Published online Oct 15, 2004. doi: 10.3748/wjg.v10.i20.2997
Revised: March 2, 2004
Accepted: March 13, 2004
Published online: October 15, 2004
AIM: To study the diagnosis of Helicobacter pylori (H pylori) infection through the determination of serum levels of anti-H pylori IgG and IgA antibodies, and the levels of anti-H pylori IgA antibodies in duodenal fluid.
METHODS: Data were collected from 93 patients submitted to upper digestive endoscopy due to dyspeptic symptoms. The patients were either negative (group A) or positive (group B) to H pylori by means of both histological detection and urease tests. Before endoscopy, peripheral blood was collected for the investigation of anti-H pylori IgG and IgA antibodies. To perform the urease test, biopsies were obtained from the gastric antrum. For the histological evaluation, biopsies were collected from the gastric antrum (greater and lesser curvatures) and the gastric body. Following this, duodenal fluid was collected from the first and second portions of the duodenum. For the serological assaying of anti–H pylori IgG and IgA, and anti-H pylori IgA in duodenal fluids, the ELISA method was utilized.
RESULTS: The concentration of serum IgG showed sensitivity of 64.0%, specificity of 83.7%, positive predictive value of 82.0%, negative predictive value of 66.6% and accuracy of 73.1% for the diagnosis of H pylori infection. For the same purpose, serum IgA showed sensitivity of 72.0%, specificity of 65.9%, positive predictive value of 72.0%, negative predictive value of 67.4% and accuracy of 69.8%. If the serological tests were considered together, i.e. when both were positive or negative, the accuracy was 80.0%, sensitivity was 86.6%, specificity was 74.2%, positive predictive value was 74.2% and negative predictive value was 86.6%. When values obtained in the test for detecting IgA in the duodenal fluid were analyzed, no significant difference (P = 0.43) was observed between the values obtained from patients with or without H pylori infection.
CONCLUSION: The results of serum IgG and IgA tests for H pylori detection when used simultaneously, are more efficient in accuracy, sensitivity and negative predictive value, than those when used alone. The concentration of IgA antibodies in duodenal fluid is not useful in identifying patients with or without H pylori.
-
Citation: Locatelli A, Catapani WR, Junior CRG, Silva CBP, Waisberg J. Detection of anti-
Helicobacter pylori antibodies in serum and duodenal fluid in peptic gastroduodenal disease. World J Gastroenterol 2004; 10(20): 2997-3000 - URL: https://www.wjgnet.com/1007-9327/full/v10/i20/2997.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i20.2997
Knowledge about peptic ulcers has changed profoundly since Warren and Marshall[1] managed to cultivate Helicobacter pylori (H pylori) from gastric biopsies. This raised great interest in the study of how it colonized the upper gastrointestinal tract and more specifically, in its direct relationship with peptic disease[1-3].
In epidemiological studies, serum tests could offer high sensitivity and specificity[4]. Serum assaying of anti-H pylori IgG and IgA antibodies could be used for the determination of prevalence of acute and chronic infections[5-7]. In general, the serum levels of anti-H pylori IgG antibodies were increased in the presence of infection and could be used as a marker. On the other hand, anti-H pylori IgA antibodies were less appropriate for this purpose[8,9], nevertheless serological findings of anti-H pylori IgA antibodies in symptomatic patients might have significant clinical values for the diagnosis of infection, especially if the patient was seronegative for IgG[10].
In treatment of peptic diseases among H pylori-infected patients, local immune responses of the host, as well as the inherent factors of the microorganism, such as the presence of cytotoxin-associated gene A (cagA) were important[11,12]. Few studies have analyzed the effect of local immune response on diseases induced by H pylori[13,14].
H pylori infection could result in a major increase in cells secreting IgA in human gastroduodenal mucosa[15,16] and usually induce high serum levels of anti-H pylori antibodies. Moreover, significant concentrations of antibodies were demonstrated in saliva, gastric fluid and feces[17]. Despite the antibody response, this microorganism has been rarely eliminated from the stomach and when it was not treated adequately the infection generally persisted in the rest of an individual’s life[18].
However, the use of serological tests based on the determination of serum levels of anti-H pylori IgG and IgA antibodies to clinically diagnose H pylori infection has not yet been fully clarified[5,7,10,16].
The objective of the present study was to analyze the use of serum levels of anti-H pylori IgG and IgA antibodies, and the levels of anti-H pylori IgA antibodies in duodenal fluid for the diagnosis of H pylori infection.
Examinations were done on 93 patients with peptic symptoms from November 2000 to September 2001.
The inclusion criteria were: adult patients with a normal endoscopic examination or showing a peptic disease[19,20]. The followings were considered to be exclusion criteria: presence of malignant disease of the upper digestive tract, previous gastrectomy, use of hormonal or non-hormonal anti-inflammatory medications, proton pump inhibitors, histamine H2 receptor blockers or antibiotics or antacids over the past twelve months, previous treatment for the elimination of H pylori over the past twelve months, presence of intestinal inflammatory disease or immunodeficiency of any nature, and pediatric age.
The patients (n = 93) were divided into two groups. Group A (n = 43) that had urease test and was histology negative for H pylori, and group B (n = 50) that had urease test and was histology positive for H pylori. These criteria (patients simultaneously positive or negative in histological and urease tests) were used for diagnosis of H pylori. In group A, 22 (51.2%) patients were males and 21 (48.8%) were females, the mean age was 34.77 ± 12.89 years (range: 17 to 71 years). In group B, 25 (50.0%) patients were males and 25 (50.0%) were females, the mean age was 41.6 ± 14.6 years (range: 21 to 71 years).
Endoscopic examination, biopsies of gastric mucosa and sampling of duodenal fluid To perform urease test, two biopsies were obtained from the gastric antrum (greater and lesser curvatures) at least 2 cm from the pylorus. For the histological analysis, four biopsies were collected, two of which from the gastric antrum (greater and lesser curvatures), and two of which from the gastric body on the anterior and posterior walls. Following this, a minimum of 2 mL of secreted duodenal fluid was collected from the first and second portions of the duodenum.
Processing of biopsy material The material collected from the gastric biopsies was placed in flasks containing 40 g/L buffered formaldehyde for fixation and then sent for histological examination. Slides were stained with hematoxylin and eosin and the modified Giemsa method (1% Lugol’s solution and 25 g/L gentian violet solution). The four biopsy fragments removed from the gastric antrum were placed in flasks containing urease medium (Probac, Sao Paulo, Brazil), with 2 mL of solution in each. The flasks were maintained at 37 °C and read by the main author 6 and 12 h later.
Determination of serum levels of anti-H pylori IgG and IgA and anti-H pylori IgA in duodenal fluid Before endoscopy, peripheral blood was collected to determine the serum levels of anti-H pylori IgG and IgA. Two-milliliter aliquots from duodenal fluid were diluted with distilled water until a final volume of 10 mL was reached. This solution was centrifuged at 1500 r/min for 10 min and the supernatant was stored at -20 °C.
ELISA method (Accubind®, Monobind, Inc., Costa Mesa, California, USA) was used to determine the levels of serum anti-H pylori IgG and IgA and anti-H pylori IgA in the duodenal fluid. The serum samples were diluted to 1/100 while the samples of duodenal fluids were diluted to 1/1000. Other steps were performed according to the instructions of manufacturer.
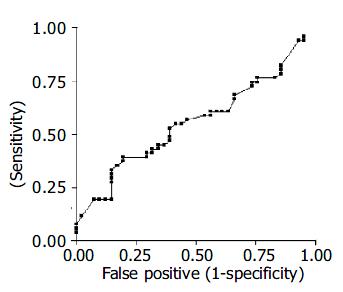
For both the anti-H pylori IgG and IgA serum antibodies, optical density values greater than 20 U/mL were considered as positive results. For the anti-H pylori IgA antibodies in the duodenal fluid, no reference optical density value for a positive or negative test was available. Thus, a receiver operating characteristic (ROC) curve was constructed, taking the results obtained in ELISA test versus the gold standard.
Statistical analysis was performed by the Mann-Whitney, Fisher’s exact and Kruskal-Wallis tests. The ROC curve was constructed in order to assess the sensitivity and specificity of anti-H pylori IgA assays in the duodenal fluid at different cutoff levels. An alpha value less than or equal to 5% was adopted for rejection of the null hypothesis. The results were expressed as mean ± SD to illustrate the distribution of variables.
The findings of endoscopic and histological examination were gastritis alone in 32 (34.4%) patients, gastritis in association with duodenal peptic ulcer and/or moderate to severe enanthematic or erosive bulboduodenitis in 18 (19.4%), gastritis in association with hiatal hernia and/or esophagitis in 16 (17.2%), gastritis and slight bulboduodenitis in 11 (11.8%), gastritis in association with gastric ulcer and duodenal ulcer simultaneously in 4 (4.3%) and gastritis and gastric polyps in one (1.0%). Eleven (11.8%) patients presented normal endoscopic and histological examination of the gastric mucosa.
The sensitivity, specificity, positive and negative predictive values and accuracy of the serum tests for anti-H pylori IgG and IgA were analyzed in relation to the gold standard. The accuracy of serum IgG levels was 73.1%, the sensitivity was 64.0%, specificity was 83.7%, positive predictive value was 82.0% and negative predictive value was 66.6%. With regard to serum IgA levels, the accuracy was 69.8%, sensitivity was 72.0%, specificity was 65.9%, positive predictive value was 72.0% and negative predictive value was 67.4%. If the serological tests were considered together, i.e. when both were positive or negative in comparison with the gold standard (Table 1), the accuracy was 80.0%, sensitivity was 86.6%, specificity was 74.2%, positive predictive value was 74.2% and negative predictive value was 86.6%.
Compared with the frequencies of individuals with positive or negative results in the serological test for identifying anti-H pylori IgG and IgA antibodies, the serological tests were efficient in the diagnosis of the presence or absence of H pylori infection. The difference in the frequencies of positive and negative test results between the groups was highly significant (Table 1). With regard to the ELISA serological tests for anti-H pylori IgG and IgA considered together (Table 1), the difference between the frequencies of patients in each group was highly significant (P < 0.0001).
In comparison between the values obtained in the ELISA tests in patients positive or negative for H pylori, the difference was significant in relation to anti-H pylori serological IgA and IgG.
Sensitivity, specificity and false positive ratio for IgA in the duodenal fluid were defined by the ROC curve at different cutoff levels, as compared to the gold standard (Figure 1).
When values obtained in the ELISA test for determination of anti-H pylori IgA antibodies in the duodenal fluids were analyzed, no significant difference (P = 0.43) was observed between the patients with or without H pylori infection.
Martin-de-Argila et al[21] concluded that ELISA serum test for the detection of anti-H pylori IgA and IgG antibodies was sensitive and specific and should preferentially be utilized in population-based studies. Peura[22] found that the sensitivity and specificity of ELISA test ranged from 75% to 95%. Perez-Perez et al[23], examined the detection of anti-H pylori IgA and IgG in serological samples from patients with positive cultures for H pylori and a histological diagnosis of gastritis, and verified that tests using both antibodies simultaneously had a sensitivity of 93.1% and a specificity of 94.4%. In our study, if the serological tests were considered together, the sensitivity was 86.6% and specificity was 74.2%. A possible explanation for this difference could be the different methods utilized as the gold standard. Indeed, the H pylori culture method performed by those authors could possibly have revealed smaller quantities of bacteria in the gastric mucosa. With few bacteria in the mucosa, the urease test and histology could give a false negative result.
Our results also pointed to an increased sensitivity when both anti-H pylori IgG and IgA were considered together, in relation to IgG or IgA alone, but the improvement in specificity occurred only in relation to IgA, and it was reduced in comparison with IgG alone. The association between IgG and IgA resulted in a marked improvement of the negative predictive value in comparison with the two assays alone, but did not offer advantages in relation to the positive predictive value.
Certainly, other variables than these might be responsible for the differences observed between the various studies, such as the severity and staging of peptic disease and the different strains of microorganism might also play a role. Strains with cytotoxin-associated gene A (cagA) exhibited more intense immunological responses[11,16,23,24].
Watanabe et al[14], found that patients who presented high titers of anti-H pylori IgA in the gastric secretion presented a lower degree of neutrophilic infiltration on histological examination. These results suggest that anti-H pylori IgA antibodies have a protective function, even though this is insufficient to completely eliminate the organism.
In the present study, when the titers of anti-H pylori IgA in the duodenal fluid in patients with and without H pylori were analyzed, no significant difference was found in these values. The ROC curve, constructed using the values obtained from the determination of duodenal anti-H pylori IgA, compared to the gold standard, showed that the levels of this immunoglobulin in the duodenal fluid presented high sensitivity and low specificity, or even the opposite, depending on the cutoff level considered. Thus, if a cutoff level of 1.0 is adopted, it can be predicted that an individual will be negative for H pylori if the anti-H pylori IgA values in the duodenal fluid are below this reference level (sensitivity 82.4%). However, at this cutoff level, individuals with anti-H pylori IgA above this value will be at increased risk of being considered as false positive, i.e. with a specificity of 14.5%, the number of false positives will reach 85.4%.
Crabtree et al[24], using ELISA method, evaluated the local immunological response of anti-H pylori IgA and IgG antibodies in patients with duodenitis. These authors observed that the local immunological response of anti-H pylori IgA antibodies was greater than that of anti-H pylori IgG antibodies. They also noted that there was a direct relationship between the titers of anti-H pylori antibodies and the severity of the inflammatory process found, or in other words, the more severe the duodenitis, the higher the levels of local antibodies and especially those of IgA. However, their investigation into these antibodies was done by evaluating the supernatant material from in vitro culture of biopsies taken from the first and second portions of the duodenum. In our study, duodenal fluid was collected from these same locations for investigating the anti-H pylori IgA antibodies. Often, studies performed in vitro could not be directly correlated with in vivo results because of the absence of other conditions frequently encountered in vivo, such as the presence of proteolytic enzymes in the intestine that could change the concentration of secreted IgA, although this immunoglobulin is very resistant to the action of these enzymes[14]. Our results expressed the concentration of IgA in the duodenal fluid, but the total amount of IgA in this fluid was unknown, because the quantity of fluid in the first and second portions of the duodenum varied between patients. Crabtree et al[24], studied anti-H pylori antibodies in the duodenal mucosa, and observed notable differences in antibody concentrations between the first and second portions of the duodenum. These authors observed that, in the second portion of the duodenum, the concentrations of anti-H pylori IgA and IgG antibodies were lower than those in the first portion. This could have had a decisive influence on our results, since the duodenal fluid presented a greater volume in the second portion of the duodenum and it was often difficult to obtain samples of duodenal fluid from the first portion.
In conclusion, the results from the present investigation demonstrate that anti-H pylori IgA and IgG serum immunoglobulins are useful in distinguishing between patients with and without H pylori infection, whereas the concentration of anti-H pylori IgA in the duodenal fluid is not useful in identification of infected or uninfected patients.
Edited by Wang XL Proofread by Chen WW and Xu FM
| 1. | Warren JR, Marshall BJ. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273-1275. [PubMed] [Cited in This Article: ] |
| 2. | Olbe L, Fandriks L, Hamlet A, Svennerholm AM, Thoreson AC. Mechanisms involved in Helicobacter pylori induced duodenal ulcer disease: an overview. World J Gastroenterol. 2000;6:619-623. [PubMed] [Cited in This Article: ] |
| 3. | Svennerholm AM, Quiding-Järbrink M. Priming and expression of immune responses in the gastric mucosa. Microbes Infect. 2003;5:731-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Jensen AK, Andersen LP, Wachmann CH. Evaluation of eight commercial kits for Helicobacter pylori IgG antibody detection. APMIS. 1993;101:795-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Andersen LP, Rosenstock SJ, Bonnevie O, Jørgensen T. Seroprevalence of immunoglobulin G, M, and A antibodies to Helicobacter pylori in an unselected Danish population. Am J Epidemiol. 1996;143:1157-1164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Morris A, Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol. 1987;82:192-199. [PubMed] [Cited in This Article: ] |
| 7. | Sobala GM, Crabtree JE, Dixon MF, Schorah CJ, Taylor JD, Rathbone BJ, Heatley RV, Axon AT. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut. 1991;32:1415-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 258] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Andersen LP, Kiilerick S, Pedersen G, Thoreson AC, Jørgensen F, Rath J, Larsen NE, Børup O, Krogfelt K, Scheibel J. An analysis of seven different methods to diagnose Helicobacter pylori infections. Scand J Gastroenterol. 1998;33:24-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Kosunen TU, Höök J, Rautelin HI, Myllylä G. Age-dependent increase of Campylobacter pylori antibodies in blood donors. Scand J Gastroenterol. 1989;24:110-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 108] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Jaskowski TD, Martins TB, Hill HR, Litwin CM. Immunoglobulin A antibodies to Helicobacter pylori. J Clin Microbiol. 1997;35:2999-3000. [PubMed] [Cited in This Article: ] |
| 11. | Rathbone BJ, Wyatt JI, Worsley BW, Shires SE, Trejdosiewicz LK, Heatley RV, Losowsky MS. Systemic and local antibody responses to gastric Campylobacter pyloridis in non-ulcer dyspepsia. Gut. 1986;27:642-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 246] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Atalay C, Atalay G, Altinok M. Serum Helicobacter pylori IgG and IgA levels in patients with gastric cancer. Neoplasma. 2003;50:185-190. [PubMed] [Cited in This Article: ] |
| 13. | Doweck J, Quintana C, Barrios A, Monastra L, Lopetegui G, Zerbo O, Schenone L, Giordano A, Valero J, Kogan Z. [Evaluation of sensitivity, specificity and predictive value of six qualitative serological methods for the detection of Helicobacter pylori antibodies]. Acta Gastroenterol Latinoam. 1997;27:259-261. [PubMed] [Cited in This Article: ] |
| 14. | Watanabe T, Goto H, Arisawa T, Hase S, Niwa Y, Hayakawa T, Asai J. Relationship between local immune response to Helicobacter pylori and the diversity of disease: investigation of H. pylori-specific IgA in gastric juice. J Gastroenterol Hepatol. 1997;12:660-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Mattsson A, Quiding-Järbrink M, Lönroth H, Hamlet A, Ahlstedt I, Svennerholm A. Antibody-secreting cells in the stomachs of symptomatic and asymptomatic Helicobacter pylori-infected subjects. Infect Immun. 1998;66:2705-2712. [PubMed] [Cited in This Article: ] |
| 16. | Xia HH, Talley NJ, Blum AL, O'Morain CA, Stolte M, Bolling-Sternevald E, Mitchell HM. Clinical and pathological implications of IgG antibody responses to Helicobacter pylori and its virulence factors in non-ulcer dyspepsia. Aliment Pharmacol Ther. 2003;17:935-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Wyatt JI, Rathbone BJ. Immune response of the gastric mucosa to Campylobacter pylori. Scand J Gastroenterol Suppl. 1988;142:44-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 156] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Luzza F, Imeneo M, Maletta M, Monteleone G, Doldo P, Biancone L, Pallone F. Isotypic analysis of specific antibody response in serum, saliva, gastric and rectal homogenates of Helicobacter pylori-infected patients. FEMS Immunol Med Microbiol. 1995;10:285-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3221] [Cited by in F6Publishing: 3366] [Article Influence: 120.2] [Reference Citation Analysis (2)] |
| 20. | Garza-González E, Bosques-Padilla FJ, Tijerina-Menchaca R, Flores-Gutiérrez JP, Maldonado-Garza HJ, Pérez-Pérez GI. Comparision of endoscopy-based and serum-based methods for the diagnosis of Helicobacter pylori. Can J Gastroenterol. 2003;17:101-106. [PubMed] [Cited in This Article: ] |
| 21. | Martín-de-Argila C, Boixeda D, Cantón R, Valdezate S, Mir N, De Rafael L, Gisbert JP, Baquero F. Usefulness of the combined IgG and IgA antibody determinations for serodiagnosis of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1997;9:1191-1196. [PubMed] [Cited in This Article: ] |
| 22. | Peura DA. Helicobacter pylori: a diagnostic dilemma and a dilemma of diagnosis. Gastroenterology. 1995;109:313-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Perez-Perez GI, Dworkin BM, Chodos JE, Blaser MJ. Campylobacter pylori antibodies in humans. Ann Intern Med. 1988;109:11-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 351] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 24. | Crabtree JE, Shallcross TM, Wyatt JI, Taylor JD, Heatley RV, Rathbone BJ, Losowsky MS. Mucosal humoral immune response to Helicobacter pylori in patients with duodenitis. Dig Dis Sci. 1991;36:1266-1273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |