Published online Dec 1, 2004. doi: 10.3748/wjg.v10.i23.3424
Revised: January 8, 2004
Accepted: January 15, 2004
Published online: December 1, 2004
AIM: To prepare the human hepatocellular carcinoma-(HCC)-targeted liposome microbubbles and to investigate their immunological properties.
METHODS: Human hepatocarcinoma specific monoclonal antibody HAb18 was attached to the surface of home-made liposome microbubbles by static attraction to prepare the targeted liposome microbubbles. The combination of HAb18 with liposome microbubbles was confirmed by the slide agglutination test and immunofluorescent assay. Their immunological activity was measured by ELISA. Rosette formation test, rosette formation blocking test and immun-ofluorescent assay were used to identify the specific binding of targeted liposome microbubbles to SMMC-7721 hepatoma cells, and cytotoxicity assay was used to detect their effect on human hepatocytes.
RESULTS: The targeted liposome microbubbles were positive in the slide agglutination test and immunofluorescent assay. ELISA indicated that the immunological activity of HAb18 on the liposome microbubbles was similar to that of free HAb18. SMMC-7721 cells were surrounded by the targeting liposome microbubbles to form rosettes, while the control SGC-7901 gastric cancer cells were not. Proliferation of SMMC-7721 cells and normal human hepatocytes was not influenced by the targeted liposome microbubbles.
CONCLUSION: The targeted liposome microbubbles with a high specific biological activity have been successfully prepared, which specifically bind to human hepatocarcinoma cells, and are non-cytotoxic to hepatocytes. These results indicate that the liposome microbubbles can be used as a HCC-targeted ultrasound contrast agent that may enhance ultrasound images and thus improve the diagnosis of HCC, especially at the early stage.
- Citation: Bian AN, Gao YH, Tan KB, Liu P, Zeng GJ, Zhang X, Liu Z. Preparation of human hepatocellular carcinoma-targeted liposome microbubbles and their immunological properties. World J Gastroenterol 2004; 10(23): 3424-3427
- URL: https://www.wjgnet.com/1007-9327/full/v10/i23/3424.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i23.3424
Targeted ultrasound imaging is a promising method of imaging diagnosis[1]. This technique can significantly improve the sensitivity and specificity of ultrasonic diagnosis. At present, several targeted ultrasound contrast agents that can enhance imaging of specific tissues, such as thrombus-specific and inflammatory tissue-specific targeted ultrasound contrast agent, have successfully been developed abroad[2-15], while study on hepatocellular carcinoma-targeted ultrasound contrast agent has not been reported.
In this study, liposome microbubbles containing fluorocarbon gases were prepared in our laboratory. Then human hepatocarcinoma specific monoclonal antibody HAb18 was attached to the surface of home-made liposome microbubbles to prepare targeted liposome microbubbles. Finally the immunological properties of targeted liposome microbubbles were investigated.
Human hepatocarcinoma specific monoclonal antibody HAb18 was kindly provided by Research Centre of Cell Engineering, the Fourth Military Medical University. Rabbit anti-mouse serum was purchased from Beijing Zhongshan Biotechnology Co.,Ltd,China. FITC conjugated sheep anti-mouse IgG and HRP conjugated sheep anti-mouse IgG were purchased from Huamei Bioengineering Company,China. MTT was purchased from Sigma. Human hepatoma cell line SMMC-7721 and human gastric carcinoma cell line SGC-7901 were maintained in our laboratory. Primary cultures of normal human hepatocytes were established from human liver tissue collected from person who died in accident.
The lipid film prepared previously in our laboratory and some mediators were mixed in proportion[16]. Then the resulting mixture was sonicated, into which perfluoropropane gas was injected. The concentration and size of the microbubbles were measured under microscope. Zeta potential of the microbubbles was determined by the Chongqing Comed Nanopharma Co., Ltd, China.
Human hepatocarcinoma specific monoclonal antibody HAb18 was added to the liposome microbubble suspension in proportion and mixed for 2 h at pH4.0, 4 °C[17]. After the mixture was separated into 2 distinct layers, the lower layer was discarded and the upper layer was washed three times with phosphate-buffered saline (PBS) to elute the free HAb18.
Slide agglutination test A drop of rabbit anti-mouse serum or normal saline was mixed with a drop of targeted liposome microbubbles or liposome microbubbles respectively for about 5 min. The results were observed under a × 100 field of microscope.
Immunofluorescent assay A 100 μL of FITC conjugated sheep anti-mouse IgG was put into 200 μL of targeted liposome microbubbles and mixed for 30 min at 4 °C. After the mixture was separated into 2 distinct layers, the lower layer was discarded and the upper layer was washed three times with PBS to elute the free FITC conjugated sheep anti-mouse IgG. Then the microbubbles were observed under fluorescence microscope.
SMMC-7721 cells (1 × 10 5) were placed in each well of a 96-well ELISA plate and cultured overnight. The cells were fixed in 0.25 g/L glutaraldehyde for 20 min. A 30 g/L of defatted milk powder was added and incubated for 1 h at 37 °C. Different concentrations of targeted liposome microbubbles or free HAb18 were added and incubated for 1.5 h at 37 °C. HRP conjugated sheep anti-mouse IgG was added and incubated for 1.5 h at 37 °C. O-phenylenediamine (OPD) and H2O2 were added and incubated for 10 min at 25 °C. A 2 mmol/L of H2SO4 was added to terminate the reaction. The optical absorbance (A) value at 490 nm was detected with ELISA.
Rosette formation test[18] Log phase SMMC-7721 cells and SGC-7901 cells were digested with 2.5 g/L trypsin. The isolated cells were adjusted to a concentration of 2 × 10 5/mL in PBS respectively. One hundred μL of targeted liposome microbubbles or liposome microbubbles was added to 200 μL of SMMC-7721 or SGC-7901 cell suspension and mixed for 30 min at 25 °C. The resulting mixture was observed under microscope and the cells that formed rosette were counted.
Rosette formation blocking test SMMC-7721 cells were mixed with different concentrations of HAb18 for 30 min at 25 °C. The mixture was centrifuged and washed with PBS to elute free HAb18. Then targeted liposome microbubbles were added to cell suspension and mixed for 30 min at 25 °C. Cells forming rosette were counted under microscope.
Immunofluorescent assay one hundred μL of targeted liposome microbubbles combined with FITC conjugated sheep anti-mouse IgG and 200 μL of SMMC-7721 cells or SGC-7901 cells were mixed for 30 min at 25 °C. The suspension was observed under fluorescence microscope.
SMMC-7721 cells or human hepatocytes (1 × 10 5 per well) were added into a 96-well culture plate. About three hours later different concentrations of targeted liposome microbubbles were added, while PBS was used as control. Three wells were used for each concentration. They were cultured in a CO2 incubator at 37 °C for 48 h, then MTT (2 g/L, 50 μL/well) was added and incubated at 37 °C for 6 h. The medium was discarded, and 100 μL/well DMSO was added. The culture plate was shaken for 10 min and the optical absorbance (A) value at 570 nm was detected with ELISA.
The average diameter of liposome microbubbles ranged from 2 to 5 μm, the concentration was 7 × 10 9 bubbles/mL (Figure 1). The mean zeta potential was -71.2 mV. Animal experiment demonstrated that the microbubbles could significantly enhance the ultrasound images of several tissues.
The average diameter of targeted liposome microbubbles was similar to that of liposome microbubbles. HAb18 did not affect the size of microbubbles. The concentration was 5 × 10 8 bubbles/mL.
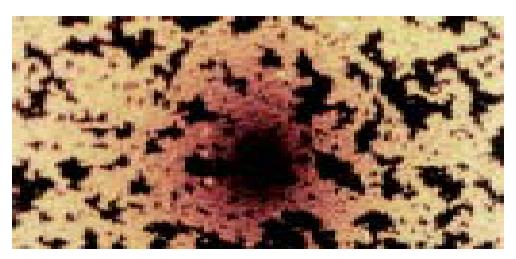
After rabbit anti-mouse serum was added, targeted liposome microbubbles agglutinated (Figure 2), but liposome microbubbles did not. While normal saline (NS) did not lead to agglutination of two kinds of microbubbles.
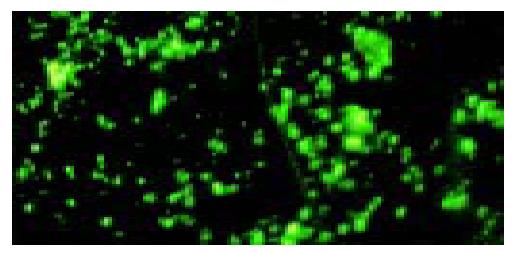
The surfaces of targeted liposome microbubbles gave out bright yellow-green fluorescence after they were stained with fluorescent agents (Figure 3), whereas liposome microbubbles did not give out any fluorescence.
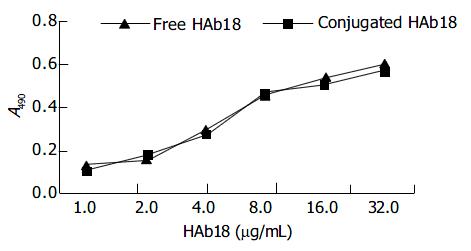
ELISA indicated that the immunological activity of HAb18 on liposome microbubbles was similar to that of free HAb18. There were no significant differences in optical absorbance (A) value between free HAb18 ground and conjugated HAb18 ground (Figure 4).
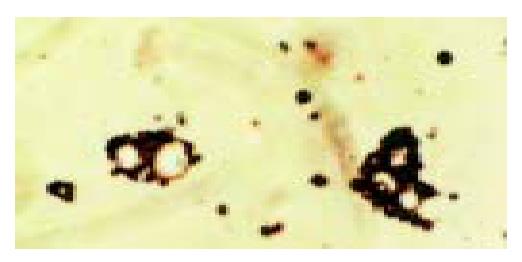
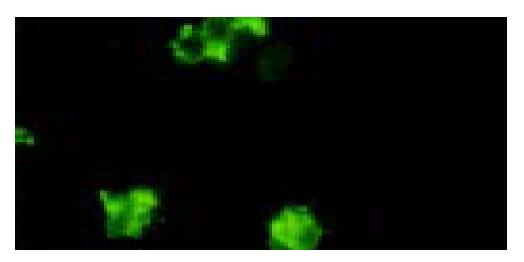
After targeted liposome microbubbles and SMMC-7721 cells or SGC-7901 cells were mixed for 30 min, SMMC-7721 cells were surrounded by targeted liposome microbubbles to form rosettes and the rate of rosettes reached 90% (Figure 5), while SGC-7901 cells were negative. Rosettes were not observed in the mixture of liposome microbubbles and SMMC-7721 cells under microscope. After SMMC-7721 cells were pretreated with different concentrations of HAb18, the rate of rosettes declined significantly, even dropped to 0. Immunofluorescent assay indicated that SMMC-7721 cells were surrounded by the microbubbles and gave out bright yellow-green fluorescence to form rosettes (Figure 6), whereas control cells were negative.
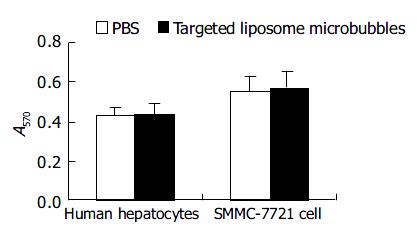
The result of MTT assay showed that the proliferation of human hepatocytes and SMMC-7721 cells were not influenced by targeted liposome microbubbles. There were no significant differences in average optical absorbance (A) value between experiment group and control group (Figure 7).
It has been nearly thirty years since the conception of ultrasound contrast imaging was suggested. Especially in recent years it has been developed rapidly and a number of ultrasound contrast agents have been described, but most of these are nontargeted. Now several targeted contrast agents that can bind to a specific acceptor have been developed[2-15,19], and it has been demonstrated that such agents can enhance ultrasound images of chosen target tissues. Skyba et al[17,20-25] observed that ultrasound could induce intravascular microbubble destruction and bioeffects, and found that the application of ultrasound to thin-shelled microbubbles flowing through small microvessels ( < 7 µm in diameter) could produce vessel wall ruptures and the microspheres in vessel could go out through the ruptured walls. Thus antibody-loaded ultrasound contrast microbubbles could also cross the ruptured microvessels and bind specifically to the target tissues.
At present, hepatocellular carcinoma (HCC) is one of the most common malignant tumors with a high incidence and mortality in the world[26]. This cancer severely threatens our health and needs to be diagnosed as early as possible, but differential diagnosis of HCC at its early stage is still difficult. So it is important to find a specific and sensitive diadynamic method for it. Studies[27] have demonstrated that human hepatocarcinoma specific monoclonal antibody HAb18 could specifically bind to hepatocellular carcinoma cells and can be used for site targeting. If the antibody was used as a target carrier to prepare targeted ultrasound contrast agent, the contrast agent could assemble in a higher concentration of hepatoma tissues and the aim of target imaging could be achieved.
In this study, we prepared and identified targeted liposome microbubbles and investigated their immunological properties. The results indicated that human hepatocarcinoma specific monoclonal antibody HAb18 was firmly attached to the surfaces of liposome microbubbles by static attraction and the immunological activity of HAb18 on liposome microbubbles was similar to that of free HAb18. These demonstrate that it is feasible to prepare targeted liposome microbubbles by static attraction. This method is simple and inexpensive. Moreover, the activity of the antibody can be retained completely. Besides, in order to investigate the properties of targeted liposome microbubbles bound specifically to target cells, rosette formation test and rosette formation blocking test as well as immunofluorescent assay were performed. These tests indicated that targeted liposome microbubbles could bind to human hepatocarcinoma cells specifically and effectively, and the binding was mediated by HAb18.
In order to evaluate the safety of targeted liposome microbubbles, we performed cytotoxicity assay. The result indicated the microbubbles did not influence the proliferation of human hepatocytes and SMMC-7721 cells. These results lay a good foundation for further study.
Edited by Zhang JZ and Wang XL Proofread by Xu FM
| 1. | Bian AN, Gao YH. Research progress of targeted ultrasound contrast. Zhonghua Chaosheng Yingxiangxue Zazhi. 2003;12:558-559. [Cited in This Article: ] |
| 2. | Unger E, Metzger P, Krupinski E, Baker M, Hulett R, Gabaeff D, Mills J, Ihnat D, McCreery T. The use of a thrombus-specific ultrasound contrast agent to detect thrombus in arteriovenous fistulae. Invest Radiol. 2000;35:86-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Unger EC, Matsunaga TO, McCreery TP, Sweitzer RH. Biomedical implications of a thrombus-specific US contrast agent. Acad Radiol. 2002;9 Suppl 1:S56-S57. [PubMed] [Cited in This Article: ] |
| 4. | Hamilton A, Huang SL, Warnick D, Stein A, Rabbat M, Madhav T, Kane B, Nagaraj A, Klegerman M, MacDonald R. Left ventricular thrombus enhancement after intravenous injection of echogenic immunoliposomes: studies in a new experimental model. Circulation. 2002;105:2772-2778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Schumann PA, Christiansen JP, Quigley RM, McCreery TP, Sweitzer RH, Unger EC, Lindner JR, Matsunaga TO. Targeted-microbubble binding selectively to GPIIb IIIa receptors of platelet thrombi. Invest Radiol. 2002;37:587-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 178] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Demos SM, Onyüksel H, Gilbert J, Roth SI, Kane B, Jungblut P, Pinto JV, McPherson DD, Klegerman ME. In vitro targeting of antibody-conjugated echogenic liposomes for site-specific ultrasonic image enhancement. J Pharm Sci. 1997;86:167-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 83] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Unger EC, McCreery TP, Sweitzer RH, Shen D, Wu G. In vitro studies of a new thrombus-specific ultrasound contrast agent. Am J Cardiol. 1998;81:58G-61G. [PubMed] [Cited in This Article: ] |
| 8. | Takeuchi M, Ogunyankin K, Pandian NG, McCreery TP, Sweitzer RH, Caldwell VE, Unger EC, Avelar E, Sheahan M, Connolly R. Enhanced visualization of intravascular and left atrial appendage thrombus with the use of a thrombus-targeting ultrasonographic contrast agent (MRX-408A1): In vivo experimental echocardiographic studies. J Am Soc Echocardiogr. 1999;12:1015-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Demos SM, Alkan-Onyuksel H, Kane BJ, Ramani K, Nagaraj A, Greene R, Klegerman M, McPherson DD. In vivo targeting of acoustically reflective liposomes for intravascular and transvascular ultrasonic enhancement. J Am Coll Cardiol. 1999;33:867-875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 191] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Christiansen JP, Leong-Poi H, Klibanov AL, Kaul S, Lindner JR. Noninvasive imaging of myocardial reperfusion injury using leukocyte-targeted contrast echocardiography. Circulation. 2002;105:1764-1767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Lindner JR, Song J, Xu F, Klibanov AL, Singbartl K, Ley K, Kaul S. Noninvasive ultrasound imaging of inflammation using microbubbles targeted to activated leukocytes. Circulation. 2000;102:2745-2750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 189] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Lindner JR, Song J, Christiansen J, Klibanov AL, Xu F, Ley K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation. 2001;104:2107-2112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 298] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 13. | Klibanov AL, Hughes MS, Villanueva FS, Jankowski RJ, Wagner WR, Wojdyla JK, Wible JH, Brandenburger GH. Targeting and ultrasound imaging of microbubble-based contrast agents. MAGMA. 1999;8:177-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Dayton PA, Ferrara KW. Targeted imaging using ultrasound. J Magn Reson Imaging. 2002;16:362-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 186] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Hamilton A, Rabbat M, Jain P, Belkind N, Huang SL, Nagaraj A, Klegerman M, Macdonald R, McPherson DD. A physiologic flow chamber model to define intravascular ultrasound enhancement of fibrin using echogenic liposomes. Invest Radiol. 2002;37:215-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Liu P, Gao YH, Tan KB, Zuo S, Liu Z. Enhanced imaging of the rabbit liver using the self-made Liposome contrast agent: an earlier experimental study. Zhongguo Chaosheng Yixue Zazhi. 2003;19:4-6. [Cited in This Article: ] |
| 17. | Shohet RV, Chen S, Zhou YT, Wang Z, Meidell RS, Unger RH, Grayburn PA. Echocardiographic destruction of albumin microbubbles directs gene delivery to the myocardium. Circulation. 2000;101:2554-2556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 345] [Cited by in F6Publishing: 304] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 18. | Kang J, Samten B, Xie S, Wei S. [Separation of human bladder cancer cells from bone marrow with a kind of immunomagnetic microspheres]. Yaoxue Xuebao. 1998;33:52-56. [PubMed] [Cited in This Article: ] |
| 19. | Leong-Poi H, Christiansen J, Klibanov AL, Kaul S, Lindner JR. Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to alpha(v)-integrins. Circulation. 2003;107:455-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 283] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 20. | Skyba DM, Price RJ, Linka AZ, Skalak TC, Kaul S. Direct in vivo visualization of intravascular destruction of microbubbles by ultrasound and its local effects on tissue. Circulation. 1998;98:290-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 366] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 21. | Price RJ, Skyba DM, Kaul S, Skalak TC. Delivery of colloidal particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation. 1998;98:1264-1267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 303] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 22. | Mukherjee D, Wong J, Griffin B, Ellis SG, Porter T, Sen S, Thomas JD. Ten-fold augmentation of endothelial uptake of vascular endothelial growth factor with ultrasound after systemic administration. J Am Coll Cardiol. 2000;35:1678-1686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Lawrie A, Brisken AF, Francis SE, Cumberland DC, Crossman DC, Newman CM. Microbubble-enhanced ultrasound for vascular gene delivery. Gene Ther. 2000;7:2023-2027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 222] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Price RJ, Kaul S. Contrast ultrasound targeted drug and gene delivery: an update on a new therapeutic modality. J Cardiovasc Pharmacol Ther. 2002;7:171-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Zhang DZ, Ren H, Wang ZG, Leng ZY, Ran HT, Zhang DF. Ultrasound enhancing the infection efficiency of adenoviral vector into the intact hepaocyte. Linchuang Chaosheng Yixue Zazhi. 2002;4:321-323. [Cited in This Article: ] |
| 26. | Qian J, Truebenbach J, Graepler F, Pereira P, Huppert P, Eul T, Wiemann G, Claussen C. Application of poly-lactide-co-glycolide-microspheres in the transarterial chemoembolization in an animal model of hepatocellular carcinoma. World J Gastroenterol. 2003;9:94-98. [PubMed] [Cited in This Article: ] |