Published online Jul 15, 2004. doi: 10.3748/wjg.v10.i14.2024
Revised: December 28, 2003
Accepted: January 8, 2004
Published online: July 15, 2004
AIM: To evaluate the effects of tanshinone II-A on inducing growth inhibition and apoptosis of human hepatocellular carcinoma (HCC) cells.
METHODS: The human hepatocellular carcinoma cell line SMMC-7721 was used for the study. The cells were treated with tanshinone II-A at different doses and different times. Cell growth and proliferation were measured by MTT assay, cell count and colony-forming assay. Apoptosis induction was detected by microscopy, DNA ladder electrophoresis and flow cytometry.
RESULTS: In MTT assay, the inhibitory effect became gradually stronger with the passage of time, 24, 48, 72 and 96 h after treatment with tanshinone II-A, and the most significant effect was observed at 72 h. On the other hand, the increase of doses (0.125, 0.25, 0.5, 1.0 mg/L tanshinone II-A) resulted in enhanced inhibitory effect. The growth and proliferation of SMMC-7721 cells were obviously suppressed in a dose- and time-dependent manner. The results of cell count were similar to that of MTT assay. In colony-forming assay, the colony-forming rates were obviously inhibited by tanshinone II-A. In tanshinone II-A group, the morphology of cellular growth inhibition and characteristics of apoptosis such as chromatin condensation, crescent formation, margination and apoptotic body were observed under light and transmission electron microscopes. DNA ladder of cells was presented in electrophoresis. The apoptosis index (AI) was 16.9% (the control group was 4.6%) in flow cytometry. The cells were arrested in G0/G1 phase, and the expressions of apoptosis-related genes bcl-2 and c-myc were down-regulated and fas, bax, p53 up-regulated.
CONCLUSION: Tanshinone II-A could inhibit the growth and proliferation of HCC cell effectively in vitro by apoptosis induction, which was associated with up-regulation of fas, p53, bax, expression and down-regulation of bcl-2 and c-myc.
- Citation: Yuan SL, Wei YQ, Wang XJ, Xiao F, Li SF, Zhang J. Growth inhibition and apoptosis induction of tanshinone II-A on human hepatocellular carcinoma cells. World J Gastroenterol 2004; 10(14): 2024-2028
- URL: https://www.wjgnet.com/1007-9327/full/v10/i14/2024.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i14.2024
Hepatocarcinoma is one of the most common causes of malignancy-related death in China. Its therapy in clinic is a big challenge. New remedial method would possibly depend on advances in basic research[1]. Recent evidence suggests that apoptosis of cell is closely related to occurrence, progress and metastasis of tumor[2]. Study on induced apoptosis of tumor cells is an important field of tumor therapy and tumor molecular biology at present. Inducing apoptosis is a new therapeutic target of cancer research. New and promising anticancer drugs could be discovered by studies on apoptosis induction[3-5]. Tanshinone II-A is an alcohol-extracted product from the root of the traditional Chinese medicine-Salvia miltiorrhiza Bunge, which is known to have anti-inflammatory, anti-oxidative and cytotoxic activities[6-9]. Traditional Chinese medicines considered as potential drug in cancer treatment. Previous studies confirmed that tanshinone II-A could induce differentiation of human cervical carcinoma cell line (ME180) and leukemia cells (NB4, HL60 and K562), reverse malignant phenotype of human hepatocarcinoma cell line (SMMC-7721)[10-16]. However, there were no studies about growth inhibition and apoptosis induction of tanshinone II-A in hepatocarcinoma cell. In this in vitro study, human hepatocarcinoma cell line SMMC-7721 was used. The growth inhibition and apoptosis induction of tanshinone II-A on SMMC-7721 cell were obviously exhibited. We conclude that the effect of growth inhibition of tanshinone II-A on hepatocarcinoma might be related to induction of cellular apoptosis through regulation of apoptosis-associated genes.
Human hepatocellular carcinoma SMMC-7721 cell line was provided by Shanghai Institute of Cancer Research[17]. The cells were grown in RPMI 1640 (Gibco) supplemented with 100 ml/L fetal bovine serum (Huamei, Chengdu, China), penicillin (100 mg/L) and streptomycin (100 mg/L) in a humidified atmosphere of 50 mg/L CO2 at 37 °C. Anti-p53, fas, c-myc, bax and bcl-2 antibodies were obtained from Sigma. Other special chemicals were purchased from Sigma (St Louis, MO, USA).
Tanshinone II-A (Tan II-A) was provided by Institute of Traditional Chinese Medicine (concentration 96%). It was dissolved in DMSO (final concentration 0.2 mL/L). The solution was filtered through a 0.22 µm micropore filter and stored at 4 °C.
SMMC-7721 cells were seeded in flasks or dishes. The Tan II-A group was treated with Tan II-A of different doses (0.125, 0.25, 0.5, 1.0 mg/L) for 24, 48, 72 and 96 h, respectively. The control group was added with equal concentration of DMSO for negative control. The cells were measured after successive 96-h treatment.
SMMC-7721 cells were seeded in 96-well microtitre plates with 1 × 103 per well and incubated for 24 h in 100 μL culture media. Then the cells were treated with 0.125, 0.25, 0.5, 1.0 mg/L of Tan II-A in the experimental group for 24, 48, 72, and 96 h respectively. MTT 100 μL (5 g/L) was added to the cells and cultivated for another 4 h. After the supernatant fluid was removed, DMSO 100 μL per well was added to the cells and shaken for 15 min. The absorbance at 570 nm was measured by an ELISA reader. At the same time, the SMMC-7721 cells without treatment were served as control. Each assay was repeated three times.
SMMC-7721 cells were treated with Tan II-A (0.125, 0.25, 0.5, 1.0 mg/L) for 96 h, respectively, then condition of the cell growth was observed under an inverse and light microscope. Number and viability of the cells were assessed by trypan blue exclusion.
Cells were seeded in a 6-well plate with 300 cells per well. After 24 h, the cells were treated with Tan II-A (0.125, 0.25, 0.5, 1.0 mg/L), and cultured in routine medium for 10 days continually. Then the cells were fixed by methanol, Giemsa staining. Finally, the sum of colony per group was counted. The inhibiting rate of colony-formation was calculated.
SMMC-7721 cells were treated with DMSO (control group) or 0.5, 1.0 mg/L Tan II-A for 48 h. The cells were gently washed with serum-free medium, and observed by inverse and light microscopy after Giemsa staining. On the other hand, some other cells were fixed with 25 g/L glutaraldehyde in 0.1 mol/L of sodium cacodylate buffer, osmicated with 10 g/L osmium tetroxide. then cell block was stained, dehydrated in graded ethanol, infiltrated with propylene oxide, and embedded overnight and incubated in a 60 °C oven for 48 h. Silver sections were cut with an Ultracut E microtome, collected on a formvar and carbon-coated grid, stained with uranyl acetate and Reynold’s lead citrate, and examined under a transmission electron microscope to identify the morphological changes of apoptosis.
After induction of apoptosis, the cells (1 × 106/group, both attached and detached cells) were washed by PBS, and fixed in ice-cold 700 mg/L ethanol for 24 h. After the ethanol was removed, the cells were rinsed in 0.2 mol/L Na2HPO4 and 0.1 mol/L citric acid (192:8, pH7.8) for 60 min at room temperature. After being centrifuged (10000 g), the supernatant was collected in Eppendorf tubes, 2.5 g/L NP-40 and 10 g/L RNase A were added at 37 °C for 30 min, then Protein K (1 g/L, Promega) was added at 50 °C for 30 min. Equal quantity of DNA was electrophoresed in 15 g/L agarose gels and stained with ethidium bromide (5 mL/L) for 2 h at 80 V. Ladder formation of oligonucleosomal DNA fragmentation was detected under ultraviolet light.
The sample preparation and measurement followed the method described in reference[15]. The cells were harvested, counted and fixed. The cell concentration was adjusted to 106/mL. According to the routine method, cell frequency distribution of each phase in cell cycle was measured. Cellular fas, p53, bax, bcl-2 and myc gene expressions were detected by immunohistochemical method on FACS-420 FCM. The results were shown with scanning figure and date.
Data were presented as the mean ± SD error of the mean. Student’s t test was used for comparison among different groups. A P value of less than 0.05 is considered statistically significant.
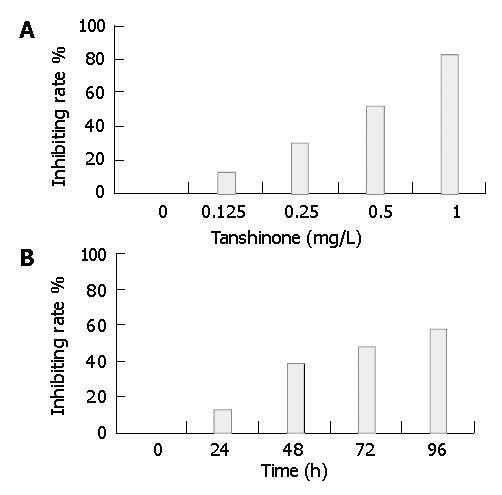
Tan II-A exhibited a statistically significant dose-dependent growth-inhibitory effect on SMMC-7721 cell evaluated by MTT assay. The inhibiting rates of cell growth were 12.2%, 30.6%, 52.4% and 82.5%, respectively when treated with Tan II-A of different doses (0.125, 0.25, 0.5, 1.0 mg/L) for 96 h (Figure 1A). Moreover, the growth-inhibitory effect of Tan II-A on the cells was found to be time-dependent. The inhibiting rates were 12.5%, 38.2%, 47.6% and 58.7%, respectively when treated separately for 24, 48, 72 and 96 h with 0.5 mg/L of Tan II-A (Figure 2B). The inhibitory effect became gradually stronger with the passage of time after treatment, and the most significant effect was observed at 72 h. The cell growth and proliferation were obviously inhibited in a dose- and time-dependent manner. The same inhibitory effect was found by cell count. In colony-forming assay, the colony-forming rates of SMMC-7721 cell were 11.6%, 22.5%, 38.5%, 42.2% in Tan II-A group and 68.2% in control group, respectively, suggesting the obviously inhibiting effect of Tan II-A on cell proliferation.
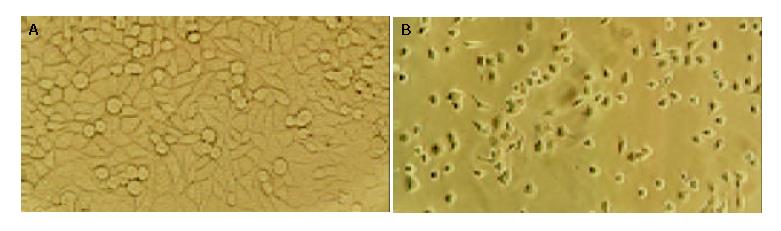
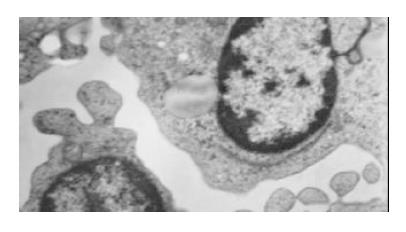
As shown in Figure 2, Tan II-A-induced morphological changes were evident by 72 h of treatment in SMMC-7721 cell. Roundish, large and serried cells (in control group, Figure 2A) became polygonal, small, detached or sparse, membranous frothed and wizened (Tan II-A group, Figure 2B) in the dishes under light microscope. The ultrastructural characteristics of apoptosis such as chromatin condensation, crescent formation, margination and apoptotic bodies were observed by electron microscopy in the Tan II-A group (Figure 3), which were more obvious in the given experimental period than that in the control group.
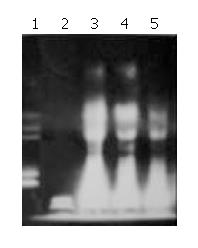
Agarose gel electrophoresis exhibited DNA ladder formation in SMMC-7721 cell after exposed to different concentration of Tan II-A for 96 h. Compared with control, the DNA ladder was more clearly observed by treatment with 0.5, 1.0 and 2.0 mg/L Tan II-A, while the DNA fragment induced by 0.125, 0.25 mg/L Tan II-A was not clear (Figure 4).
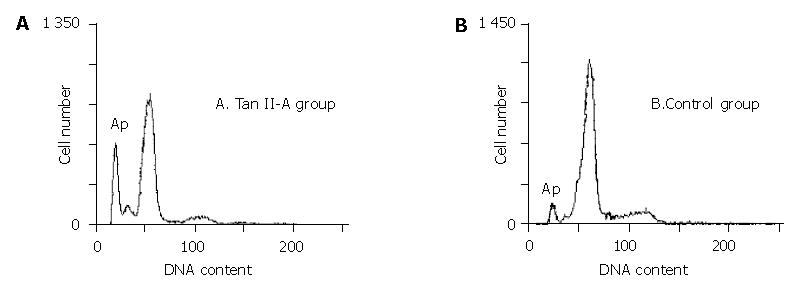
In order to determine the effect of Tan II-A on proliferation and apoptosis in SMMC-7721 cell, the cells were exposed to 0.5 mg/L Tan II-A for 96 h. Cell cycle distribution, cell proliferation and apoptotic damage of DNA were determined on a flow cytometer. The result showed the accumulation of cells in G0/G1 and a corresponding reduction in percentages of cells in S and G2/M phase, and decreased proliferation index (PI). The cells in sub-G1 phase were increased. An apoptotic peak was detected (Table 1, Figure 5)
The expressions of apoptosis associated genes fas, bax, p53 were up-regulated. The expressions of bcl-2 and c-myc were down-regulated (Table 2). The tabulated percentages were an average calculated on the results of three separate experiments. The values were presented as mean ± SE (n = 3).
Cellular proliferation and apoptosis in normal tissue maintain a balance. Unlimited growth and proliferation, but blocked apoptosis are main characteristics of malignant tumor cell. So inhibiting proliferation by apoptosis induction of cancer cell is a new method for cancer control and therapy[18-20].
On the basis of previous studies, we chose different concentrations of non-cytotoxic Tan II-A to study Tan II-A induced-apoptosis in vitro. MTT, cell count assay and colony-forming assay showed that 0.25, 0.5, 1.0 mg/L Tan II-A obviously inhibited the growth and proliferation of SMMC-7721, and the inhibiting effect was in a dose- and time-dependent manner. The most significant effect was observed at 72 h by 1.0 mg/L Tan II-A. Flow cytometer showed that Tan II-A produced significant cell cycle arrest in G0/G1, decreased proliferation index (PI) as evidence of an antiproliferation effect. The cellular growth and inhibition could be observed by morphology. The morphological characteristics of cellular growth inhibition were also observed under light microscope. Our results demonstrated that Tan II-A inhibited growth and proliferation of SMMC-7721 cells[21,22].
Apoptosis, or programmed cell death, has an essential role in controlling cell number in many developmental and physiological settings and in chemotherapy-induced tumour-cell killing. The execution of apoptosis may be initiated by many different signals, either from within or outside the cell involving ligand-receptor interactions, as has been shown for Fas/Fas-ligand, or potentially by more unspecific signals such as DNA damage. During the modulation phase of apoptosis many different genes such as p53, c-myc or Bcl-2/Bax have been shown to be able to shift the balance either to cellular proliferation or death. Cellular growth and proliferation are closely related to apoptosis[23-25].
The detection of apoptotic cell by microscopy is based on several well-established morphological features. These features include condensation of chromatin and cytoplasm as well as fragmentation of the cells that lead to the appearance of membrane bound apoptotic bodies[26]. Those ultrastructural characteristics apoptosis, the DNA ladder and increased cells in sub-G1 phase were observed in SMMC-7721 cells treated with 0.5, 1.0 mg/L Tan II-A.
Apoptosis-associated genes play a major role in apoptosis, participating in the initiation and progress of programmed cell death. Bcl-2 has been identified as an apoptosis inhibitor, and shown to cooperate with c-myc in immortalizing cells. Under certain conditions, constitutive expression of c-myc induces apoptosis and can be suppressed by bcl-2. It appears that the c-myc cooperating oncogenic activity of bcl-2 is related to its inhibition of apoptotic pathways[26]. Bax protein inhibits the function of bcl-2 leading to increased apoptosis.
The tumor suppressor gene p53, a transcription factor, has been identified as a participant in the cellular DNA damage response. Upon DNA damage, p53 up-regulation causes G1 arrest. The apoptosis promoting capacity of p53 is presumably due to its ability to activate bax, a gene that encodes an inhibitor of bcl-2. Fas (CD95/APO-1) is a death-promoting receptor that belongs to the tumor necrosis factor (TNF) receptor family, and induces apoptosis through different ways[27].
In the present study, we observed down-regulation of bcl-2 and c-myc expression, which is in keeping with the role of bcl-2 in blocking apoptosis and c-myc in promoting proliferation. At the same time, the expressions of apoptosis associated genes fas, bax, p53 were up-regulated. These suggested that Bcl-2, c-myc, fas, bax and p53 were involved in apoptosis of hepatocarcinoma cells induced by Tan II-A. Parts of these mechanisms were related to inhibition of cell growth. Some results of our study on inducing apoptosis in human hepatocellular carcinoma cell are consistent with other researches[28].
Tan II-A inhibits growth and proliferation of hepatocarcinoma cells by inducing apoptosis. Tan II-A might have different mechanisms in inducing apoptosis and inhibiting proliferation of cancer cells. Other possible mechanisms of the action of Tan II-A need to be further investigated. We consider that Tan II-A could be a new prospective, highly effective and low toxic anticancer drug.
Edited by Chen WW Proofread by Zhu LH and Xu FM
| 1. | Kanzler S, Galle PR. Apoptosis and the liver. Semin Cancer Biol. 2000;10:173-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Carson DA, Ribeiro JM. Apoptosis and disease. Lancet. 1993;341:1251-1254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 490] [Cited by in F6Publishing: 522] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 3. | Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013-2026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 16] [Reference Citation Analysis (0)] |
| 4. | Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456-1462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4724] [Cited by in F6Publishing: 4648] [Article Influence: 160.3] [Reference Citation Analysis (0)] |
| 5. | Lee SM, Li ML, Tse YC, Leung SC, Lee MM, Tsui SK, Fung KP, Lee CY, Waye MM. Paeoniae Radix, a Chinese herbal extract, inhibit hepatoma cells growth by inducing apoptosis in a p53 independent pathway. Life Sci. 2002;71:2267-2277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Li ZT, Yang BJ, Ma GE. [Chemical studies of Salvia miltiorrhiza f. alba]. Yaoxue Xuebao. 1991;26:209-213. [PubMed] [Cited in This Article: ] |
| 7. | Lin TJ. [Antioxidation mechanism of schizandrin and tanshinonatic acid A and their effects on the protection of cardiotoxic action of adriamycin]. Shengli Kexue Jinzhan. 1991;22:342-345. [PubMed] [Cited in This Article: ] |
| 8. | Liang Y, Yang YM, Yuan SL. Studies on Pharmic mechanism and clinic application of Tanshinone. Traditional Herbal Drugs. 2000;31:304-306. [Cited in This Article: ] |
| 9. | Wu WL, Chang WL, Chen CF. Cytotoxic activities of tanshinones against human carcinoma cell lines. Am J Chin Med. 1991;19:207-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 102] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Yuan S, Huang G, Wang X. [The differentiation-inducing effect of tanshinone and retinoic acid on human cervical carcinoma cell line in vitro]. Zhonghua Zhongliu Zazhi. 1995;17:422-424. [PubMed] [Cited in This Article: ] |
| 11. | Liang Y, Yang Y, Yuan S, Liu T, Jia Y, Xu C, Niu T, Qin H, Qin P. [Terminal differentiation of human acute promyelocytic leukemia (APL) cells induced by Tanshinone II A in primary culture]. Huaxi Yike Daxue Xuebao. 2000;31:207-210. [PubMed] [Cited in This Article: ] |
| 12. | Yoon Y, Kim YO, Jeon WK, Park HJ, Sung HJ. Tanshinone IIA isolated from Salvia miltiorrhiza BUNGE induced apoptosis in HL60 human premyelocytic leukemia cell line. J Ethnopharmacol. 1999;68:121-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Sung HJ, Choi SM, Yoon Y, An KS. Tanshinone IIA, an ingredient of Salvia miltiorrhiza BUNGE, induces apoptosis in human leukemia cell lines through the activation of caspase-3. Exp Mol Med. 1999;31:174-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Wang X, Yuan S, Wang C. [A preliminary study of the anti-cancer effect of tanshinone on hepatic carcinoma and its mechanism of action in mice]. Zhonghua Zhongliu Zazhi. 1996;18:412-414. [PubMed] [Cited in This Article: ] |
| 15. | Yuan S, Wang Y, Chen X, Song Y, Yang Y. [A study on apoptosis of nasopharyngeal carcinoma cell line induced by Tanshinone II A and its molecular mechanism]. Huaxi Yike Daxue Xuebao. 2002;33:84-6, 90. [PubMed] [Cited in This Article: ] |
| 16. | Yuan SL, Huang RM, Wang XJ, Song Y, Huang GQ. Reversing effect of Tanshinone on malignant phenotypes of human hepatocarcinoma cell line. World J Gastroenterol. 1998;4:317-319. [PubMed] [Cited in This Article: ] |
| 17. | Ai ZW. [Reversing effect of retinoic acid on some phenotypes of human hepatocarcinoma cell line]. Zhonghua Zhongliu Zazhi. 1991;13:9-12. [PubMed] [Cited in This Article: ] |
| 18. | Marx J. Cell death studies yield cancer clues. Science. 1993;259:760-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 91] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Sakuma H, Yamamoto M, Okumura M, Kojima T, Maruyama T, Yasuda K. High glucose inhibits apoptosis in human coronary artery smooth muscle cells by increasing bcl-xL and bfl-1/A1. Am J Physiol Cell Physiol. 2002;283:C422-C428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Tsuda H, Sata M, Ijuuin H, Kumabe T, Uchida M, Ogou Y, Akagi Y, Shirouzu K, Hara H, Nakashima Y. A novel strategy for remission induction and maintenance in cancer therapy. Oncol Rep. 2002;9:65-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Hu W, Kavanagh JJ. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol. 2003;4:721-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 376] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 22. | Giridharan P, Somasundaram ST, Perumal K, Vishwakarma RA, Karthikeyan NP, Velmurugan R, Balakrishnan A. Novel substituted methylenedioxy lignan suppresses proliferation of cancer cells by inhibiting telomerase and activation of c-myc and caspases leading to apoptosis. Br J Cancer. 2002;87:98-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Fimognari C, Nüsse M, Cesari R, Iori R, Cantelli-Forti G, Hrelia P. Growth inhibition, cell-cycle arrest and apoptosis in human T-cell leukemia by the isothiocyanate sulforaphane. Carcinogenesis. 2002;23:581-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 157] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 24. | Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581-8589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 659] [Cited by in F6Publishing: 668] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 25. | Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169-1178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1816] [Cited by in F6Publishing: 1819] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 26. | Barnhart BC, Lee JC, Alappat EC, Peter ME. The death effector domain protein family. Oncogene. 2003;22:8634-8644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Lyu SY, Choi SH, Park WB. Korean mistletoe lectin-induced apoptosis in hepatocarcinoma cells is associated with inhibition of telomerase via mitochondrial controlled pathway independent of p53. Arch Pharm Res. 2002;25:93-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Rahman MA, Dhar DK, Masunaga R, Yamanoi A, Kohno H, Nagasue N. Sulindac and exisulind exhibit a significant antiproliferative effect and induce apoptosis in human hepatocellular carcinoma cell lines. Cancer Res. 2000;60:2085-2089. [PubMed] [Cited in This Article: ] |