Published online Oct 15, 2004. doi: 10.3748/wjg.v10.i20.2958
Revised: January 3, 2004
Accepted: January 8, 2004
Published online: October 15, 2004
AIM: To develop an efficient animal colitis-associated carcinogenesis model and to detect the expression of β -catenin and p53 in this new model.
METHODS: Dysplasia and cancer were investigated in mice pretreated with a single intraperitoneal injection of 20 mg/kg body mass of 1,2-dimethylhydrazine prior to three repetitive oral administrations of 30 g/L dextran sulfate sodium to give conditions similar to the clinically observed active and remission phases. Immunohistochemical staining of β - catenin and p53 was performed on paraffin-imbedded specimens of animals with cancer and/or dysplasia, those without dysplasia and the normal control animals.
RESULTS: At wk 11, four early-invasive adenocarcinomas and 36 dysplasia were found in 10 (90.9%) of the 11 mice that underwent 1,2-dimethylhydrazine-pretreatment with 3 cycles of 30 g/L dextran sulfate sodium-exposure. Dysplasia and/or cancer occurred as flat lesions or as dysplasia-associated lesion or mass (DALM) as observed in humans. Colorectal carcinogenesis occurred primarily on the distal portion of the large intestine. No dysplasia and/or cancer lesion was observed in the control groups with 1,2-dimethylhydrazine pretreatment or 3 cycles of 30 g/L dextran sulfate sodium exposure alone. Immunohistochemical investigation revealed that β -catenin was translocated from cell membrane to cytoplasm and/or nucleus in 100% of cases with dysplasia and neoplasm, while normal membrane staining was observed in cases without dysplasia and the normal control animals. Nuclear expression of p53 was not detected in specimens.
CONCLUSION: A single dose of procarcinogen followed by induction of chronic ulcerative colitis results in a high incidence of colorectal dysplasia and cancer. Abnormal expression of β -catenin occurs frequently in dysplasia and cancer. This novel mouse model may provide an excellent vehicle for studying colitis-related colon carcinogenesis.
- Citation: Wang JG, Wang DF, Lv BJ, Si JM. A novel mouse model for colitis-associated colon carcinogenesis induced by 1,2-dimethylhydrazine and dextran sulfate sodium. World J Gastroenterol 2004; 10(20): 2958-2962
- URL: https://www.wjgnet.com/1007-9327/full/v10/i20/2958.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i20.2958
The incidence of colorectal cancer (CRC) has been increasing in patients with ulcerative colitis (UC) and the risk of CRC increases with increased extent and duration[1-4]. The mechanisms underlying the frequent development of CRC in patients with UC are still unknown.
Through the study of animal models which do not allow experiments in humans, we could better understand the cause and mechanisms of various diseases. There are many animal ulcerative colitis models, but only a few of them are applicable to the study of dysplasia-cancer sequence. Among them, the most widely used is a mouse model with dextran sulfate sodium (DSS). DSS could be used to induce UC in mice. Acute colitis was observed by administration of 50-100 g/L DSS to mice for 4-9 d[5-8]. A chronic colitis could be induced by feeding 30-50 g/L DSS in drinking water for 7 d followed by 7-14 d of water[6,8-10]. In recent years, some researchers have described the occurrence of dysplasia and/or cancers in mice when they are subjected to repeated administration of DSS in a long term[11-13]. Before the occurrence of cancer, the features of colitis in this model were very similar to those in patients in terms of both clinical and histopathological characteristics, i.e. diarrhea, occult blood, melena, mucosal inflammatory cell infiltration, crypt abscess formation, and mucosal erosion. But this kind of models needs a long period to be established and the incidence of induced tumors is relatively low.
1,2-dimethylhydrazine (DMH) is a toxic environmental pollutant which was reported as a specific colon procarcinogen. Animal studies showed that experimental colonic tumors induced by DMH were of epithelial origin with a similar histology, morphology and anatomy to human colonic neoplasms[14]. This procarcinogen could thus provide an adequate model for studying colorectal cancer. However, mutiple treatments with DMH (20-40 mg/kg body mass) and a long-term experimental period are needed to induce large bowel neoplasms[15-17].
We present here a newly developed colitis-associated CRC mouse model in which dysplasia and cancer developed within 10 wk when mice were given a single, low dose of DMH followed by three repeated administrations of 30 g/L DSS in drinking water. We used DSS to induce recurrence-remission cycle of chronic colitis which was similar to humans and added a low dose of DMH, in order to shorten CRC development period since chronic exposure to a small amount of environmental carcinogens is also a main cause of human CRC. In addition, we found dysplasiae and cancers in this model showed positive reactivity for β -catenin, but not for p53.
Forty-five specific pathogen-free BALB/c male mice weighing 25-30 g (Slaccas Experimental Animal Co. Ltd. Shanghai, China), 7 wk of age, were used. They were housed in plastic cages (5 or 6 mice/cage) with wood shavings under standard laboratory conditions (24 ± 0.5 °C temperature, 50 ± 10% humidity, and 12 h of light from 06.00 to 18.00). All mice were permitted free access to a commercial diet and DSS-supplemented or normal drinking tap water in bottles at the Animal Laboratory Center of our hospital.
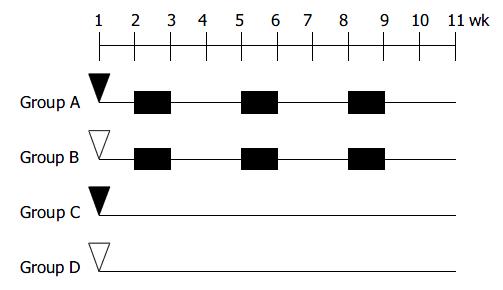
The design for inducing colitis-associated dysplasia and/or cancer is shown in Figure 1. At the age of 8 wk, the animals were divided into one experimental group (group A) and 3 control groups (groups B-D), 10-12 mice each group. The animals in group A were subjected to three cycles of alternating administration of distilled water containing 30 g/L synthetic dextran sulfate sodium (DSS; mol mass 5000; Wako Pure Chemical Industries, Led. Japan) for 7 d followed by distilled water for the subsequent 14 d after intraperitoneal pretreatment with 20 mg/kg 1,2-dimethylhydrazine (DMH; Sigma-Aldrich Corp. St. Louis. MO. USA). For comparison, control groups B to D received each of the treatment alone or maintained as untreated control. Three mice from group A, B, and D were sacrificed during the experiment. Fourty-two mice were anesthesized with ether and sacrificed at the age of 18 wk.
Changes in body weight were recorded every week throughout the experiment. Occult blood was examined on d 3 or 4 of DSS feeding, when DSS feeding was stopped, and once a week thereafter. Presence of gross blood and stool consistency were observed daily. The individuals who examined the mice were blinded as to the experimental group to which the animals belonged.
After death, the entire colorectum from the colocecal junction to the anal verge was examined. Their length was measured , and then the specimen was opened longitudinally and washed with saline. After colorectum was macroscopically inspected, it was immediately fixed in a 40 g/L formaldehyde buffer solution (pH 7.2).
Part of the colon was divided into three equal portions (proximal, middle and distal). Five-six pieces/portion and 14-17 pieces/colorectum were stained with hematoxylin and eosin (H&E) for histological processing.
The severity of UC of each colon was histologically graded on a scale from 0-4 and expressed using the pathological index corresponding to the following modified standard scoring system[18]: 0, normal; 1, focal inflammatory cell infiltration including polymorphonuclear leucocytes; 2, gland loss with inflammatory cell infiltration or crypt abscess formation; 3, mucosal uleration, or five or more foci of gland loss with inflammatory cell infiltration; 4, two or more areas of mucosal ulceration.
Dysplasia (low and high grades) was scored according to the criteria described by Riddell et al[19]. Cancer was divided into early invasive and advanced cancer. Early invasive cancer was defined as cancer cells invading into muscularis mucosa and/or into submucosa. Advanced cancer was defined as cancer cells invading into muscularis propria or beyond.
A single experienced pathologist reviewed all cases blindly.
To detect the expression of β -catenin and p53, we utilized the two step immunostaining technique. Four μm think tissue sections were dewaxed and rehydrated through changes of xylene and graded alcohol, then to water. Endogenous peroxidase activity was blocked by incubating the sections with 30 g/L hydrogen peroxidase for 15 min. Heat-mediated antigen retrieval was performed by heating the sections (immersed in 0.01 mol/L citrate buffer, pH 6.0) in a microwave oven (750 W) for 15 min. The slides were then washed with phosphate-buffered saline (PBS) before incubated with primary antibody overnight at 4 °C. The antibody to β -catenin (Santa Cruz Biotechnology, Inc. USA) was a mouse monoclonal IgG1 antibody corresponding to amino acids 680-781 and was used at a dilution of 1:800. p53 antibody (Santa Cruz Biotechnology, Inc. USA) was a rabit polyclonal antibody that reacts with both wild-type and mutant p53 and was used at a dilution of 1:200. After washed with PBS, the slides were incubated for 30 min with the EnVision + peroxidase reagent (Zhongshan Biological Technology Co., LTD, Beijing, China). After further washed in PBS, the slides were developed with 3,3’-diaminobenzidine (DAB; Sigma-Aldrich Corp. St. Louis. MO. USA) for 5 min, and the reaction was terminated in water. The slides were then counterstained with hematoxylin, dehydrated in alcohol, and evaluated under a light microscope. Positive controls for β - catenin expression were normal human colonic epithelia. Human colonic adenocarcinoma were used as positive controls for p53 antibody. Omission of the primary antibody of β -catenin and p53 was used as a negative control.
Lesions were classified as positive for β -catenin if cytoplasmic/ nuclear staining was detected (1 + : ≤ 50% cells positive, 2 + : ≥ 50% cell positive). Staining for p53 was considered positive if nuclear expression was detected in more than 10% of cells. Two experienced pathologists who were blinded to the specimen independently examined slides, and a high level of concordance (90%) was achieved. In case of disagreement, the slides were reviewed and a consensus view was achieved.
Statistical analysis was carried out by SPSS 11.5 for windows statistic software. Variance tests and One-way ANOVA test were used to compare the means of weight, length of large bowel in different groups, the number of neoplasms, and pathological index in different sites. P < 0.05 was considered statistically significant.
At the end of the first DSS treatment period, 75.0% (18/24) mice in groups A and B had diarrhea and occult blood or gross blood in the feces, and these signs disappeared after the mice drank distilled water for 14 d. However, during the second and third administrations of DSS, only 45.5% (10/22) and 36.4% (8/22) mice had diarrhea or occult blood, respectively.
Body growth rate was slightly lower in the mice that received 3 cycles of DSS treatment. The mean body weight of group A and B at the end of study was significantly smaller that that of group D (P < 0.05, Table 1).
Although the colorectal length was not affected by DMH-treatment alone, it was shortened by DSS treatment. The mean length of colorectum of mice in groups A and B was significantly shorter than that of mice in group D (P < 0.01, Table 1).
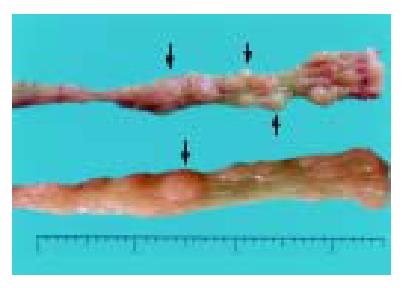
Group A mice that received DMH pretreatment and repeated administrations of 30 g/L DSS developed mutiple tumous in the colorectal region. Ten of 11 (90.9%) animals were detected to have at least one dysplasia and/or cancer lesion. Gross lesions were noted in 5 animals (all in group A). These lesions were dome shaped and ranged 2 to 4 mm in size, which appeared in the distal portion of the large intestine mainly, then in the middle portion, and none in the proximal part. (Table 2, Figure 2).
| Group number | Mice | Number of dysplasiae/cancers | Pathology score of UC | ||||
| Proximal | Middle | Distal | Proximal | Middle | Distal | ||
| A | 11 | 0.55 ± 0.82 | 0.73 ± 0.79 | 2.36 ± 1.12a | 1.82 ± 0.40 | 1.72 ± 0.46 | 1.82 ± 0.40c |
| B | 11 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 2.00 ± 0.63 | 1.82 ± 0.40 | 2.09 ± 0.54c |
| C | 10 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| D | 10 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
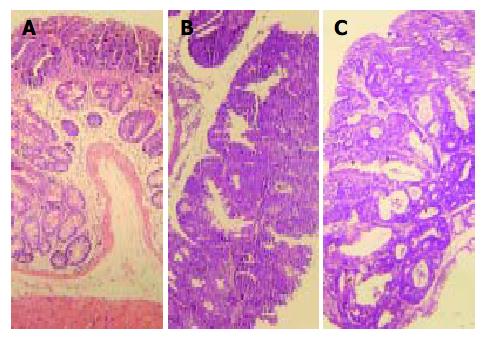
Most tumor tissues were tubular lesions with atypical severe cellular and structural high-grade dysplasia. A small number of lesions revealed relatively moderate dysplasia. Ten dysplasiae were categorized as low-grade dysplasia (Figure 3A), and 26 dysplasiae as high-grade dysplasia (Figure 3B), 22 out of 36 (61.1%) dysplasia lesions were DALMs. Four out of 26 tumours on the distal portion of the large intestine were confirmed to be early invasive adenocarcinomas (Figure 3C), and 3 of them arose within a DALM. No signs of tumor development were detected in control groups B to D.
Among the animals with dysplasia and/or cancer, the incidence of dysplasia and/or cancer was 15.0% (6/40), 20.0% (8/40) and 65.0% (26/40) in the proximal, middle and distal colon segments, respectively, 4 of 10 (40.0%) animals with dysplasia and/or cancer had lesions limited to only one colon segment, 3 animals (30.0%) had lesions in two different segments, and 3 animals (30.0%) had lesions in all three segments. Of the 3 animals with cancers, one had two synchronous cancers.
The mice that received repeated administrations of 30 g/L DSS showed mild colitis regardless of DMH pretreatment. Mice in group A and B demonstrated mutiple foci of gland loss with inflammatory cell infiltration, but not many crypt abscess formations and mucosal ulcerations. Pathological scores in these two groups were statically significantly higher than those in group D (Table 2). However, there was no significant relationship between pathology scores among three portions of group A and B. This did not correlate well with the locations of CRC that developed mainly on the distal segment of colorectum.
Four cancers, 25 dysplasiae (10 of low-grade, 15 of high-grade), 10 negative dysplasiae and 10 control animals were studied for β -catenin and p53 expression, respectively.
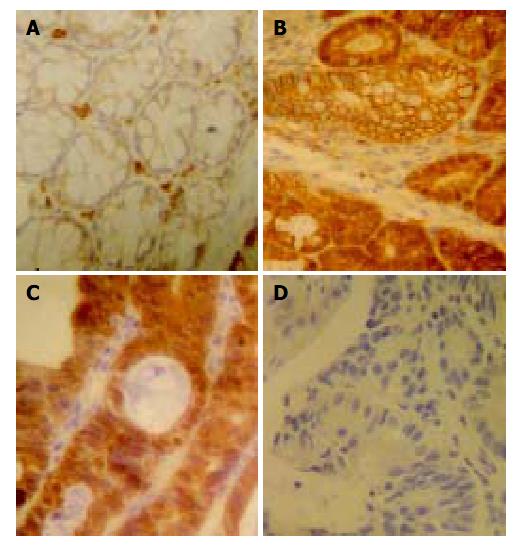
In normal colon epithelial cells, β -catenin was mainly localized at the membranes of cell-cell borders (Figure 4A). Strong (2 + ) β -catenin expression was seen in nuclei and cytoplasms of cancer cells of 4 specimens (Figure 4C). Although the intensity was relatively weaker than that in carcinoma cells, dysplastic cells showed positivity for β -catenin in their nuclei, cytoplasms, and cell membranes (Figure 4B). The intensity was greater in high-grade dysplasiae (73.3% were 2 + ) than in low-grade dysplasiae (30.0% were 2 + ). In addition, positive reaction against β -catenin antibody was found in vascular endothelia and infiltrated inflammatory cells. However, nuclear p53 staining was not seen in tumor cells and dysplastic cells (Figure 4D).
The results of our experiment indicate that neither DMH pretreatment nor repeated administration of 3% DSS induced any tumorous lesions in the colorectum. However, their combination induced 4 invasive adenocarcinomas and 36 lesions with dysplasia in 11 mice within the relatively short term of 10 wk. Therefore, a clear synergism between the two agents was established. The histopathology of dysplasia and cancer in this model was very similar to that seen in humans. First, the animals developed dysplasia in both flat mucosa and DALM identical to that seen in humans. Second, early invasive cancer was revealed in 4 out of 40 lesions, and the data provided evidence of a dysplasia-adenocarcinoma sequence in this experimental system. Finally, the distribution of dysplasia/ cancer was also similar. Colon cancers in patients with UC developed mainly on the left side of the large intestine and transverse colon. In our experiment, DMH and DSS induced colitis-related tumors dominant in the distal part of colon followed by in the middle part. In human UC, dysplasia was found in two or more segments in 42%-75% of the cases and the incidence of mutiple synchronous cancers was reported to vary from 22%-50%[20-22]. In the current study, the result also showed that dysplasia was present in two or more segment in 60% of the animals and the incidence of synchronous cancers was 33.3%. However, we finished the experiment in 10 wk and only induced four early invasive cancers.
β -catenin played a role in both cell adhesion and intracellular signaling[23,24]. Cytoplasmic/nuclear translocation of β -catenin was reported in human colitis-associated neoplasms[25-27] and DSS-induced CRC mice models[28,29]. In our study, we also found aberrant β -catenin expression immunohistochemical in dysplasia and cancer in mice treated with DMH and DSS. Furthermore, We noticed that cancinoma, high- and low-grade dysplasiae had different intensities and distributions of β -catenin. However, we don’t know whether the translocation of β -catenin was due to loss of APC function or a direct mutation of β -catenin itself. This change might be associated with the progression from dysplasia to cancer. Our model are disimilar regarding the role of p53. Nuclear expression of p53 was a relatively early event in UC-related CRC compared with sporadic colon neoplasia[30-32]. However, in the current study, p53 immunohistochemical expression was not detected in colonic dysplasia and cancer. This findings might be due to the absence or low frequency of p53 mutations in colitis-related cancer mouse model. It is also possible that p53 mutations occurred at a later stage of cancer.
In conclusion, this study provides a novel colitis-associated mouse colon neoplasm model which features a single dose of procarcinogen followed by induction of chronic UC in a relatively short term. Futher studies on molecular mechanism or chemopreventive agents of this model may help us better understand CRC in patients with UC.
The authors wish to thank Dr. Mei Jin, Department of Pathology, Sir Run Run Shaw Hospital, Zhejiang University, Hangzhou, China, for her technical assistance in immunohistochemical analysis. We are also very grateful to Ming-Juan Jin, Department of Epidemiology and Public Health Statistics, Medical College of Zhejiang University, Hangzhou, China, for her statistical analysis.
Edited by Ren SR and Wang XL Proofread by Xu FM
| 1. | Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228-1233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1294] [Cited by in F6Publishing: 1161] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 2. | Delcò F, Sonnenberg A. A decision analysis of surveillance for colorectal cancer in ulcerative colitis. Gut. 2000;46:500-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1985] [Cited by in F6Publishing: 1942] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 4. | van Hogezand RA, Eichhorn RF, Choudry A, Veenendaal RA, Lamers CB. Malignancies in inflammatory bowel disease: fact or fiction? Scand J Gastroenterol Suppl. 2002;48-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694-702. [PubMed] [Cited in This Article: ] |
| 6. | Kullmann F, Messmann H, Alt M, Gross V, Bocker T, Schölmerich J, Rüschoff J. Clinical and histopathological features of dextran sulfate sodium induced acute and chronic colitis associated with dysplasia in rats. Int J Colorectal Dis. 2001;16:238-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Aghdassi E, Carrier J, Cullen J, Tischler M, Allard JP. Effect of iron supplementation on oxidative stress and intestinal inflammation in rats with acute colitis. Dig Dis Sci. 2001;46:1088-1094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Hans W, Schölmerich J, Gross V, Falk W. The role of the resident intestinal flora in acute and chronic dextran sulfate sodium-induced colitis in mice. Eur J Gastroenterol Hepatol. 2000;12:267-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-249. [PubMed] [Cited in This Article: ] |
| 10. | Clapper ML, Adrian RH, Pfeiffer GR, Kido K, Everley L, Cooper HS, Murthy S. Depletion of colonic detoxication enzyme activity in mice with dextran sulphate sodium-induced colitis. Aliment Pharmacol Ther. 1999;13:389-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Okayasu I, Yamada M, Mikami T, Yoshida T, Kanno J, Ohkusa T. Dysplasia and carcinoma development in a repeated dextran sulfate sodium-induced colitis model. J Gastroenterol Hepatol. 2002;17:1078-1083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Mitamura T, Sakamoto S, Sassa S, Suzuiki S, Kudo H, Okayasu I. The more an ulcerative colitis is repeated, the more the risk of colorectal carcinogenesis is increased in mice. Anticancer Res. 2002;22:3955-3961. [PubMed] [Cited in This Article: ] |
| 13. | Seril DN, Liao J, Ho KL, Yang CS, Yang GY. Inhibition of chronic ulcerative colitis-associated colorectal adenocarcinoma development in a murine model by N-acetylcysteine. Carcinogenesis. 2002;23:993-1001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Ma Q, Hoper M, Anderson N, Rowlands BJ. Effect of supplemental L-arginine in a chemical-induced model of colorectal cancer. World J Surg. 1996;20:1087-1091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Tsunoda A, Shibusawa M, Tsunoda Y, Yokoyama N, Nakao K, Kusano M, Nomura N, Nagayama S, Takechi T. Antitumor effect of S-1 on DMH induced colon cancer in rats. Anticancer Res. 1998;18:1137-1141. [PubMed] [Cited in This Article: ] |
| 16. | Balansky R, Gyosheva B, Ganchev G, Mircheva Z, Minkova S, Georgiev G. Inhibitory effects of freeze-dried milk fermented by selected Lactobacillus bulgaricus strains on carcinogenesis induced by 1,2-dimethylhydrazine in rats and by diethylnitrosamine in hamsters. Cancer Lett. 1999;147:125-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Schmelz EM, Sullards MC, Dillehay DL, Merrill AH. Colonic cell proliferation and aberrant crypt foci formation are inhibited by dairy glycosphingolipids in 1, 2-dimethylhydrazine-treated CF1 mice. J Nutr. 2000;130:522-527. [PubMed] [Cited in This Article: ] |
| 18. | Onderdonk AB, Bartlett JG. Bacteriological studies of experimental ulcerative colitis. Am J Clin Nutr. 1979;32:258-265. [PubMed] [Cited in This Article: ] |
| 19. | Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton SR, Morson BC. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931-968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1348] [Cited by in F6Publishing: 1179] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 20. | Connell WR, Lennard-Jones JE, Williams CB, Talbot IC, Price AB, Wilkinson KH. Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology. 1994;107:934-944. [PubMed] [Cited in This Article: ] |
| 21. | Taylor BA, Pemberton JH, Carpenter HA, Levin KE, Schroeder KW, Welling DR, Spencer MP, Zinsmeister AR. Dysplasia in chronic ulcerative colitis: implications for colonoscopic surveillance. Dis Colon Rectum. 1992;35:950-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 104] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Vatn MH, Elgjo K, Bergan A. Distribution of dysplasia in ulcerative colitis. Scand J Gastroenterol. 1984;19:893-895. [PubMed] [Cited in This Article: ] |
| 23. | Ilyas M, Tomlinson IP. The interactions of APC, E-cadherin and beta-catenin in tumour development and progression. J Pathol. 1997;182:128-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 24. | Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787-1790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2986] [Cited by in F6Publishing: 3023] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 25. | Tomlinson I, Ilyas M, Johnson V, Davies A, Clark G, Talbot I, Bodmer W. A comparison of the genetic pathways involved in the pathogenesis of three types of colorectal cancer. J Pathol. 1998;184:148-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 26. | Brabletz T, Jung A, Kirchner T. Beta-catenin and the morphogenesis of colorectal cancer. Virchows Arch. 2002;441:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Mikami T, Mitomi H, Hara A, Yanagisawa N, Yoshida T, Tsuruta O, Okayasu I. Decreased expression of CD44, alpha-catenin, and deleted colon carcinoma and altered expression of beta-catenin in ulcerative colitis-associated dysplasia and carcinoma, as compared with sporadic colon neoplasms. Cancer. 2000;89:733-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 28. | Cooper HS, Murthy S, Kido K, Yoshitake H, Flanigan A. Dysplasia and cancer in the dextran sulfate sodium mouse colitis model. Relevance to colitis-associated neoplasia in the human: a study of histopathology, B-catenin and p53 expression and the role of inflammation. Carcinogenesis. 2000;21:757-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 490] [Cited by in F6Publishing: 515] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 30. | Kern SE, Redston M, Seymour AB, Caldas C, Powell SM, Kornacki S, Kinzler KW. Molecular genetic profiles of colitis-associated neoplasms. Gastroenterology. 1994;107:420-428. [PubMed] [Cited in This Article: ] |
| 31. | Brentnall TA, Crispin DA, Rabinovitch PS, Haggitt RC, Rubin CE, Stevens AC, Burmer GC. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology. 1994;107:369-378. [PubMed] [Cited in This Article: ] |
| 32. | Ilyas M, Talbot IC. p53 expression in ulcerative colitis: a longitudinal study. Gut. 1995;37:802-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |