diagnosis. The complexity of this clinical workup is probably due to the rarity of the disease by itself and also because of the uncommon clinical presentation. Most probably, the presence of a marked weight loss is the only symptom that could have oriented the diagnostic workup towards the suspicion of a neoplastic disease.
Indolent NHL is generally considered incurable. The CHOP, a first generation chemotherapy regimen, still represents the standard chemotherapy regimen for NHL treatment[20]. It obtains a complete response in about 45-53% of cases, with a long-term survival of 30-37%, and few and acceptable side-effects. The disease localization affects treatment choice, but generally the correct therapeutic approach to the extra-nodal NHL requires the integration of chemotherapy, radiotherapy and surgery.
Several regimens have been commonly so far used in the therapy of nodal and extra-nodal NHL. The response rate to the treatment is initially greater than 50% but the response and its duration decrease with subsequent chemotherapy. However, treatment has never been shown to extend overall survival.
The poly-chemotherapy with CHOP regimen has been considered as the standard first-line treatment for advanced large B-cell lymphoma[21] as no improvement in failure-free survival or overall survival, but increased toxic effects with newer regimens were shown with the less complicated and less expensive regimen of CHOP[24]. New strategies are being developed to improve the prognosis of these patients and the association of the CHOP regimen with rituximab, a human-mouse chimeric monoclonal anti-CD20 antibody that kills CD20-positive cells by activation of complement-dependent and antibody-dependent cell-mediated cytotoxicity, has been recently proposed[22-24].
In our case the choice of the CHOP regimen was funded and we obtained a complete remission. The use of the CHOP, a first generation regimen, should not be discontinued, since there is no improvement from the use of newer regimens[21]. However, the association of CHOP with mechanism-based drugs such as rituximab may further improve treatment outcomes especially in aggressive disease[22].
In this setting it appears clear that a correct pre- and post-treatment evaluation is mandatory to assess patient outcome, to evaluate the relapse risk and to establish the prognosis and in predicting survival and eventually to plan the follow-up[25]. Thus, it seems necessary to use more than one tool to evaluate disease extension and after the therapy residual disease.
The role of the functional metabolic imaging through FDG-PET has gained relevance in the staging and evaluation of response of various solid and hematologic malignancies. FDG-PET has recently emerged as an useful prognostic tool also in patients with aggressive lymphomas[13,14,26]. This kind of investigation performed few cycles after the chemotherapy can predict relapse risk[27,28]. The vast majority of patients with positive FDG-PET scans present poorer clinical outcomes as compared to patients with negative scans[28]. In patients where FDG-PET showed residual disease after treatment, relapses were reported in 100% of the cases, whereas a long-term survival was seen in more than 80% of patients with negative PET results[28].
Providing that these observations will obtain a confirmation, by FDG-PET we would be able to customize for the single patient with aggressive lymphoma, the appropriate treatment and follow-up. The patient of this case report underwent two FDG-PET and in both cases the negative result corresponded to a remission of the disease.
Besides its rarity and complexity, this case suggests that the use of FDG-PET in assessing the response to chemotherapy of extra-nodal NHL must be implemented and that FDG-PET could be the most useful predictive tool in assessing patient outcome. The use of morphologic tools (contrast-enhanced CT or magnetic resonance) in association with a functional metabolic imaging test appears to be mandatory also in the evaluation of extra-nodal aggressive NHL.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Hennessy BT, Hanrahan EO, Daly PA. Non-Hodgkin lymphoma: an update. Lancet Oncol. 2004;5:341-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Rossi D, Gaidano G. Molecular heterogeneity of diffuse large B-cell lymphoma: implications for disease management and prognosis. Hematology. 2002;7:239-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Pileri SA, Zinzani PL, Went P, Pileri A, Bendandi M. Indolent lymphoma: the pathologist's viewpoint. Ann Oncol. 2004;15:12-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Chiu BC, Weisenburger DD. An update of the epidemiology of non-Hodgkin's lymphoma. Clin Lymphoma. 2003;4:161-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Wotherspoon AC. Gastric lymphoma of mucosa-associated lymphoid tissue and Helicobacter pylori. Annu Rev Med. 1998;49:289-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 89] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | DeVita VT, Hellman S, Rosenberg SA, eds . Cancer: principles and practice of oncology, 6th edn. Philadelphia, PA: Lippincott Williams & Wilkins 2001; . [Cited in This Article: ] |
| 7. | Jaffe ES, Harris NL, Diebold J, Muller-Hermelink HK. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. A progress report. Am J Clin Pathol. 1999;111:S8-12. [PubMed] [Cited in This Article: ] |
| 8. | Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol. 1998;16:2780-2795. [PubMed] [Cited in This Article: ] |
| 9. | Marcus R. Current treatment options in aggressive lymphoma. Leuk Lymphoma. 2003;44 Suppl 4:S15-S27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, Glick JH, Coltman CA, Miller TP. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993;328:1002-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1566] [Cited by in F6Publishing: 1454] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 11. | Rankin SC. Assessment of response to therapy using conventional imaging. Eur J Nucl Med Mol Imaging. 2003;30 Suppl 1:S56-S64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Vinnicombe SJ, Reznek RH. Computerised tomography in the staging of Hodgkin's disease and non-Hodgkin's lymphoma. Eur J Nucl Med Mol Imaging. 2003;30 Suppl 1:S42-S55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Otsuka H, Graham M, Kubo A, Nishitani H. Clinical utility of FDG PET. J Med Invest. 2004;51:14-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology. 2004;231:305-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 584] [Cited by in F6Publishing: 532] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 15. | Kasamon YL, Wahl RL, Swinnen LJ. FDG PET and high-dose therapy for aggressive lymphomas: toward a risk-adapted strategy. Curr Opin Oncol. 2004;16:100-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Arbuck SG, Ivy SP, Setser A. The Revised Common Toxicity Criteria: Version 2.0. CTEP Website. Available from: http: //ctep.info.nih.gov. [Cited in This Article: ] |
| 17. | Green S, Weiss GR. Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Invest New Drugs. 1992;10:239-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 468] [Cited by in F6Publishing: 481] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 18. | Waldron JA, Magnifico M, Duray PH, Cadman EC. Retroperitoneal mass presentations of B-immunoblastic sarcoma. Cancer. 1985;56:1733-1741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 19. | Crump M, Gospodarowicz M, Shepherd FA. Lymphoma of the gastrointestinal tract. Semin Oncol. 1999;26:324-337. [PubMed] [Cited in This Article: ] |
| 20. | Seymour JF. New treatment approaches to indolent non-Hodgkin's lymphoma. Semin Oncol. 2004;31:27-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Fisher RI, Shah P. Current trends in large cell lymphoma. Leukemia. 2003;17:1948-1960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Coiffier B. Effective immunochemotherapy for aggressive non-Hodgkin's lymphoma. Semin Oncol. 2004;31:7-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Davis TA, Grillo-López AJ, White CA, McLaughlin P, Czuczman MS, Link BK, Maloney DG, Weaver RL, Rosenberg J, Levy R. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin's lymphoma: safety and efficacy of re-treatment. J Clin Oncol. 2000;18:3135-3143. [PubMed] [Cited in This Article: ] |
| 24. | Rastetter W, Molina A, White CA. Rituximab: expanding role in therapy for lymphomas and autoimmune diseases. Annu Rev Med. 2004;55:477-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 172] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 25. | Wooldridge JE, Link BK. Post-treatment surveillance of patients with lymphoma treated with curative intent. Semin Oncol. 2003;30:375-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Montravers F, McNamara D, Landman-Parker J, Grahek D, Kerrou K, Younsi N, Wioland M, Leverger G, Talbot JN. [(18)F]FDG in childhood lymphoma: clinical utility and impact on management. Eur J Nucl Med Mol Imaging. 2002;29:1155-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Blum RH, Seymour JF, Wirth A, MacManus M, Hicks RJ. Frequent impact of [18F]fluorodeoxyglucose positron emission tomography on the staging and management of patients with indolent non-Hodgkin's lymphoma. Clin Lymphoma. 2003;4:43-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Schot B, van Imhoff G, Pruim J, Sluiter W, Vaalburg W, Vellenga E. Predictive value of early 18F-fluoro-deoxyglucose positron emission tomography in chemosensitive relapsed lymphoma. Br J Haematol. 2003;123:282-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |