Published online Apr 14, 2005. doi: 10.3748/wjg.v11.i14.2101
Revised: March 20, 2004
Accepted: April 13, 2004
Published online: April 14, 2005
AIM: To study the effects of chromic-P32 phosphate (32P colloids) interstitial administration in Pc-3 implanted pancreatic carcinoma, and investigate its anticancer mechanism.
METHODS: Ninety-eight tumor bearing nude mice were killed at different time points after the injection of 32P colloids to the tumor core with observed radioactivity. The light microscopy, transmission electron microscopy (TEM) and immuno-histochemistry and flow cytometry were used to study the rates of tumor cell necrosis, proliferating cell nuclear antigen index, the micro vessel density (MVD). The changes of the biological response to the lymphatic transported 32P colloids in the inguinal lymph node (ILN) were dynamically observed, and the percentage of tumor cell apoptosis, and Apo2.7, caspase-3, Bcl-2, Bax-related gene expression were observed too.
RESULTS: The half-life of effective medication is 13 d after injection of 32P colloids to the tumor stroma, in 1-6 groups, the tumor cell necrosis rates were 20%, 45%, 65%, 70%, 95% and 4%, respectively (F = 4.14-105.36, P<0.01). MVD were 38.5±4.0, 28.0±2.9, 17.0±2.9, 8.8±1.5, 5.7±2.3 and 65.0±5.2 (t = 11.9-26.1, P<0.01), respectively. Under TEM fairly differentiated Pc-3 cells were found. Thirty days after medication, tumors were shrunk and dried with scabs detached, and those in control group increased in size prominently with plenty of hypodermic blood vessels. In all animals the ILN were enlarged but in medicated animals they appeared later and smaller than those in control group. The extent of irradiative injury in ILN was positively correlated to the dosage of medication. Typical tumor cell apoptosis could be found under TEM in animals with intra-tumoral injection of low dosed 32P colloids. The peak of apoptosis occurred in 2.96 MBq group and 24 h after irradiation. In the course of irradiation-induced apoptosis, the value of Bcl-2/Bax was down regulated; Apo2.7 and caspase-3 protein expression were prominently increased dose dependently.
CONCLUSION: 32P colloids intra-tumor injection having prominent anticancer effectiveness may reveal the ability of promoting cell differentiation. The low dose 32P colloids may induce human pancreatic carcinoma Pc-3 implanted tumor cell apoptosis; Apo2.7, caspase-3, Bcl-2 and Bax protein participated in regulating the process of irradiation induced cell apoptosis.
- Citation: Liu L, Feng GS, Gao H, Tong GS, Wang Y, Gao W, Huang Y, Li C. Chromic-P32 phosphate treatment of implanted pancreatic carcinoma: Mechanism involved. World J Gastroenterol 2005; 11(14): 2101-2108
- URL: https://www.wjgnet.com/1007-9327/full/v11/i14/2101.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i14.2101
High malignant pancreatic carcinoma mostly not diagnosed until in medium or late stage with the mortality remains high. Measures in clinical treatment of tumors have greatly advanced as the development of new preparations in cell growth inhibition, gene therapy, 125I seeds, sustained release chemotherapy seeds, etc., but a great deal of problems remain in the treatment of most solid tumor. The effectiveness of radioactive nuclide has not been fully recognized yet among the measures of conservative therapy. The aim of this study was to observe the biological distribution of chromic-P32 phosphate (Cr32PO4, 32P colloids) in tumor and regional lymph nodes, as well as its anticancer effect and the dose/time-dependent relationship after intra-tumoral injection of 32P colloids to treat the human pancreatic carcinoma cell (Pc-3) bearing models, and to explore its mechanism. Based on this experiment we further studied the effect of low dose 32P colloids on inducing apoptosis of transplanted Pc-3 cell in nude mice, and exploring its possible mechanism.
Medicine Medical 32P colloids were provided by Zhonghe Gaotong Isotope Co Ltd. Colloid particles 20-50 nm in diameter had stable property, specific radioactivity ratio 222-370 MBq/mL, radiochemical purity ≥98%, pH 6.0-8.0, 32P physical half-life 14.28 d, pure β-emitter, β ray mean energy 0.695 MeV (maximum energy 1.711 MeV), penetrating distance in soft tissue 3-4 mm on average.
Animal models After culturing and multiplying the human pancreatic carcinoma cell, Pc-3 2×106 cells were planted into each of the 10 BALB/c-nu/nu nude mice (male, mean body weight 20 g, 6-wk old, from Experimental Center, Shanghai Institute of Oncology, as in all of the following experiments) on the dorsum hypodermically. About 10 d after implantation, the tumor mass attained the diameter of 1.0 cm, the animals were killed by cervical dislocation. Under aseptic condition the tumor mass was dissected and sliced into pieces of 2.0 mm in diameter, rinsed in RPMI 1640 solution (no bovine serum) then the tumor tissue was implanted into the right axilla of 93 BALB/c-nu/nu nude mice subcutaneously. The animals were to be used in experiment as the tumor grew to 0.8-1.0 cm in diameter after 10 d.
Experiment and grouping Forty-eight tumor bearing nude mice were randomly allocated into eight groups, six mice in each group. 32P colloids in 3.7, 7.4, 14.8, 18.5, 29.6 and 0 (cold colloid as control) MBq, in the volume of 0.05-0.1 mL, were injected respectively to 1st-6th groups, then killed at the 14th d after medication. The 7th and 8th groups, 14.8 MBq and placebo were medicated respectively and bred for 30 d on average (maximum 40 d). Fifty tumor bearing nude mice were randomly allocated into 10 groups (n = 5). To the mice in 9th-14th groups 32P colloids of 0.37, 0.74, 1.48, 2.96, 5.92 and 0 MBq, were injected respectively, and then killed 24 h after medication. 32P colloids 1.48 MBq were injected to animals in 15th-19th group and then killed at 6, 12, 24, 36, and 48 h after medication respectively. Groups 11 and 17 were the same group. From the resected tumor masses the percentage of tumor cell apoptosis and necrosis, the changes in ultra structure and Apo2.7, caspase-3, Bcl-2, Bax-related gene expression were observed.
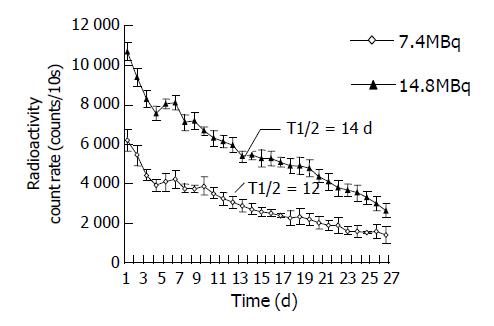
Estimation of the half-life of effectiveness In groups two and seven, the radioactivity counts on tumor surface were measured daily by ZC-201 surveying instrument with probe at fixed spatial geometric position, 6 cm above the tumor surface for 10 s, mean value of three measurements taken as the final value. Consecutive measurement was done for 14 and 28 d respectively (n = 12 before 14 d, n = 6 after 14 d). The effective half-life of intra-tumoral injection of 32P was calculated.
Pathological observation In 1st-8th groups, the tumor mass and bilateral inguinal lymph nodes (ILN) were dissected, sampled in several sites from each tissue, two pieces of tumor and normal tissues without necrosis under naked eye, 1 mL3 in size were taken, fixed in 2% glutaraldehyde, dehydrated, sliced in super-thin thickness, double stained with lead-uranium, then observed under TEM H-600. The remaining tumor or normal tissues were fixed in 10% formaldehyde immediately, embedded in paraffin then prepared for light microscopy (Olympus). The morphological changes of tumor were observed and the rate of tumor cell necrosis was calculated on 14th d. The whole course changes of bilateral groin lymph nodes in nude mice, 32P colloids transported in the lymphatic ducts and influence on the changes in ILN were dynamically observed. All of the paraffin slides were made to undergo microwave retrieval with tissue antigen, and dyed with SABC method. Estimation of positive expression of mouse against human proliferating cell nuclear antigen (PCNA) was done. When brown yellow colored granules presented in the tumor cell nuclei without plasma staining, it is defined as positive expression. Method of PCNA index (PI) calculation: Firstly under low and high power microscopes suitable visual fields with plenty of tumor cells were selected, then the cell number under high power microscope counted. From 5 to 10 high power visual fields in different tissues the number of positive cells and their PI were noted. PI = number of PCNA positive cells/number of tumor cells counted×100%. Cluster of differentiation 34 (CD34) is the specific surface marker of endothelial cell in tumor blood vessels. The positive expression signifies the formation of micro blood vessels. Methods of counting the micro blood vessel were that each slide was examined blindly by two expert pathologists, 10 visual fields with micro vessel density (MVD) under low power (×100) magnification were randomly selected, then the MVD under high power magnification counted, the mean value of MVD calculated. Any yellow stained cell or cell cluster signified as one MVD value.
Estimation of cell apoptosis and necrosis by Annexin V-FITC/PI double parameters flow cytometry was prepared A piece of fresh tumor 5-8 mm in diameter was cut, rinsed with PBS solution, the single cell suspension was prepared through digestion, grinding and filtration. Annexin V-FITC 5 μL (10 μg/mL) and PI 5 μL (50 μg/mL) were added to 100 μL cell suspension, kept at room temperature (20-25 °C), protected from light for 15 min, then assessed with flow cytometry, the data analyzed by Cellquest software program. For estimation of protein caspase-3 and Apo2.7, to each of 100 μL single cell suspension 10 μL of rabbit anti-caspase-3 polyclonal antibody and 20 μL mouse anti-Apo2.7 polyclonal antibody were added respectively, protected from light, reacted 20 min at 4 °C, rinsed twice with PBS; diluted with 1 mL PBS; and assessed by flow cytometry with exciting wave length 488 nm. Corresponding negative control was prepared. Percentage of positive cells were acquired and analyzed by Cellquest software program. Bax, Bcl-2 protein was estimated by immuno-histochemistry. The positive expression of Bax, Bcl-2 protein was the presentation of brown granules distributed in the cytoplasm of Pc-3 cells. Two expert pathologists examined each slide blindly through randomly counting 200 clear nucleated tumor cells. The percentage of positive cell in the total cells counted was calculated, and then the mean value was taken.
Observation under electron microscopy Samples were taken from several sites of the tumor prepared routinely by the super thin slides in 0.05 μm thickness, under H-600 model transmission electron microscopy (TEM), the changes in ultra structure of the tumor cell and morphology of the apoptosis cell were observed.
Estimating the dose absorbed Based on the equation of absorbed dose in β-internal irradiation the absorbed dose was calculated[1,2]:
D0=1.443 (Σ△i)C0Teff[1-e-(0.693/Teff)t] cGy------------(1)
where C0 means when t=0, the concentration of β radioactive nuclide in tissue (kBq/g); △i is the coefficient of the balanced absorbed dose in i type ray for releasing radioactive nuclide, expressed as g·cGy/kBq·h; △i of 32P is 0.04; Teff is the half-life of effectiveness; D0 is the absorbed dose in tissue at t time expressed as cGy (1 Gy = 100 cGy).
Analysis of variance was conducted after the rate of tumor cell necrosis and apoptosis in different dose group was converted to its square root, when the differences revealed statistical significance; Student-Newman-Keuls’ method (SNK) was employed. The value of MVD is expressed as mean value plus standard deviation (mean±SD), t -test between groups was conducted.
The effective half-life of 32P colloids 7.4 and 14.8 MBq groups were 12 and 14 d respectively, 13 d on average, equal to 312 h (Figure 1). The related data brought to equation (1), an equation for estimating the absorbed dose of interstitially injected 32P colloids was obtained.
Dβ = 19.78C0(1-e-0.002t)-----------(2)
General presentation Ten days after implantation of tumor cells, the tumor presented as a prominent solid nodular mass on the body surface, covered with smooth, light red colored skin. One week later, in groups 6 and 8 the tumor evidently increased in size with thinning of the superficial skin in bright and red color, in some cases ulceration on the covering skin occurred. In groups 1-5, 2-3 d after 32P colloids administration, the nude mice showed slightly decreased in appetite, local swollen and redness appeared on the injection sites, vanished 24 h later. In about 3-5 d, spotted ulceration appeared on tumor surface. At beginning tumor growth in therapeutic groups were comparatively slower than those in control group, after 7 d, ecchymosis appeared on the tumor surface, followed by cystic change of the tumor, rupture and bleeding took place, gradually shrunk and finally healed with scab. In groups 4, 5 and 7, 10-14 d after medication, scattered small petechiae appeared on the dorsum of six nude mice, in group 7 about 20 d later the petechiae diminished gradually, tumor size decreased then dried and the scab shed.
Light microscopy and immuno-histochemistry Animals in groups 1-6 were executed on 14th d, the weight of tumor, macroscopically average absorbed dosage in tumor tissues, rate of tumor cell necrosis, value of MVD and PI are shown in Table 1. The Pc-3 cells of control group 11 were densely arranged with plenty of blood vessels. In medication groups there revealed necrosis in different extents, or some loosely arranged tumor cells remained. In low dose groups there were remaining actively proliferated tumor cells and lymphocyte infiltration; and in high dose group, most of the tumor cells damaged instead by fibrotic connective tissues. In medicated nude mice their tissues of main viscera such as liver, lung, spleen, etc., were essentially normal, but in one case of group 5, epithelial anaphase and pearl formation occurred.
| Group | Injected dose(MBq) | Absorbed dose (Gy) | 14 d tumorweight (g) | Tumor cell necroticrate(%) | F | SNK1 | MVD2 | PI |
| 6 | 3.7 | 248.7 | 2.16±0.15 | 20 | 4.14 | A | 38.5±4.0 | 70 |
| 7 | 7.4 | 497.4 | 1.60±0.12 | 45 | 33.57 | B | 28.0±2.9 | 65 |
| 8 | 14.8 | 852.7 | 1.13±0.10 | 65 | 44.62 | C | 17.0±2.9 | 45 |
| 9 | 18.5 | 1147.8 | 0.86±0.09 | 70 | 54.93 | C | 8.8±1.5 | 20 |
| 10 | 29.6 | 2023.4 | 0.30±0.05 | 95 | 105.36 | D | 5.7±2.3 | 10 |
| 11 | 0 | – | 2.84±0.17 | 4 | – | E | 65.0±5.2 | 92 |
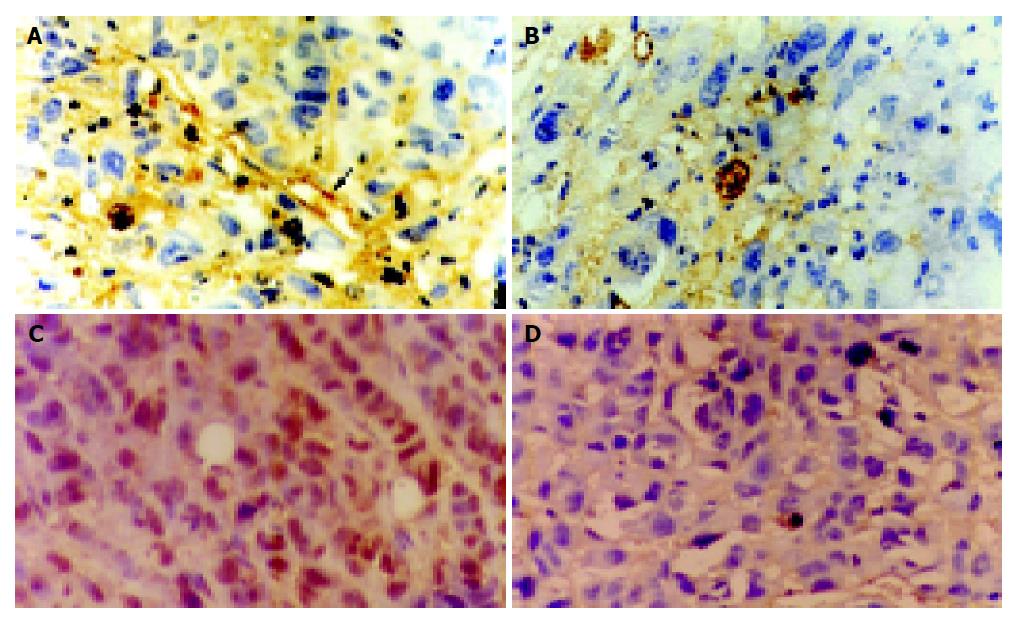
Results of CD34 and PCNA assay were shown in Figure 2. In control group CD34 was highly expressed, the tumor field showed plenty of stained micro-vessels, and poly-nucleated, deep brown yellow colored PCNA tumor cells; in medication groups, CD34 expression lowered prominently, tumor cells loosely arranged with scanty of micro-vessels stained or micro-vessels were not found. Only a few PCNA cell had nuclei stained light brown-yellow.
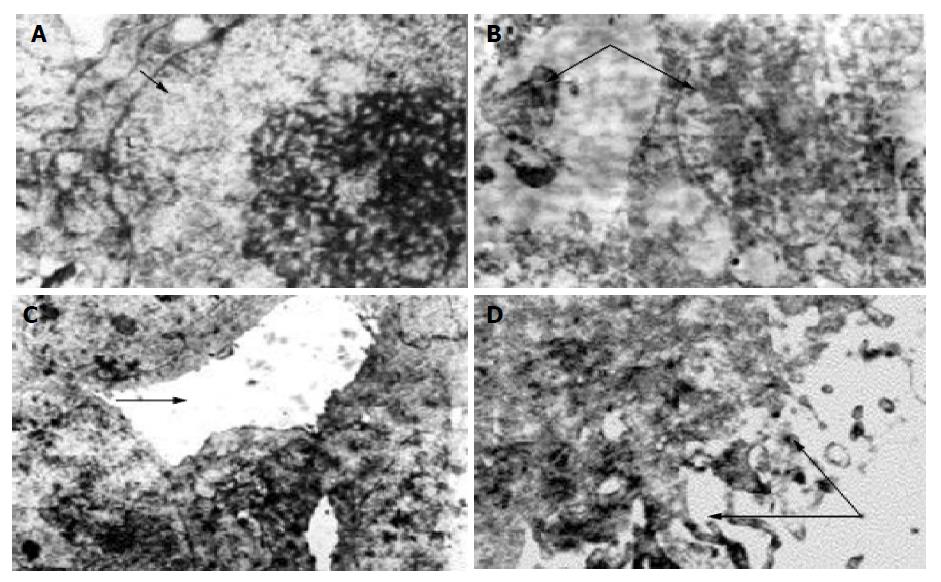
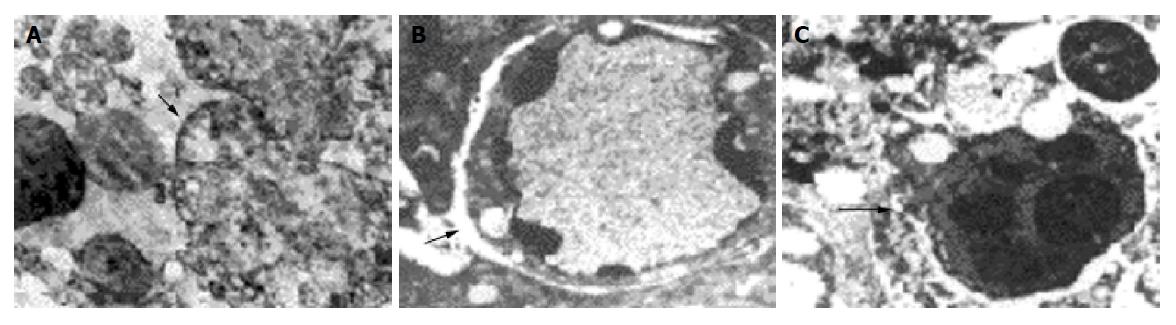
Transmission electron microscopy In control group the characteristic presentation of actively growing tumor cell was: large nuclei, poly-nucleoli, scanty cytoplasm, and some nuclear division might be found. There were few microvilli on the cell surface, bridging the connection between the neighboring cells with formation of pseudo-lumen. In medication groups with differences in absorbed dosages their presentations were: (1) Large numbers of necrotic Pc-3 tumor cells, breaking down into debris, a lot of vacuoles in the cytoplasm, in some cells only the contour of nucleus remained. Some apoptotic tumor cells could be found accompanied with shrunken and broken nuclei, bleeding in interstitium, many neutrocytes infiltrated between the necrosed cells, many collagen fibers and fibroblasts presented in the interstitium. (2) The remaining tumor cells showed swollen mitochondria, distension of endoplasmic reticulum with many lipid droplets, collagen fiber hyperplasia, bleeding and inflammatory cell infiltration in the interstitium. (3) Tumor cells have small nuclei and plenty of cytoplasm in which were presented many mitochondria and free ribosomes, many microvilli on the cell surface. (4) Some tumor cells were well-differentiated (Figure 3), some glandular lumen-like structure presented between the cells, many long microvilli presented on the cell surface. (5) No tumor cells were found in dry scab of the tumor debris, and in the muscular tissue around the tumor there were longitudinal myocomma, neutrocytes in interstitial tissue, and normal nervous structure.
Influence of 32P colloids on ILN (Figure 4) Twelve days after medication bilateral superficial ILN were palpable, while in control group the ILN were palpable 6 d earlier than those in medication group. The characteristics of swelling of ILN were control group>medicated mice, low dose group>high dose group. Under light microscope, lymphocytes uniformly distributed in ILN of group 6 (control group), manifested as normal glandular tissue without abnormalities in lipid tissue and blood vessels; in groups 1-5 the changes in ILN are positively correlated to the dosage of medication. Many plasma cells (rich in rough endoplasmic reticulum) in ILN of groups 1 and 2, showed chronic lymphadenitis; in groups 1-4, apoptotic cells could be found in connective tissue and endothelium of lymph sinus, and lymphocytes loosely distributed in group 5. The vital and denatured Pc-3 cells were found in ILN of control and medicated mice respectively.
Percentages of apoptosis and necrosis of tumor cells (Tables 2 and 3) In the dose range 0.37-5.92 MBq apoptosis rate tended to increase with the increase of dose absorbed, similar tendency occurred in necrotic rate. The apoptosis rate of the same dose (1.48 MBq) group gradually increased in the time range 6-24 h along with the prolongation of time, attained the peak at 24 h without evident plateau, and then tending to decrease. There were statistically significant differences in rates of cell apoptosis and necrosis between each dosage group and the control group. As compared with group 14, in groups 9-13 the expression of Pc-3 cell Apo2.7 protein and caspase-3 enhanced prominently, and increased along the increase of absorbed dose, existed a dose-response dependent relationship, the correlation coefficient r are 0.774 and 0.768 (n = 30, P<0.01); Bcl-2/Bax ratio is evidently lower than that of control group (F = 35.95, P<0.01).
| Group (n = 5) | Radioactivity(MBq) | Tumor absorbeddose (Gy) | Pc-3 cell apoptosisrate (%) rate | SNK | Pc-3 cell necrosis(%) | SNK | Apo2.7 positive (%) | Caspase-3positive (%) | Bcl-2/Bax |
| 14 | 0 | 0.00±0.00 | 0.67±0.15 | A | 1.8±0.5 | A | 4.9±1.8 | 7.69±1.5 | 2.07±0.85 |
| 9 | 0.37 | 2.89±0.20 | 10.09±1.84 | B | 2.2±0.6 | B | 8.3±2.4 | 10.43±2.6 | 1.64±0.62 |
| 10 | 0.74 | 5.78±0.25 | 17.36±4.33 | C | 6.0±1.1 | B | 13.6±2.3 | 13.46±2.9 | 1.02±0.52 |
| 11 | 1.48 | 11.56±1.29 | 21.85±3.04 | D | 11.7±0.5 | C | 15.0±3.2 | 14.92±4.1 | 0.81±0.32 |
| 12 | 2.96 | 23.12±3.12 | 33.67±3.69 | E | 13.1±1.0 | D | 16.3±3.1 | 18.68±4.6 | 0.37±0.12 |
| 13 | 5.92 | 46.23±4.20 | 27.76±4.09 | F | 23.4±2.6 | D | 27.3±4.9 | 25.57±5.3 | 0.07±0.03 |
| Group (n = 3) | Time (h) | Tumor absorbed dose (Gy) | Pc-3 apoptosis rate1(%) | SNK |
| 15 | 6 | 7.25±2.58 | 10.00±3.02 | A |
| 16 | 12 | 13.73±2.43 | 17.93±2.24 | B |
| 17 | 24 | 24.82±5.14 | 33.85±4.54 | C |
| 18 | 36 | 46.06±8.55 | 27.85±3.04 | D |
| 19 | 48 | 47.43±11.60 | 18.41±5.40 | E |
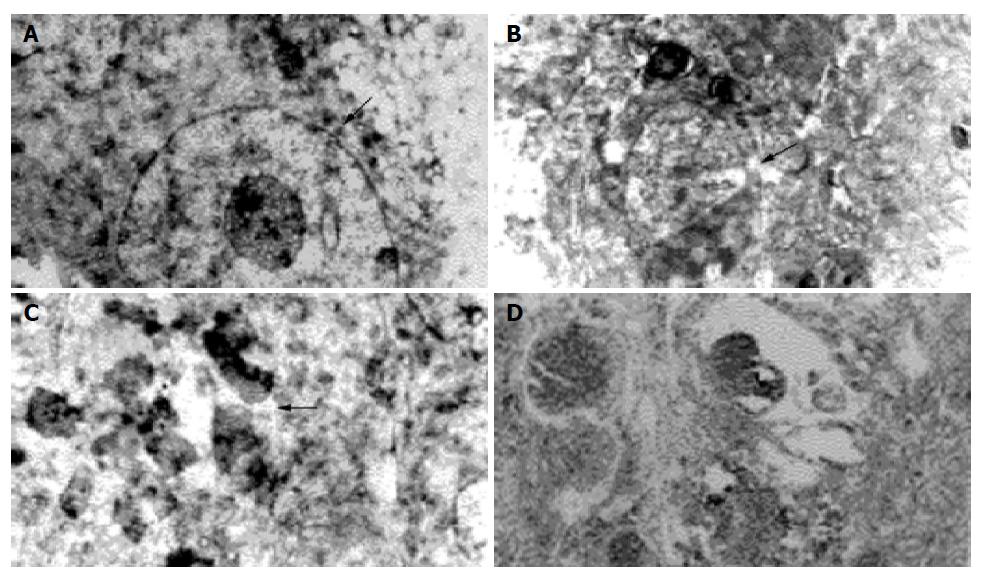
Transmission electron microscopy Normal Pc-3 cell was round in shape with large nucleus and several nucleoli, scanty cytoplasm, chromatin evenly distributed in the cell. The ultra structure unchanged, neither apoptosis nor necrosis was found in Pc-3 cell when the absorbed dose 2.89±0.20 Gy. Apoptosis cell and some necrotic picture could be found at absorbed dose 5.78±0.25 Gy (Figure 5). A great number of Pc-3 cells necrosed when absorbed dose 46.23±4.20 Gy. The characteristics of apoptosis cells were the volume of cell nucleus diminished, chromatin densely aggregated at periphery, vacuoles presented on some cell membrane, encapsulated plasmid and debris formed apoptosis body, structure of cell membrane and organelle remained intact. The characteristics of necrosed cells were condensed, ruptured or lyzed nucleus, prominently swollen mitochondria, broken cristae or vacuolation, rupture of cell membrane, and damaged organelle.
After interstitial injection of 99Tcm-sulfur colloids, the mechanism of their transportation in the regional lymphatic system has been applied to locate the pioneer lymph nodes in breast cancer[3]. Some authors have reported that phosphous-32 chromic phosphate in conjunction with platinum analog chemotherapy intraperitoneal injection for treatment of disseminated ovarian cancer in 30 cases the survival rate at 3 year was 63%[4]. In the recent years, some preliminary reports on the treatment of refractory malignant solid tumors or metastatic tumors have been published[5-8].
Radioactive nuclide internal irradiation in treating tumor has the character of continuity of radiation effective time and accumulation of response. 32P in a pure β-emitter by its direct or indirect effects of radioactive ionization destroys tumor cell membrane and structure of organelle membrane, leading to cell necrosis or apoptosis, finally inhibiting the proliferation of tumor cells. It was confirmed that: (1) the positive correlation of dose-response and time-response existed in the rates of tumor cell necrosis. Reciprocal relationship occurred between PI value and absorbed dose of tumor, suggesting PCNA of Pc-3 cell under the effect of 32P β ray, participating in DNA synthesis, is directly or indirectly inhibited, thereby the DNA synthesis and cell proliferation suppressed. (2) In low dosage group, the morphological characters of highly differentiated pancreatic carcinoma were observed. This is the phenomenon similar to 32P glass micro sphere interventional treatment of liver cancer[9] Further improved that irradiation in suitable dosage has the effect of inducing differentiation on tumor cells. (3) Neogenesis of blood vessel in tumor is one of the important premises in growth, infiltration and metastasis of malignant tumor. Quantitative analysis of CD34 expression in Pc-3 tumor tissue revealed MVD in treating groups was prominently lower than that of control group, and existed dose-response relationship with the absorbed dose, this suggests 32P colloids may inhibit the angiopoiesis of tumor by blocking the blood vessels in the lesions and their surroundings thus suppressing growth of Pc-3 cell and blood-borne metastasis, leading to death of tumor cells.
Lymphatic metastasis is the main manner of early metastasis in most malignant tumors. 32P colloids intratumoral injection is superior to other radionuclide preparations such as seeds[10] and microsphere[11-13], with the advantage of treating solid tumor as well as the early lymphatic metastasis at the same time. This study confirmed that: (1) In early implanted tumor, even with intact capsule, lymphatic metastasis already occurred. After the effect of 32P irradiation on ILN the tumor cells denatured and died. This explained the transport route of colloids in local lymphatic system after interstitial medication was basically similar to the dispersion route of primary tumor by the lymphatic ducts. (2) In medicated mice, the occurrence of ILN swelling was later in time and smaller in size than that in control; the extent of ILN swelling in medicated mice was reciprocally related to the dosage. This showed 32P had certain abilities of inhibiting the progress of lymphatic metastasis and exerting pursuing effects on occult metastatic foci in lymphatic drainage area of neighboring tumor. (3) The degree of irradiative injury to ILN became more severe with the increasing of 32P colloids activity injected to the tumor, after 4 wk the tissues repaired to normal. This explained the positive correlation between 32P colloids in ILN and the injected activity in tumor; irradiated injury to ILN was reversible after interstitial medication to tumor. (4) After the tumor mass received different irradiation doses, the picture of cell apoptosis was found in the sinus of ILN, suggesting irradiation at low dose had the effect of inducing apoptosis in actively proliferating cells. 32P colloids exerted influence on the morphology of ILN suggesting this therapeutical measure not only affects the primary tumor lesion but also prevention and treatment of tumor with occult lymphatic metastasis.
The conception of ionized irradiation in inducing cell apoptosis received increasingly great concern. We have studied the gene regulating mechanism of low dose interstitial injection of 32P colloids in inducing apoptosis of implanted human pancreatic carcinoma Pc-3 cells in nude mice. The experience confirmed: (1) After β ray 3-50 Gy internal irradiation for 6-48 h, apoptotic cells were found in Pc-3 cells, indicating these tumor cells are irradiation susceptible cells, sensitive to low dose irradiation. Based on the differences of sensitivity of tumor and normal tissues in inducing apoptosis, we might selectively induce cell apoptosis with low dose irradiation, eliminating the tumor cells, at the same time protecting normal cells[14], minimizing the adverse injury in internal irradiation therapy. (2) When the absorbed dose was at 2.89±0.20 to 23.12±3.12 Gy, the increase of percentage in cell apoptosis was parallel to the increase of dosage, this conclusion coincides with in vitro or other experiments[15]. As the absorbed dose increased continuously to 46.23±4.20 Gy, the apoptosis rate was prominently lower than that of low dosage group, there was clearly dose-response relationship between them, and the cell necrosis rate was positively correlated to the absorbed dose. The changes of apoptosis and necrosis are related to the intensity of injury and the continuity of stimuli, suggesting the onset of apoptosis and necrosis and their regulating mechanism are related to the time and intensity thresholds of the external injury. (3) The irradiation induced cell apoptosis was related to regulation of several genes, in which the closely related genes are p53, Bcl-2 and Bax, etc. Gene Bcl-2 is a kind of apoptosis inhibiting gene, gene Bax is a kind of apoptosis promoting gene, introduction of Bax gene may accelerate cell apoptosis, and mutation of gene Bax may inhibit apoptosis. Gene Bcl-2/Bax gave important effects on regulating cell apoptosis. Generally the ratio of Bcl-2/Bax decides whether the cell is apoptosis or not[16]. The results of this experiment showed that in the process of β radiation induced apoptosis of Pc-3 cells, genes Bcl-2 and Bax participated in regulation, prominently down regulation of Bcl-2/Bax ratio. This injury of DNA molecule resulted from ionized irradiation, activated p53 exerted transcription effect to activate Bax gene, and elevate Bax mRNA and protein expression, forming Bax homologous dimerism polymer. The ratio of Bcl-2/Bax lowered, cell apoptosis was induced. (4) Recent study discovered that mitochondria event was the key event in cell apoptosis; the most important process was the activation of caspase proteinase family. Caspase-3 is the most important responsive caspase in the family, responsible for the cleavage of all or part of the proteinase in the process of cell apoptosis, after activation of caspase cells will irreversibly progress to apoptosis[17,18]. In this experiment we found that caspase-3 protein expression was prominently increased after the action of 32P colloids; it confirmed the mechanism of initiating apoptosis by the action of 32P colloids. (5) Apo2.7 protein is a new quantitative marker of cell apoptosis, reacting with the membranous protein of 38 ku mitochondria, and denoting in early stage of cell apoptosis related to molecular cascade of cell apoptosis[19]. From the results of this experiment, Apo2.7 protein expression was evidently enhanced, and showed dose-response relation with the absorbed dose. (6) Cell apoptosis is a very complicated biological process involving multiple regulating genes and signal transmitting paths. Different dose of irradiation induced cell apoptosis underwent different mechanisms, but the same dose of irradiation might have several pathways to arouse apoptosis. In this experiment it was found that at a particular time point (24 h), following the increase of absorbed dose in tumor, the expression of apoptosis related gene was up-regulated (caspase-3, Apo2.7) or down-regulated (Bcl-2/Bax), the rate of apoptosis did not raise when the absorbed dose increased, but decreased in the high dose group. It is possible other than the above mentioned regulating genes, some unknown apoptosis regulating genes participated in this process, which needs to be further studied.
Biological distribution of 32P colloids greatly influenced absorbed dose in tumor and the toxic side effects. This study[20] confirmed that: radioactive nuclides after interstitial injection of 32P colloids, are mainly condensed in the tumor mass with prolonged duration, and the amount distributed in the organs other than tumor was very less. This study confirmed that the effective half-life of 32P colloids in tumor is nearly the same to its physical half-life. The characteristic curve of radioactivity counting rate on tumor surface manifested as: within 4 d after injection the radioactivity decreased exponentially, then 2 d later raised again, presented as a small peak in the curve, followed by a tendency of continuously gradual decline. After interstitial medication of 32P colloids, they were engulfed and transported by phagocytes through the lymphatic circulation to the drained lymph nodes or micro-vessels, and finally attained by the tumor tissue rich in blood supply, the circulation was repeated continuously, the total body radioactivity decreased concomitantly with elapse of time and excretion, the radioactivity decreased rapidly in normal tissues and organs, while in the tumor mass the ratio of its radioactivity to the total activity of whole body was continuously elevated. Therefore T/N ratio was high, signifying the irradiative dose was highly acquired by the tumor, scarcely distributed to other parts of the body. In this experiment we found that under the condition of high dose ionized irradiation, coagulative necrosis happened in Pc-3 cells with fair tumor inhibition response, but scattered small petechiae presented on the dorsum of six experimental nude mice and some mice getting emaciated distinctly. Morphological examination revealed fibrotic changes in lung, anaplasia in the epiderma covering the tumor. As the absorbed dose was ≤853 Gy, it attained ideal anti-cancer effectiveness, no general toxic side effects were found in the experimental animals. Therefore it is important to choose the suitable dose of interstitial medication, so as to attain the maximum effect on tumor cells and minimize irradiative injury to other tissues and organs as far as possible.
| 1. | Tang ZY. Modern Oncology. 2nd Ed. Shanghai: Shanghai University of Medical Science Press 2000; 1132-1134. [Cited in This Article: ] |
| 2. | Qin MX, Diao GP. Clinical Interventional Nuclear Medicine. 1st Ed. Tianjin Scientific Technology Press 1996: 11-13. . [Cited in This Article: ] |
| 3. | Shen K, Nirmal L, Han Q, Wu J, Lu J, Zhang J, Liu G, Shao Z, Shen Z. Sentinel lymph node biopsy in breast cancer. Zhonghua WaiKe ZaZhi. 2002;40:347-350. [PubMed] [Cited in This Article: ] |
| 4. | Pattillo RA, Collier BD, Abdel-Dayem H, Ozker K, Wilson C, Ruckert AC, Hamilton K. Phosphorus-32-chromic phosphate for ovarian cancer: I. Fractionated low-dose intraperitoneal treatments in conjunction with platinum analog chemotherapy. J Nucl Med. 1995;36:29-36. [PubMed] [Cited in This Article: ] |
| 5. | Dempke W, Firusian N. Treatment of malignant pericardial effusion with 32P-colloid. Br J Cancer. 1999;80:1955-1957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Firusian N, Dempke W. An early phase II study of intratumoral P-32 chromic phosphate injection therapy for patients with refractory solid tumors and solitary metastases. Cancer. 1999;85:980-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 7. | Order SE, Siegel JA, Principato R, Zeiger LE, Johnson E, Lang P, Lustig R, Wallner PE. Selective tumor irradiation by infusional brachytherapy in nonresectable pancreatic cancer: a phase I study. Int J Radiat Oncol Biol Phys. 1996;36:1117-1126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Order SE, Court WS. Models of clinical infusional brachytherapy. Int J Radiat Oncol Biol Phys. 2001;50:279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 9. | Liu L, Jiang Z, Teng GJ, Song JZ, Zhang DS, Guo QM, Fang W, He SC, Guo JH. Clinical and experimental study on regional administration of phosphorus 32 glass microspheres in treating hepatic carcinoma. World J Gastroenterol. 1999;5:492-505. [PubMed] [Cited in This Article: ] |
| 10. | Zelefsky MJ, Hollister T, Raben A, Matthews S, Wallner KE. Five-year biochemical outcome and toxicity with transperineal CT-planned permanent I-125 prostate implantation for patients with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2000;47:1261-1266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 214] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Liu L, Teng GJ, Jiang Z, He SC, Xie SL, Wu LS, Song FJ, Guo JH, Fang W, Zhao CG. Preliminary clinical application of phosphorus-32 glass microspheres in interventional treating hepatocellular carcinoma. Hejishu. 2000;23:316-322. [Cited in This Article: ] |
| 12. | Liu L, Teng GJ, Zhang DS, Song JZ, He SC, Guo JH, Fang W. Toxicology of intrahepatic arterial administration of interventional phosphorus-32 glass microspheres to domestic pigs. Chinese Med J. 1999;112:632-636. [Cited in This Article: ] |
| 13. | Nijsen F, Rook D, Brandt C, Meijer R, Dullens H, Zonnenberg B, de Klerk J, van Rijk P, Hennink W, van het Schip F. Targeting of liver tumour in rats by selective delivery of holmium-166 loaded microspheres: a biodistribution study. Eur J Nucl Med. 2001;28:743-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Fan ZP. Ionized radiation and cell apoptosis. Haijun Yixue Zazhi. 2000;21:83-85. [Cited in This Article: ] |
| 15. | Akagi Y, Ito K, Sawada S. Radiation-induced apoptosis and necrosis in Molt-4 cells: a study of dose-effect relationships and their modification. Int J Radiat Biol. 1993;64:47-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Lee JU, Hosotani R, Wada M, Doi R, Kosiba T, Fujimoto K, Miyamoto Y, Tsuji S, Nakajima S, Nishimura Y. Role of Bcl-2 family proteins (Bax, Bcl-2 and Bcl-X) on cellular susceptibility to radiation in pancreatic cancer cells. Eur J Cancer. 1999;35:1374-1380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Kirsch DG, Doseff A, Chau BN, Lim DS, de Souza-Pinto NC, Hansford R, Kastan MB, Lazebnik YA, Hardwick JM. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J Biol Chem. 1999;274:21155-21161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 350] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 18. | Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5130] [Cited by in F6Publishing: 5034] [Article Influence: 193.6] [Reference Citation Analysis (0)] |
| 19. | Zhang C, Ao Z, Seth A, Schlossman SF. A mitochondrial membrane protein defined by a novel monoclonal antibody is preferentially detected in apoptotic cells. J Immunol. 1996;157:3980-3987. [PubMed] [Cited in This Article: ] |
| 20. | Gao W, Liu L, Yin QH, Wang ZZ, Wang Y, Duan MH, Huang Y, Zhang DS, Li C. Biodistribution pharmacokinetics and toxic effect of P-32 chromic phosphate colloids interstitially injected into human pancreatic cancer (Pc-3) bearing nude mice. Zhonghua Fangsheyixue Yu Fanghuzazhi. 2004;24:23-25. [Cited in This Article: ] |