Published online May 21, 2005. doi: 10.3748/wjg.v11.i19.2900
Revised: June 25, 2004
Accepted: July 15, 2004
Published online: May 21, 2005
AIM: To investigate the characteristics of the progression of islet β cell function in Chinese latent autoimmune diabetes in adult (LADA) patients with glutamic acid decarboxylase antibody (GAD-Ab) positivity, and to explore the prognostic factors for β cell function.
METHODS: Forty-five LADA patients with GAD-Ab positivity screened from phenotypic type 2 diabetic (T2DM) patients and 45 T2DM patients without GAD-Ab matched as controls were followed-up every 6 mo. Sixteen patients in LADA1 and T2DM1 groups respectively have been followed-up for 6 years, while 29 patients in LADA2 and T2DM2 groups respectively for only 1.5 years. GAD-Ab was determined by radioligand assay, and C-peptides (CP) by radioimmune assay.
RESULTS: The percentage of patients whose fasting CP (FCP) decreased more than 50% compared with the baseline reached to 25.0% at 1.5th year in LADA1 group, and FCP level decreased (395.8±71.5 vs 572.8±72.3 pmol/L, P<0.05) at 2.5th year and continuously went down to the end of follow-up. No significant changes of the above parameters were found in T2DM1 group. The average decreased percentages of FCP per year in LADA and T2DM patients were 15.8% (4.0-91.0%) and 5.2% (-3.5 to 35.5%, P = 0.000) respectively. The index of GAD-Ab was negatively correlated with the FCP in LADA patients (rs = -0.483, P = 0.000). The decreased percentage of FCP per year in LADA patients were correlated with GAD-Ab index, body mass index (BMI) and age at onset (rs = 0.408, -0.301 and -0.523 respectively, P<0.05). Moreover, GAD-Ab was the only risk factor for predicting β cell failure in LADA patients (B = 1.455, EXP (B) = 4.283, P = 0.023).
CONCLUSION: The decreasing rate of islet β cell function in LADA, being highly heterogeneous, is three times that of T2DM patients. The titer of GAD-Ab is an important predictor for the progression of islet β cell function, and age at onset and BMI could also act as the predictors.
- Citation: Yang L, Zhou ZG, Huang G, Ouyang LL, Li X, Yan X. Six-year follow-up of pancreatic β cell function in adults with latent autoimmune diabetes. World J Gastroenterol 2005; 11(19): 2900-2905
- URL: https://www.wjgnet.com/1007-9327/full/v11/i19/2900.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i19.2900
Latent autoimmune diabetes in adults (LADA), screened from phenotypic type 2 diabetic (T2DM) patients by glutamic acid decarboxylase antibody (GAD-Ab) and characterized by slowly progressive islet β cell dysfunction, initially presents as non-insulin-dependent and easily misdiagnosed as T2DM[1-4]. Studies have shown that islet β cell function of LADA patients decreases with different velocity until being insulin-dependent and some factors can predict the failure rate of islet function[5-9]. However, the characteristics and risk factors of progression of β cell function in LADA remain unclear in Chinese. We measured GAD-Ab, as markers of autoimmunity, and fasting C-peptide (FCP), as a marker of β cell destruction in LADA patients and studied them prospectively for 6 years in order to unveil the unknown changes of β cell function in LADA patients. In this way we can offer suitable therapy including insulin or immune intervention for LADA at an early time to preserve their islet function.
We screened phenotypic T2DM, from endocrine ward and outpatient clinic in our hospital, with GAD-Ab. We would diagnose a phenotypic T2DM as LADA according to the criteria[10] of diagnosis of LADA as follows: (1) >25 year of age at onset; (2) no ketosis within half a year after diagnosis; (3) GAD-Ab positive. If the LADA patient still preserved some β cell function (defined as FCP>100 pmol/L), the patient would be enrolled in our follow-up study. From 1996 to 2001, we had screened 45 LADA patients, including 28 males and 17 females, with a mean age of 48.5±11.6 years (range 28-68 years). Mean FCP and 2-h-postprandial C-peptide (CP) level was 546.1±284.7 pmol/L (111-1194 pmol/L) and 1098.0±669.0 pmol/L (186.0-2317.0 pmol/L) respectively and median of GAD-Ab was 0.49±0.08 (0.05-1.91) at the beginning of the follow-up. Twenty-seven patients received insulin therapy, 13 were given sulfonylurea and biguanide, 4 were given biguanide alone and 1 was on diet control. Six patients were with family history of T2DM. Meanwhile, 45 other T2DM patients (included 31 males and 14 females), with a mean age of 48.3±10.2 years (range 26-68 years), with no history of ketosis or ketonic acidosis and was GAD-Ab negative were enrolled in this study as matched controls. Their mean FCP and 2-h-postprandial CP level were 753.0±401.2 pmol/L (270.7-2356.6 pmol/L) and 1469.8±462.0 pmol/L (540.0-3 972.0 pmol/L) respectively. Twenty-one of these patients received insulin therapy, 20 were given both sulfonylurea and biguanide, 4 were given biguanide alone. Five patients were with family history of T2DM. LADA and T2DM patients were followed every 6 mo (except the 24th mo). Fasting blood glucose, 2-h-postprandial glucose, HbA1c, FCP, 2hCP and GAD-Ab index were determined for all patients at each visitation. LADA1 (n = 16) and T2DM1 (n = 16) group patients had been followed for 72 mo (entered in 1996-1998), while LADA2 (n = 29) and T2DM2 (n = 29) for 18 mo (entered in 1999-2001). The patients with undetectable FCP/2hCP during the follow-up were excluded.
We established a healthy control group from epidemiological surveys in Changsha and sampled 188 healthy individuals to define the normal range of islet autoantibodies including GAD-Ab. One hundred and eighty-eight subjects were without family history of diabetes or autoimmune diseases (99 males and 89 females) with a mean age of 35.2±13.5 years (range 1.5-68.0 years) and with normal fasting and postprandial blood glucose levels.
GAD-Ab is determined by radioligand assays[11,12] in our laboratory and the assay was evaluated in the 3rd Diabetes Autoantibody Standardization Program (DASP 2003) sponsored by the Immunology of Diabetes Society (IDS) with 82% sensitivity and 98% specificity. The GAD65-Ab[13] plasmid (kindly provided by Dr. W.A. Hagopian, Seattle, WA, USA) is used in coupled in vitro transcription and translation (TnT, Promega, Madison, WI, USA) in the presence of 35S-methionine as previously described[14]. A human serum with high levels of immunoprecipitating autoantibodies was used as index positive control, and healthy serum from screening is used as negative control. All samples are tested in duplicate. Results are expressed as a normalized index, which is calculated using the formula: index = (unknown sample CPM-index negative control CPM)/(index positive control CPM-index negative control CPM). The inter-assay coefficients of variation were 7.1-10.8% (n = 5), and the intra-assay CV were 4.9-8.3% (n = 5) respectively. Based on the data from 188 healthy blood donors whose GAD-Ab indices ranged from -0.118 to 0.052, the cut-off for positivity was 0.052 (99.5 percentile).
CP was determined by radioimmune assay using a commercial kit (DePu Company, Germany). The inter-assay coefficients of variance were 9.1-15.0% (n = 3), and the intra-assay coefficients of variance were 3.7-8.6% (n = 3).
All data were expressed as mean±SD. All statistics were performed by using statistical procedure of social science (SPSS), including Student’s t test, Mann-Whitney U test, ANOVA or Friedman test, Pearson or nonparametric correlation analyses, stepwise multiple linear regressions in multivariate analysis, survival and Cox stepwise regression analysis. P values less than 0.05 were considered significant.
Baseline characteristics of the subjects are summarized in Table 1. LADA and T2DM patients were matched with age, sex, age at onset, duration, body mass index (BMI) and HbA1c. Two-hour-postprandial CP was significantly lower in LADA1 than T2DM1 group (P<0.05) and FCP and 2hCP were both lower in LADA2 than T2DM2 group (P<0.05, P<0.01).
| LADA1 | T2DM1 | LADA2 | T2DM2 | |
| Number | 16 | 16 | 29 | 29 |
| Sex (male/female) | 11/5 | 12/4 | 18/11 | 19/10 |
| Age (yr) | 47.7±11.2 | 47.0±7.9 | 49.0±12.0 | 52.2±10.9 |
| Age at onset (yr) | 44.9±10.5 | 44.2±7.4 | 46.0±11.1 | 49.9±10.1 |
| Duration (yr) | 2.7±0.9 | 2.8±0.5 | 3.0±0.6 | 2.2±0.4 |
| BMI (kg/m2) | 20.7±2.9 | 21.8±1.7 | 21.4±3.3 | 22.5±1.9 |
| FBG (mmol/L) | 14.5±5.3 | 13.7±1.7 | 14.2±5.6 | 13.5±3.1 |
| HbA1c (%) | 10.1± 0.6 | 9.9±1.9 | 9.2±2.2 | 8.6±2.0 |
| Family history (%) | 18.8 | 12.5 | 10.3 | 10.3 |
| Insulin therapy (%)FCP (pmol/L) | 56.3 (9/16) | 37.5 (6/16) | 65.5 (19/29) | 51.7 (15/29) |
| Postprandial-2-h | 572.8±72.3 | 714.6±63.7 | 531.3±53.2b | 772.7±86.1 |
| C-peptide (pmol/L) | 885.1±147.7a | 1196.7±104.2 | 1240.0±140.5b | 1659.9±188.5 |
Patients in the LADA1 group were followed for 5 years (from 4 to 6 years), LADA2 for 1.3 years (from 0.5 to 1.5 years).
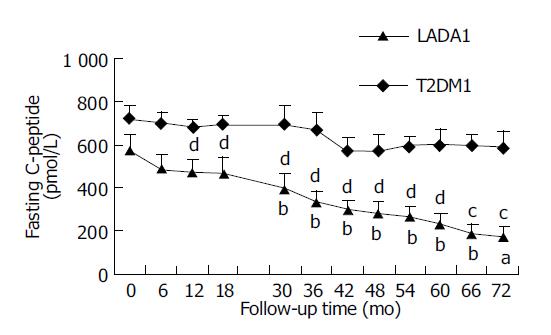
Figure 1 shows that FCP levels were not significantly different between LADA1 and T2DM1 patients at entry (P>0.05). In the LADA1 patients, CP levels did not change significantly during the 1st year of follow-up, but FCP declined from the 30th mo (P<0.05). T2DM1 patients did not show any significant decrease during the follow-up period (P>0.05).
The FCP, which was always lower in LADA2 than T2DM2 patients, did not change significantly, compared with their baseline data during their 1.5-year follow-up period in the two groups (P>0.01 and 0.01).
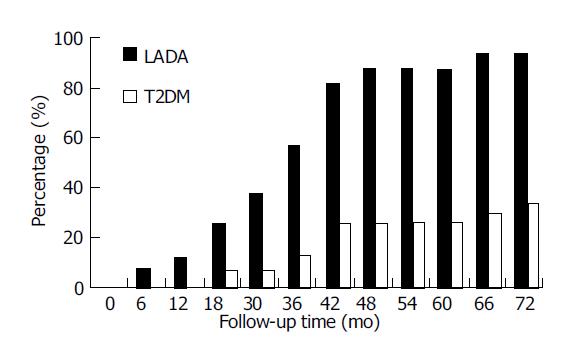
The proportion of LADA1 patients with islet function decreased more than 50% (Figure 2) The proportion of LADA1 patients whose islet function decreased by larger than 50% went up with time. This proportion in FCP increased from 6.25% at the 6th mo to 25.0% at the 18th mo (P<0.05) and reached to 93.8% at the 5.5th-6th year (P<0.01). While in T2DM1 patients, only 30.0% at the 5.5th-6th year (P<0.05).
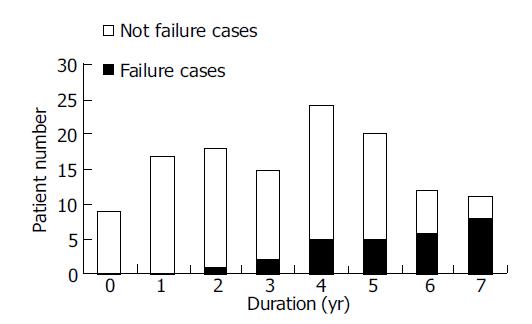
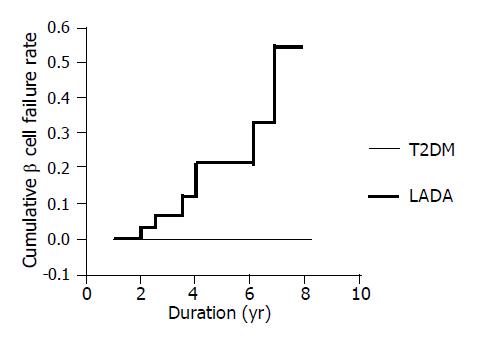
The relation between complete β cell failure and duration (Figure 3) There were eight LADA patients who progressed complete β cell failure (undetectable FCP) for about 4.4±1.9 years (2-6.8 years) after diagnosis until now. The frequency of β cell failure increased from 0 of 9 (0/9) patients at diagnosis to 8 of 11 (8/11) patients after 7 years. None of the T2DM patients developed complete β cell failure during the follow-up.
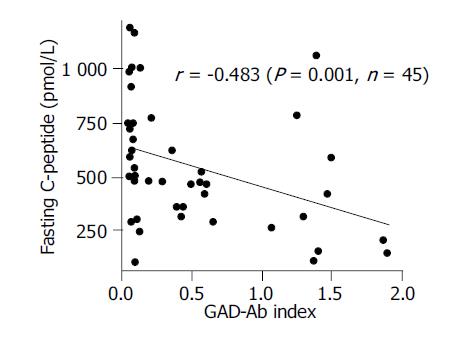
The relation between FCP and GAD-Ab index in LADA patients Baseline FCP levels of 45 LADA patients were negatively correlated with GAD-Ab index (r = -0.483, P = 0.001) (Figure 4), which indicated that the much higher titer of GAD-Ab that the LADA patient had, the more faster the failure of islet β cell function would occur.
Correlation analysis of the average decreased percentages of FCP per year in LADA patients (Table 2) We could calculate the average decreased percentages of FCP per year in 45 LADA patients through dividing the decreased value of FCP during the follow-up period with their initial FCP. As the follow-up times of LADA1 and LADA2 group were different, we further divided the above percentage with corresponding follow-up time for each patient. Then we got 15.8%/year (4.0-91.0%/year) as the average decreased percentage of FCP per year for LADA patients and 5.2%/year (-3.5 to 35.5%/year) for T2DM and their difference was significant (P<0.001). We also analyzed the correlation between the variables at entry with the above percentage in LADA group and found that the decreased percentage of FCP per year were correlated with GAD-Ab index, BMI and age at onset (rs = 0.408, -0.301 and -0.523 respectively, P<0.05).
| GAD-Ab Index | Age at onset (yr) | BMI (kg/m2) | Sex (m/f) | Duration (yr) | Family history | FBG (mmol/L) | HbA1c (%) | |
| Correlation efficient | 0.408 | –0.301 | –0.523 | 0.19 | –0.084 | –0.198 | 0.132 | –0.035 |
| P | 0.006 | 0.047 | 0.000 | 0.216 | 0.590 | 0.197 | 0.430 | 0.835 |
Multivariate analysis for β cell function in LADA patients A multiple stepwise regression analysis was used to identify the factors (age at onset, duration of disease, BMI, sex, HbA1c, FBS, family history and therapy protocol) to predict β cell failure in LADA patients. In analysis, duration of disease, GAD-Ab index, age at onset, and BMI by turns (according to their effects on FCP) were significant risk factors and the regression equation was as follows: y = -3.390*duration-3.153*GAD-Ab index+2.834*age at onset+2.264*BMI (F = 8.978, P = 0.000).
Survival analysis of β cell failure in LADA and T2DM patients (Figure 5) In order to further characterize the different prognosis of β cell function between LADA and T2DM patients, survival analysis was introduced to our study. We supposed the patient that developed β cell failure as “dead”, otherwise, as “survival” at different period of duration of disease (called “survival time”). We found two significantly different survival curves in the two groups and the cumulative proportion of β cell failure increased from 0.074 at the 2nd year to 0.462 at the 8th year of duration in LADA patients that was gradually rising with the duration; however, that of T2DM remained unchangeable in the whole period.
Risk factor analysis for β cell failure with Cox stepwise regression model GAD-Ab titer, age at onset, sex, BMI, FBS and HbA1c of patients at the diagnosis were introduced as covariates with “survival time” (defined as “the time before β cell failure”) and Cox setpwise regression model was used to identify the risk factor for β cell failure in LADA patients. With forward stepwise method, most factors were excluded and GAD-Ab titer was the only variable that entered the equation (B = 1.455, EXP (B) = 4.283, P = 0.023), which meant that the odds of β cell failure will increase 4.283 times with the increase of the GAD-Ab titer.
There has been four prospective studies with follow-up time for more than 5 years on LADA patients, including Finland’s 10-year study reported by Niskanen et al[5], in 1995, UKPDS’ 6-year by Turner et al[15], in 1997, Goetz et al’s[6], 5-year report on Americans in 2002 and 12-year study in Sweden by Borg et al[7], in 2002. Among the above studies, only the latter two were focusing on the relationship between β cell function and islet autoantibodies. Compared with the above reports, although there were some disadvantages such as small sample, we could still find some priorities in the study which included (1) more frequent, which meant more accurate to reflect the tiny change of islet β cell function; (2) analyze β cell function with different parameters, such as the changes of FCP value and its proportion, the changes from overall and from individual separately, correlation, multivariate regression analysis, survival analysis and Cox regression analysis; (3) using new index to assess the change of islet function such as “the proportion of LADA patients with islet function decreased more than 50%” and “the average decreased percentage of FCP per year”.
We found that FCP and 2-h PCP of LADA patients declined slowly during the follow-up. Though T2DM patients also showed a decreasing trend, there were no significant changes. Borg et al[7], reported that FCP of diabetics with GAD-Ab positivity decreased obviously, while those without GAD-Ab did not show any change during the 12-year prospective study, corresponding well with our results. We have observed that (1) the change of FCP value, which showed that FCP remained stable during the 1.5th year of follow-up, but began to decrease at the 2.5th year in LADA1 group; (2) the proportion of LADA patients with islet function decreased more than 50%, which already reached to 37.5% at 1.5th year (equivalent 3-5 years after diagnosis) in LADA patients, and the difference was significant compared with the baseline, while the T2DM patients did not show such changes until the 5.5-6th year (equivalent 8-9 years after diagnosis). In a word, the declining of β cell function developed about 3-4 years earlier in LADA than in T2DM patients.
LADA patients also showed heterogeneous descending rates of β cell function in the study. Firstly, the FCP in some patients presented a linearly decreasing way and even decreased to lower than 121.0 pmol/L (decreased 63.2%) in half a year and some progressed slowly that FCP maintained 680.0 pmol/L with 9-year duration and decreased more than 50% (65.4%) compared with baseline until 12.5 years later. Secondly, “the average decreased percentages of FCP per year (%/yr)” was calculated to reflect the decreasing rate of islet function. FCP of 45 LADA patients decreased 15.8%/year averagely and with the quickest 91%/year, the slowest 5.2%/year. However, that of T2DM patients was 5.2%/year which amount to one-third (1/3) of LADA’s and was similar with 4.5%/year in 6 years after diagnosis reported by UKPDS[16]. Thirdly, undetectable FCP was defined as β cell failure[7] and no one developed at entry, but the failure frequency increased to 1/18 at the 2nd year and to 8/11 at the 7th year. The fastest declining one needed only 2 years to lose insulin secretion function; nevertheless some patients still preserved β cell function even 15 years after diagnosis. The β cell failure in LADA patients experienced 4.4±1.9 years averagely and no one failed in T2DM patients during our current study. In addition, β cell failure was supposed as “dead” (otherwise “survival”) and then, we made a survival analysis in the two groups and found that β cell cumulative failure rate was rising with duration in LADA patients. In contrast, β cell failure did not occur with any T2DM patient at all times.
GAD-Ab, as the major immune marker for the diagnosis of LADA, and its predictive value for the failure of β cell function had been confirmed in many studies[2,5-8,15,17-20]. Moreover, the predictive value was related with the titer of GAD-Ab[7,8,15,17,19]. Borg et al[7], and Gottsater et al[17], found that GAD-Ab in high levels usually predicts much faster decrease of β cell function. The predictive value, highest with GAD-Ab titer more than 60 U, the second with titer from 20 to 60 U/L and worst with titer less than 20 U/L had been reported by UKPDS[15]. While there were still some studies[21] that suggested that GAD-Ab did not directly induce β cell failure. With GAD-Ab assay we identified the two groups of patients, and they showed completely different characteristics in the progression of β cell function, confirming the predictive value of GAD-Ab for islet function in LADA patients. We found that the initial GAD-Ab levels were negatively correlated with FCP, and antibody levels were positively associated with the average decreased percentages of FCP per year (%/yr). At last, the risk factor analysis of β cell failure in LADA with Cox stepwise regression model also revealed that GAD-Ab titer was the only factor responsible for β cell failure. All these suggested that GAD-Ab, as the most classic marker for diagnosing LADA, also provided the most important predictive value for the loss or failure of β cell function. It suggested that stronger immune damage results in faster loss of islet β cell function. Of 16 LADA1 patients, 56.3% (9/16) used insulin at the beginning of follow-up, and the proportion rose to 90% (9/10) at the end of follow-up, while only 37.5% (6/16) used insulin in T2DM1 subjects at entry and 44.4% (4/9) turned to insulin dependency at the end of follow-up. This not only reflected some different characteristics of two groups in progression of islet beta-cell function, but also demonstrated the predictive value of GAD-Ab for insulin requirement in LADA patients[6,12,17,18,22,23].
In this study, age at onset, BMI of LADA patients were negatively correlated with the average decreased percentages of FCP per year, which meant that much younger and thinner the patients were, much faster decrease of islet function would happen. It was similar with UKPDS and other reports[15,17,21,24] that the patients with younger age and smaller BMI at onset may present a linear and fast failure of β cell function, otherwise much slower. But someone[15] also pointed out that BMI was of no prognostic importance for islet function in LADA patients older than 45 years. Other factors including sex, family history, FBS and HbA1c did not show any significant relation with FCP in our study, which indicated that they were not key points for the β cell failure. To search for the major factors to predict islet function in LADA, a multiple regression analysis[25-27] had been applied. We identified GAD-Ab, age at onset, BMI and duration of disease as the best predictors. Duration, which was not linearly related with β cell function, entered the regression equation and it suggested that duration could predict β cell function in coordination with other factors. This also indicated the importance of assessing islet function with all the parameters combined together. Nevertheless, Cox stepwise regression model suggested that, among the above factors, only GAD-Ab could truly predict β cell failure in LADA patients, which further testified that this antibody not only was an important marker for the diagnosis of LADA but its titer could also predict the progress and prognosis of islet β cell function in LADA patients.
As β cell function of LADA was in a relatively steady state at early 3-5 years after diagnosis and duration was the important predictor for the deterioration of islet function, immune interventions, including insulin therapy, should be carried out as early as possible, especially for the patients with high titer of GAD-Ab, lower BMI and younger age at onset to delay the failure of β cell function[28-31].
We thank Dr. William A. Hagopian (University of Washington, Seattle, WA) for kindly providing the human GAD65 cDNA, and Dr. Thomas Dyrberg (Novo, Bagsvaerd, Denmark) for technical assistance in autoantibodies assay.
Co-first-authors: Lin Yang and Zhi-Guang Zhou
Co-correspondents: Lin Yang
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Tuomi T, Groop LC, Zimmet PZ, Rowley MJ, Knowles W, Mackay IR. Antibodies to glutamic acid decarboxylase reveal latent autoimmune diabetes mellitus in adults with a non-insulin-dependent onset of disease. Diabetes. 1993;42:359-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 304] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Hagopian WA, Karlsen AE, Gottsäter A, Landin-Olsson M, Grubin CE, Sundkvist G, Petersen JS, Boel E, Dyrberg T, Lernmark A. Quantitative assay using recombinant human islet glutamic acid decarboxylase (GAD65) shows that 64K autoantibody positivity at onset predicts diabetes type. J Clin Invest. 1993;91:368-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 142] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Gottsäter A, Landin-Olsson M, Fernlund P, Lernmark A, Sundkvist G. Beta-cell function in relation to islet cell antibodies during the first 3 yr after clinical diagnosis of diabetes in type II diabetic patients. Diabetes Care. 1993;16:902-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 80] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Tuomi T, Carlsson A, Li H, Isomaa B, Miettinen A, Nilsson A, Nissén M, Ehrnström BO, Forsén B, Snickars B. Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes. 1999;48:150-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 337] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | Niskanen LK, Tuomi T, Karjalainen J, Groop LC, Uusitupa MI. GAD antibodies in NIDDM. Ten-year follow-up from the diagnosis. Diabetes Care. 1995;18:1557-1565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 103] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Goetz FC, Roel J, Jacobs DR, Barbosa J, Hannan P, Palmer J, Hagopian W. Declining beta-cell function in type 2 diabetes: 5-year follow-up and immunologic studies of the population of Wadena, MN. Metabolism. 2002;51:144-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Borg H, Gottsäter A, Fernlund P, Sundkvist G. A 12-year prospective study of the relationship between islet antibodies and beta-cell function at and after the diagnosis in patients with adult-onset diabetes. Diabetes. 2002;51:1754-1762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Borg H, Gottsäter A, Landin-Olsson M, Fernlund P, Sundkvist G. High levels of antigen-specific islet antibodies predict future beta-cell failure in patients with onset of diabetes in adult age. J Clin Endocrinol Metab. 2001;86:3032-3038. [PubMed] [Cited in This Article: ] |
| 9. | Zimmet PZ, Tuomi T, Mackay IR, Rowley MJ, Knowles W, Cohen M, Lang DA. Latent autoimmune diabetes mellitus in adults (LADA): the role of antibodies to glutamic acid decarboxylase in diagnosis and prediction of insulin dependency. Diabet Med. 1994;11:299-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 266] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Zhou ZG, Wu HW. Diagnosis and treatment of latent autoimmune diabetes in adults (LADA). Zhonghua Neifenmi Daixie Zazhi. 1998;14:1-2. [Cited in This Article: ] |
| 11. | Petersen JS, Hejnaes KR, Moody A, Karlsen AE, Marshall MO, Høier-Madsen M, Boel E, Michelsen BK, Dyrberg T. Detection of GAD65 antibodies in diabetes and other autoimmune diseases using a simple radioligand assay. Diabetes. 1994;43:459-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 128] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Huang G, Zhou ZG, Peng J, Yan X, Zhu XP, Yang L, Li X, Wang JP, Jiang TJ. Detection of GAD-Ab index in diabetic patients using 35S-labeled recombinant human GAD65 antigen. Zhonghua Heyixue Zazhi. 2003;23:82-86. [Cited in This Article: ] |
| 13. | Falorni A, Ortqvist E, Persson B, Lernmark A. Radioimmunoassays for glutamic acid decarboxylase (GAD65) and GAD65 autoantibodies using 35S or 3H recombinant human ligands. J Immunol Methods. 1995;186:89-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 142] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Woo W, LaGasse JM, Zhou Z, Patel R, Palmer JP, Campus H, Hagopian WA. A novel high-throughput method for accurate, rapid, and economical measurement of multiple type 1 diabetes autoantibodies. J Immunol Methods. 2000;244:91-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Turner R, Stratton I, Horton V, Manley S, Zimmet P, Mackay IR, Shattock M, Bottazzo GF, Holman R. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet. 1997;350:1288-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 529] [Cited by in F6Publishing: 539] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 16. | U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995;44:1249-1258. [Cited in This Article: ] |
| 17. | Gottsäter A, Landin-Olsson M, Lernmark A, Fernlund P, Sundkvist G, Hagopian WA. Glutamate decarboxylase antibody levels predict rate of beta-cell decline in adult-onset diabetes. Diabetes Res Clin Pract. 1995;27:133-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Seissler J, de Sonnaville JJ, Morgenthaler NG, Steinbrenner H, Glawe D, Khoo-Morgenthaler UY, Lan MS, Notkins AL, Heine RJ, Scherbaum WA. Immunological heterogeneity in type I diabetes: presence of distinct autoantibody patterns in patients with acute onset and slowly progressive disease. Diabetologia. 1998;41:891-897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Törn C, Landin-Olsson M, Lernmark A, Palmer JP, Arnqvist HJ, Blohmé G, Lithner F, Littorin B, Nyström L, Scherstén B. Prognostic factors for the course of beta cell function in autoimmune diabetes. J Clin Endocrinol Metab. 2000;85:4619-4623. [PubMed] [Cited in This Article: ] |
| 20. | Törn C, Landin-Olsson M, Lernmark A, Scherstén B, Ostman J, Arnqvist HJ, Björk E, Blohmé G, Bolinder J, Eriksson J. Combinations of beta cell specific autoantibodies at diagnosis of diabetes in young adults reflects different courses of beta cell damage. Autoimmunity. 2001;33:115-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Rattarasarn C, Diosdado MA. Clinical characteristics, and time course of pancreatic beta-cell function and glutamic acid decarboxylase antibodies in Thai patients with adult-onset Type 1 diabetes: distinction between patients of rapid- and slow-onset. Horm Metab Res. 1999;31:311-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Littorin B, Sundkvist G, Hagopian W, Landin-Olsson M, Lernmark A, Ostman J, Arnqvist HJ, Blohmé G, Bolinder J, Eriksson JW. Islet cell and glutamic acid decarboxylase antibodies present at diagnosis of diabetes predict the need for insulin treatment. A cohort study in young adults whose disease was initially labeled as type 2 or unclassifiable diabetes. Diabetes Care. 1999;22:409-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Falorni A, Gambelunghe G, Forini F, Kassi G, Cosentino A, Candeloro P, Bolli GB, Brunetti P, Calcinaro F. Autoantibody recognition of COOH-terminal epitopes of GAD65 marks the risk for insulin requirement in adult-onset diabetes mellitus. J Clin Endocrinol Metab. 2000;85:309-316. [PubMed] [Cited in This Article: ] |
| 24. | Decochez K, Keymeulen B, Somers G, Dorchy H, De Leeuw IH, Mathieu C, Rottiers R, Winnock F, ver Elst K, Weets I. Use of an islet cell antibody assay to identify type 1 diabetic patients with rapid decrease in C-peptide levels after clinical onset. Belgian Diabetes Registry. Diabetes Care. 2000;23:1072-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Chiu KC, Lee NP, Cohan P, Chuang LM. Beta cell function declines with age in glucose tolerant Caucasians. Clin Endocrinol (Oxf). 2000;53:569-575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Bonfanti R, Bazzigaluppi E, Calori G, Riva MC, Viscardi M, Bognetti E, Meschi F, Bosi E, Chiumello G, Bonifacio E. Parameters associated with residual insulin secretion during the first year of disease in children and adolescents with Type 1 diabetes mellitus. Diabet Med. 1998;15:844-850. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Skyler JS, Rabinovitch A. Cyclosporine in recent onset type I diabetes mellitus. Effects on islet beta cell function. Miami Cyclosporine Diabetes Study Group. J Diabetes Complications. 1992;6:77-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Pozzilli P, Di Mario U. Autoimmune diabetes not requiring insulin at diagnosis (latent autoimmune diabetes of the adult): definition, characterization, and potential prevention. Diabetes Care. 2001;24:1460-1467. [PubMed] [Cited in This Article: ] |
| 29. | Kobayashi T, Nakanishi K, Murase T, Kosaka K. Small doses of subcutaneous insulin as a strategy for preventing slowly progressive beta-cell failure in islet cell antibody-positive patients with clinical features of NIDDM. Diabetes. 1996;45:622-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 83] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Kobayashi T, Maruyama T, Shimada A, Kasuga A, Kanatsuka A, Takei I, Tanaka S, Yokoyama J. Insulin intervention to preserve beta cells in slowly progressive insulin-dependent (type 1) diabetes mellitus. Ann N Y Acad Sci. 2002;958:117-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Li X, Zhou ZG, Huang G, Peng J, Yang L, Wang JP, Yang L. Rosiglitazone combined with insulin preserves Islet β Cell Function in LADA: A preliminary study. Zhongguo Tangniaobing Zazhi. 2003;11:242-246. [Cited in This Article: ] |