Published online Apr 26, 2019. doi: 10.12998/wjcc.v7.i8.917
Peer-review started: January 14, 2019
First decision: January 30, 2019
Revised: March 11, 2019
Accepted: March 16, 2019
Article in press: March 16, 2019
Published online: April 26, 2019
Celiac disease (CeD) is an autoimmune disorder, mainly affecting the small intestine, triggered by the ingestion of gluten with the diet in subjects with a specific genetic status. The passage of gluten peptides through the intestinal barrier, the uptake by antigen presenting cells and their presentation to T cells represent essential steps in the pathogenesis of the disease. CeD prevalence varies in different populations, but a tendency to increase has been observed in various studies in recent years. A higher amount of gluten in modern grains could explain this increased frequency, but also food processing could play a role in this phenomenon. In particular, the common use of preservatives such as nanoparticles could intervene in the pathogenesis of CeD, due to their possible effect on the integrity of the intestinal barrier, immune response or microbiota. In fact, these alterations have been reported after exposure to metal nanoparticles, which are commonly used as preservatives or to improve food texture, consistency and color. This review will focus on the interactions between several food additives and the intestine, taking into account data obtained in vitro and in vivo, and analyzing their effect in respect to the development of CeD in genetically predisposed individuals.
Core tip: Celiac disease (CeD) is a common autoimmune disorder caused by the ingestion of gluten. Its frequency has been increasing, and several factors have been analyzed as possible triggers; among them also food additives should be taken into account. Several nanoparticles are used as food additives or preservatives, and they can interact with the intestine or the immune system, increasing, in theory, the immune response towards gluten. The scope of this review is to analyze the data present in the literature with respect to the pathogenetic mechanisms involved in the development of CeD.
- Citation: Mancuso C, Barisani D. Food additives can act as triggering factors in celiac disease: Current knowledge based on a critical review of the literature. World J Clin Cases 2019; 7(8): 917-927
- URL: https://www.wjgnet.com/2307-8960/full/v7/i8/917.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i8.917
Celiac disease (CeD) is a multifactorial disorder, characterized by the presence of an autoimmune response that mainly involves the small intestine, triggered by the ingestion of gluten from wheat, barley, and rye in genetically predisposed individuals. CeD genetic background is quite complex, and partially still unknown. About 40% of the genetic predisposition relies on genes localized in the human leukocyte antigen (HLA) region, mainly on those encoding for specific class II HLA molecules, namely DQ2.5 and DQ8 heterodimers. The combination of the HLA alleles DQA1*0501 and DQB1*0201 generates the HLA-DQ2.5 heterodimer, which is detected in more than 90% of Caucasian CeD patients (either in cis or in trans), whereas the remaining patients carry the HLA-DQ8 heterodimer, encoded by DQA1*03 (α chain) and DQB1*0302 (β chain). However, the presence of the DQ2 heterodimer is not sufficient for the development of CeD. In fact, the HLA-DQ2 haplotype is present in 30%-35% of the Caucasian population (in which CeD has a high prevalence), but only 2%-5% of gene carriers develop CeD[1]. Using the Genome Wide Association Study approach, several additional loci have been identified as predisposing to CeD, but in total they account for about 50% of the genetic component.
Although the genetic background is essential for the development of CeD, research has started to focus on the possible environmental factors (apart from gluten) that could trigger the disorder. This could be quite important, since several data suggest that the prevalence of CeD is increasing, and cases are now reported even in populations that were thought to have a negligible prevalence of this disease. A study performed in United Kingdom showed a four-fold increase in CeD incidence rate over a period of 22 years, but regional differences were present[2]. Even if this increased rate could be explained by a different awareness of the problem by physicians, or by the use of an easier serological diagnosis and a casefinding approach, there is still some evidence which suggests that this raise in the prevalence/incidence of the disease is a real phenomenon. Evaluation in a Scottish pediatric population revealed that, in two decades, the incidence of children with nonclassical CeD had increased dramatically (attributable to better diagnosis), but also the number of patients with classical manifestations had quadrupled (thus suggesting a real variation in CeD frequency)[3]. Moreover, similar data have been observed in Finland as well as in the United States[4-6].
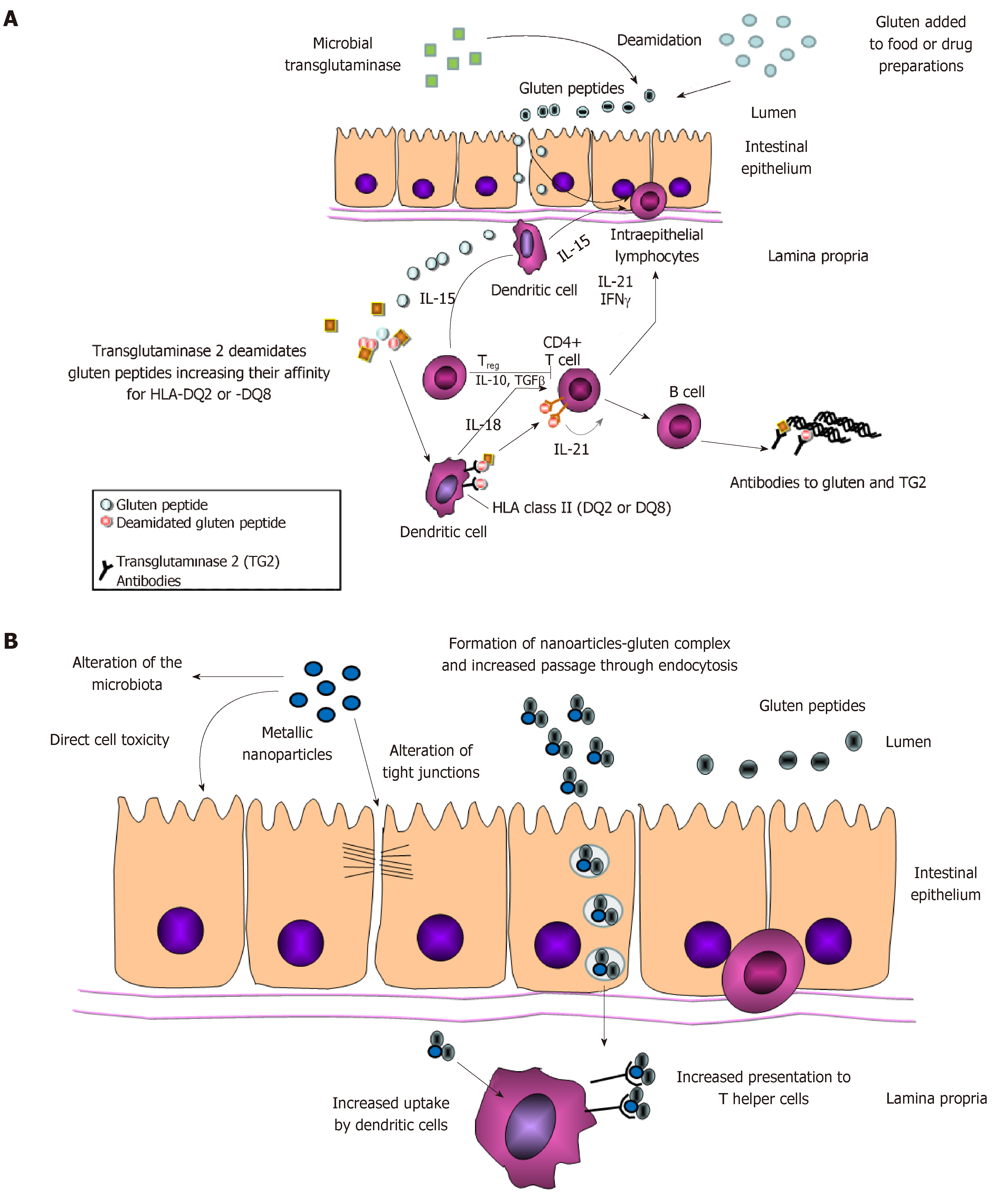
To identify the possible additional environmental causes it is necessary to dissect the various steps involved in CeD pathogenesis. The ingested gluten undergoes digestion, which generates several small peptides, including the 33 mer peptide (residues 57 to 89 of α-gliadin), a celiac “superantigen” able to stimulate T cells[7], or the 31-43 peptide (from residues 31 to 43 of α-gliadin), which can have a toxic effect on intestinal mucosa[8]. However, in order to trigger the autoimmune response these peptides need to cross the gastrointestinal barrier and reach the lamina propria. This passage can take place using two different routes, namely the transcellular and the paracellular one. The first is a vesicle-mediated passage which involves endocytosis on the luminal side of enterocytes, followed by transcytosis and release on the basolateral side. Thus, in theory, substances which are able to make the gluten peptides more prone to be captured on the apical side and transported could play a role in the pathogenesis of CeD. Conversely, paracellular transport depends on tight junctions (TJ) and the correct expression/interaction of the proteins that maintain junction functionality. Therefore, agents able to induce inflammation and/or cytokine release could cause the rearrangement of proteins such as ZO-1 or occludin, causing a loss of function of TJ and, in turn, an increased paracellular passage of lumen substances, such as gluten peptides.
Once gluten peptides have crossed the intestinal barrier, they are further processed in the submucosa; due to their high content in glutamine, proline and hydrophobic amino acid residues, these peptides are excellent substrates for transglutaminase 2 (TG2) which deamidates them. This processing increases negative charges, allowing the gluten peptides to bind more strongly to HLA-DQ2 (or HLA-DQ8). Better antigen presentation results in CD4+ Th1 T-cell activation that, in turn, will cause activation of intraepithelial lymphocytes, crypt hyperplasia and villus atrophy, as well as B cell stimulation and the production of auto-antibodies directed against deamidated gliadin peptides and TG2 (Figure 1A).
Given these data, food additives could have a role in triggering the development of CeD if they are able to alter the gastrointestinal barrier, antigen presentation or the activation of the immune system. Although there are currently few experimental published data that specifically address the interaction between food additives and CeD, there are at least three possible categories that should be analyzed, namely transglutaminase, gluten nanoparticles and metallic nanoparticles.
The use of transglutaminase in food processing belongs to the various techniques that industries in the field currently use to modify the proteins present in aliments. Microbial transglutaminases (mTGs), like human ones, catalyzes acyl transfer, deamidation and crosslinking between glutamine (acyl donor) and lysine (acceptor). These reactions can profoundly modify a large amount of proteins constituting food matrices and this, in turn, can improve several food properties such as texture and stability; more interestingly, these changes can take place without affecting other food characteristics like taste or nutritional value. For these reasons, the possible ingestion of microbial tranglutaminases, due to its use in food processing, has been recognized as safe by Food and Drug Administration[9].
Currently mTGs are is used in several processed foods since, among others, their use increases the water retention capacity of proteins, fact that could help to increase the juiciness of products such as meat, or emulsion properties that are important in food characterized by creamy texture (e.g., yogurt)[10]. Moreover, bacterial transglutaminase treatment has also been applied to cereal proteins (including wheat protein); its use can improve stability, elasticity and water retention of the dough, and for this reason it has also been employed in the preparation of gluten-free food[11-12].
Due to the pivotal role of transglutaminase in the pathogenesis of CeD, its use in food preparation has raised some concern. Transglutaminases can act on gluten peptides, making them more immunogenic, but it must also be remembered that TG2 is itself an autoantigen, and the ingestion of mTGs could also generate an autoimmune response through a molecular mimicry mechanism. The comparison of the primary and tertiary structure of a commonly used mTGs with TG2 reveals little homology, although both are able to bind gluten peptides using similar aminoacids[13]. Moreover, mTGs can deamidate gluten peptides, making them more immunogenic, as assessed in an in vitro system that employed gluten-specific T cells isolated from the duodenum of celiac patients[14].
Few papers have tried to assess the possible correlation between the use of bacterial transglutaminase and CeD, but most of them are only based on peptide-patients’ antibody interaction. An initial investigation performed using sera from nine celiac patients suggested that treatment of wheat with mTGs increases the IgA-based reactivity, and to a lesser degree when mTGs were used to treat gluten-free bread[15]. Matthias et al[16] evaluated the presence of antibodies directed against either human or bacterial transglutaminase (alone or bound to gluten peptides) in pediatric patients with or without CeD. In the serum of CeD patients, they could detect antibodies against mTGs, although prevalently IgG rather than IgA (as commonly observed against TG2), whereas they were not present in controls. The authors also found a correlation between serum levels of antibodies against mTG-peptides and TG2-peptides, as well as between these serum titer and intestinal damage, and they suggested a causal role of this food supplement in the development of CeD. Different results were observed by Ruh et al[17], who extracted gliadin from pasta treated or untreated with mTGs and employed it to assess possible reactivity with circulating antibodies present in CeD patients. The authors detected a huge variation among patients, but no difference in reactivity between the two types of gliadin. These results were also confirmed by Heil et al[18].
On the contrary, in theory, the use of mTGs could also be useful to decrease the immunogenicity of gluten, but in order to do so the enzyme has to be used in association with acyl-acceptor molecules such as lysine[19]. This pre-treatment of gluten could in fact block the aminoacids that are the usual target of TG2, thus preventing the modifications that increase the affinity of gluten peptides for the DQ2 molecule[13,20]. Moreover, experiments performed ex vivo on duodenal biopsies of CeD patients showed that the modification of gluten by mTGs with L-lysine prevented pro-inflammatory cytokine production[21,22]. Gluten transamidation by mTGs could thus be used to produce flour of bread with less immunoactive gluten peptides[23,24], but there are still some issues that need to be clarified, due to the affinity of mTGs for the aminoacids usually targeted by TG2 and to the possibility that TG2 overcomes the modification induced by mTGs.
Gluten-based nanoparticles have been mainly developed as a tool for drug delivery, and have been tested in particular for hydrophobic drugs[25]. However, there is another use that could be potentially problematic, i.e., the development of coating matrices for paper and cardboard used for food packaging. Plant-derived proteins have good film-forming properties, are biodegradable, and can be produced with moderate costs, facts that make them suitable for coating food containers. Some authors have also combined gluten with nanocellulose and titanium dioxide in order to obtain nanocomposites able to increase the resistance of paper. These na-nocomposites also have an antibacterial activity, a quality that might be very attractive for food-preserving packages[26]. As will be mentioned later, the issue regarding these nanomaterials is that data about the possible release of nanoparticles in food are needed.
Nowadays several nanoparticles (NPs) are intentionally added to food, beverages and their packages[27], mainly to preserve aliments[28,29] or to improve their organoleptic properties (such as taste, consistency and appearance). Consequently, in recent years, an increase of toxicological studies on food nanoparticles has been registered. Although NP can enter the body through several routes, according to the Nanotechnology Consumer Product Inventory (CPI) enlisted in 2014, one of the major NP points of entry is the gastrointestinal system[30]. They also reported that nanomaterials are particularly present in commercial food or food-related products under the form of metallic nanoparticles (mNP), of which Ag (E174), TiO2 (E171), ZnO, Au (E175) and SiO2 (E551) NPs are the most popular. Briefly, AgNPs are particularly used as antimicrobial agent in aliments/beverages, their packages and in agriculture[29]; ZnONPs are also used as strong antibacterial agents, but they can also be used as a dietary supplement; E171 is used as a whitening agent in pharmaceutical, dairy and pastry products; AuNP is mostly present as a contaminant from dental restoration material or agriculture-derived products (such as seeds)[31,32]; SiO2NP is employed to improve the organoleptic properties of food and its nutritional values.
In order to evaluate the possible effects of ingested NP, there are several factors that should be taken into account: (A) NP dimensions: several studies reported as the size of food mNP might alter their uptake from intestinal cells[33-35]. The smaller the mNP, the faster and easier will be its passage through the mucous layer and its passage into the mucosa either by transcellular or paracellular transport; (B) Core material: it could determine whether NPs remain intact or partially digested by the intestinal fluids. An important concern is in fact the propensity of NPs to be dissolved and release heavy metals, which in turn affects NP toxicity. In this sense, AgNP, ZnONP and CuONP are regarded as the most dangerous food nanoparticles[36,37]. NP core composition also determines the chemical reactivity, substance adsorption on NP surface, and possibly the epithelial translocation route[38,39]; (C) Aggregation/agglomeration state: NPs can arrive into the gut as single entities or in clusters (agglomerates or aggregates[40]). This feature depends on the NP composition, but also on the physiochemical properties of the environment. It has been reported that the degree of aggregation/agglomeration of SiO2-, Ag- and aluminium-NPs can change in artificial mouth, gastric and intestinal conditions[41-43]. At the same time, this factor also affects NP uptake and toxicity, as demonstrated by McCracken et al[44] and Albanese et al[45]; (D) Gastrointestinal environment and food: Physiochemical features of food, beverages, and the gut are important factors that influence NP stability, size, surface composition and aggregation/agglomeration state[41,43,44,46,47]. Wang et al[48] and Cao et al[49] demonstrated a higher oxidative stress-related toxicity exerted by ZnONP when associated with Vitamin C and palmitic oil, respectively; on the contrary the presence of flavonoids or quercetin seems to protect against AgNP toxicity[50,51].
Although the daily consumption of metallic NPs is usually thought to be trivial, this is not the case, in particular if TiO2 is taken into account. Early studies suggested that average daily human consumption of TiO2 was 5.4 mg per person[52], 0.035 mg/kg of body weight (b.w.)/d[53] and 5 mg/person[54]. More recent papers, however, estimated a daily intake of 1–2 mg TiO2/kg b.w. for United States children under 10 years of age, and 0.2-0.7mg TiO2/kg b.w. for other United States consumers[55], whereas EFSA data reported a range between 0.2 and 0.4 mg/kg b.w. in infants and the elderly, and 5.5-10.4 mg/kg b.w. in children, depending on the exposure[56]. Although these data should be corrected for the percentage on TiO2 NPs present in the E171, it must be noted that these quantities are not far from the estimates for the lowest observed adverse effect level (LOAEL) of 5 mg/kg body weight/d derived for nano‐ TiO2 by the European Commission’s Scientific Committee on Consumer Safety[57].
The effects of mNP that could have a role in CeD development involve three different aspects, namely the impairment of the intestinal barrier, the interaction with the immune system and the possible effect on microbiota (Figure 1B).
The first layer of the small intestinal barrier is a very thin (approximately 20 micron) layer of mucus, composed of mucin glycoproteins and antimicrobial agents such as secretory IgA. The second layer is a continuous and tight epithelium, composed of several specialized cells: at the bottom of the crypts reside stem and Paneth cells, whereas enterocytes, goblet and enteroendocrine cells are mainly in the villi. What makes the epithelium a selective barrier is the presence of highly dynamic intercellular junctions, adherent junctions (AJ) and TJ being the most representative. AJ are composed of transmembrane proteins cadherine, which are connected between them extracellularly, and with the catenin proteins in the cells. Catenins are in turn linked to the acti-myosin complex. TJ are formed by occludins, claudins and JAM-A proteins that interact with zonula occludens proteins and catenins in the intracellular space. Therefore AJ, TJ and actin cytoskeleton form a complex that can regulate the permeability (paracellular route) of the intestinal barrier, following intracellular or extracellular signals.
A growing number of diseases have recently been associated with intestinal barrier alterations, particularly related to TJ dysfunction. This finding can be easily explained: gastrointestinal barrier permeability alterations can increase the cut-off of molecules passing into the submucosa. In physiological conditions, only small molecules with a molecular weight of about 600da can pass the barrier, but these alterations result in the passage of immunogenic molecules, the activation of the immune system and the establishment of an inflammatory state. Since inflammatory mediators are also known to affect the intestinal barrier, a mild inflammatory status could eventually lead to a stronger disruption of the barrier itself[58]. Particularly important in this sense is the association of a leaky barrier with inflammatory bowel diseases (IBD) and several autoimmune diseases, such as CeD[58-60]. To develop CeD, gluten peptides have to pass into the submucosa. Therefore, any factors which are able to alter the intestinal barrier permeability, allowing an higher passage of these peptides into the submucosa, may increase the number of predisposed subjects developing the disease.
In 2015 Lerner and Matthias[61] observed that the increase in the incidence of autoimmune diseases (considering also CeD among others) paralleled with the growing use of food additive in the industry. They therefore postulated that the permeability alterations induced by food additives could be associated with the increment in incidence of autoimmune diseases. Although the author did not refer directly to the mNP, several studies have been performed on their impact on the GI barrier. Results showed that mNP can alter the intestinal permeability both directly, by altering the TJ or inducing epithelial cell death[34,62-64], or indirectly, by inducing inflammation or oxidative stress that in turn can impair TJ and permeability[58,65]. In this context, the work of Ruiz et al[66] is interesting. It looked at the impact of TiO2NP both in vivo (mice with DSS-induced ulcerative colitis) and in vitro (intestinal epithelial cells and macrophages). TiO2NP oral administration worsened the already established colitis through inflammasome activation. Also, in vitro stimulations induced IL-1β and IL18 increment, as well as higher epithelial permeability driven by the activation of the inflammasome pathway. These results clearly associate the consumption of mNP with an increase of the intestinal permeability, but only when there is a pre-existent tendency to develop it.
However, even if the studied mNP does not induce permeability alteration, it has to be considered that the mNPs may absorb the protein itself on its surface and therefore behave as a “Trojan horse”, increasing the amount of immunogenic molecules that arrive into the submucosa[67,68]. Thus, in the case of CeD, food NPs could bind gliadin peptides and help them to cross the intestinal barrier, probably using the endocytotic pathway. Several studies are needed to test this hypothesis, since no data are currently available on this topic. Moreover, it will be necessary to take into account the interaction with other food components[69,70], and with the intestinal mucus[71], since both components can alter NPs uptake by enterocytes.
On the other hand, it must be underlined that NPs can play a role in the pathogenesis of other gastroenterological disorders, and concerns have also been raised for several evidences that linked NPs, particularly the whitening agent E171(TiO2NP), to IBD development[66,72,73].
mNPs can interact with cells involved in innate and adaptive immune response in several organs, altering cytokine production, activation of cell surface receptors and/or cell maturation (including the ability of cells to present antigens)[74-77]. Nanoparticles can be recognized as foreign materials and eliminated by the immune system, but they can also trigger an excessive activation of immune responses. This could be useful should NP be used as an adjuvant in vaccinations, but could be detrimental in case of autoimmune disorders. In particular, the binding of gliadin peptides to food NP could represent a way by which these specific antigens can be taken up in great quantity by antigen presenting cells, thus increasing the activation of the autoimmunity process. Several studies have been performed on macrophage-like cell lines to assess the effect of metallic NPs, analyzing the cytotoxicity as well as differences in cytokines production; AgNPs were able to increase the production of IL-8[78,79], which also depended on NP size[78] whereas TiO2 NPs increased the secretion of TNF-α and IL-6[80]. Interestingly, Au-NPs induced an alteration in phagocytosis without variation in cytotoxicity or cytokine gene expression[81], whereas a similar effect by TiO2 NPs was associated with an inflammatory response[82]. Transcription profiling on a macrophage cell line treated with different NPs revealed a particular expression pattern, thus suggesting that each metallic NP can trigger a specific response, also depending on the chemical characteristics of the nanoparticle itself[83]. Silver NPs have been demonstrated to be able to interact with human monocytes, increasing the production and release of IL-1β, even after the exposure to very low concentrations[84]. Ag-NP were also able to cause superoxide production, as well as the formation of inflammasome. Metallic NPs also altered the expression of adhesion molecules and chemokine receptor type 4 on the surface of human peripheral lymphocytes[85]; interestingly, these effects were independent from any sign of cytotoxicity, suggesting that the response to NP exposure can be more subtle and mainly related to gene expression variations. However, NPs can also interact with cells involved in adaptive immune response, and in vitro data showed that TiO2 NPs can induce maturation of dendritic cells through the activation of Nf-kB pathway[86], a process which is essential for antigen presentation to T helper cells. Again, this process could be important in CeD, since antigen presentation by dendritic cells represents an essential step for the activation of the autoimmune response.
Microbiota plays an important role in maintaining the homeostasis of a healthy gut. Alterations in microbiota composition have been reported both in pediatric as well as adult CeD patients if compared to controls[87-89], although it is currently still unknown whether these changes are causative of the disease or a consequence of mucosal alterations.
However, microbiota can be altered by exposure to dietary mNP. In vitro experiments performed on a colon-like microbial community showed that exposure to small quantities of E171 (comparable to the amount present in two pieces of chewing gum) was sufficient to alter the phylogenetic composition[90]. Significant changes in the phyla were also observed in mice treated for 28 d with TiO2NP, with variations induced by both forms of TiO2NP, namely rutilium and anatase[91].
As already mentioned, silver nanoparticles are employed as antibacterial agents, and thus it should be expected that they can alter the microbiota composition. In fact, in mice exposed to increasing doses of AgNP for 28 d, a disturbed bacterial evenness (αdiversity) and populations (β-diversity) was detected by Next Generation Sequencing. This effect was also dose-dependent. Ag NP increased the ratio between Firmicutes (F) and Bacteroidetes (B) phyla, results similar to those observed in presence of inflammation[92]. Variation in microbiota composition were also observed by another group, although results were different regarding the phyla, possibly due to the different experimental design (rats treated for 14 d)[93].
Last but not least, it must be emphasized that within our gut there is also a viral component that interacts with the microbiota and the intestinal mucosa. Although studies evaluating the possible effect of food NP on this component are scanty, initial data obtained in vitro suggest that Ag-NP can alter the abundance of several viral species[94]. Since these species can be hosted by different categories of bacteria (commensal or pathogenic), changes in intestinal viriome can, in turn, cause alteration in the microbiota itself.
Food additives could play an important role in the pathogenesis of CeD, either altering gliadin peptides properties or interacting with the intestinal environment, at the barrier level or with the immune system. Moreover, the increasing use of prepared food and, in turn, the augmented ingestion of NPs, could be an additional factor in triggering the development of CeD in genetically predisposed individuals. For this reason, in vitro and in vivo studies to evaluate these possible interactions are needed.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sergi C S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ
| 1. | Romanos J, Rosén A, Kumar V, Trynka G, Franke L, Szperl A, Gutierrez-Achury J, van Diemen CC, Kanninga R, Jankipersadsing SA, Steck A, Eisenbarth G, van Heel DA, Cukrowska B, Bruno V, Mazzilli MC, Núñez C, Bilbao JR, Mearin ML, Barisani D, Rewers M, Norris JM, Ivarsson A, Boezen HM, Liu E, Wijmenga C; PreventCD Group. Improving coeliac disease risk prediction by testing non-HLA variants additional to HLA variants. Gut. 2014;63:415-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | West J, Fleming KM, Tata LJ, Card TR, Crooks CJ. Incidence and prevalence of celiac disease and dermatitis herpetiformis in the UK over two decades: population-based study. Am J Gastroenterol. 2014;109:757-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 182] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 3. | White LE, Merrick VM, Bannerman E, Russell RK, Basude D, Henderson P, Wilson DC, Gillett PM. The rising incidence of celiac disease in Scotland. Pediatrics. 2013;132:e924-e931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Lohi S, Mustalahti K, Kaukinen K, Laurila K, Collin P, Rissanen H, Lohi O, Bravi E, Gasparin M, Reunanen A, Mäki M. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007;26:1217-1225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 516] [Cited by in F6Publishing: 497] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 5. | Riddle MS, Murray JA, Porter CK. The incidence and risk of celiac disease in a healthy US adult population. Am J Gastroenterol. 2012;107:1248-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Almallouhi E, King KS, Patel B, Wi C, Juhn YJ, Murray JA, Absah I. Increasing Incidence and Altered Presentation in a Population-based Study of Pediatric Celiac Disease in North America. J Pediatr Gastroenterol Nutr. 2017;65:432-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Shan L, Molberg Ø, Parrot I, Hausch F, Filiz F, Gray GM, Sollid LM, Khosla C. Structural basis for gluten intolerance in celiac sprue. Science. 2002;297:2275-2279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1153] [Cited by in F6Publishing: 1042] [Article Influence: 47.4] [Reference Citation Analysis (1)] |
| 8. | Picarelli A, Di Tola M, Sabbatella L, Anania MC, Di Cello T, Greco R, Silano M, De Vincenzi M. 31-43 amino acid sequence of the alpha-gliadin induces anti-endomysial antibody production during in vitro challenge. Scand J Gastroenterol. 1999;34:1099-1102. [PubMed] [Cited in This Article: ] |
| 9. | Food and Drug Administration. Transglutaminase GRAS Notification, Washington, DC, 2001. Available from: https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm154631.htm. [Cited in This Article: ] |
| 10. | Romeih E, Walker G. Recent advances on microbial transglutaminase and dairy application. Trends in Food Science & Technology. 2017;62:133e140. [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Gharibzahedi SMT, Yousefi S, Chronakis IS. Microbial transglutaminase in noodle and pasta processing. Crit Rev Food Sci Nutr. 2017;1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Mazzeo MF, Bonavita R, Maurano F, Bergamo P, Siciliano RA, Rossi M. Biochemical modifications of gliadins induced by microbial transglutaminase on wheat flour. Biochim Biophys Acta. 2013;1830:5166-5174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Gianfrani C, Siciliano RA, Facchiano AM, Camarca A, Mazzeo MF, Costantini S, Salvati VM, Maurano F, Mazzarella G, Iaquinto G, Bergamo P, Rossi M. Transamidation of wheat flour inhibits the response to gliadin of intestinal T cells in celiac disease. Gastroenterology. 2007;133:780-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Dekking EHA, Van Veelen PA, de Ru A, Kooy-Winkelaar EMC, Gröneveld T, Nieuwenhuizen WF, Koning F. Microbial transglutaminases generate T cell stimulatory epitopes involved in celiac disease. J Cereal Sci. 2008;47:339-346. [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Cabrera-Chávez F, Rouzaud-Sández O, Sotelo-Cruz N, Calderón de la Barca AM. Transglutaminase treatment of wheat and maize prolamins of bread increases the serum IgA reactivity of celiac disease patients. J Agric Food Chem. 2008;56:1387-1391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Matthias T, Jeremias P, Neidhöfer S, Lerner A. The industrial food additive, microbial transglutaminase, mimics tissue transglutaminase and is immunogenic in celiac disease patients. Autoimmun Rev. 2016;15:1111-1119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Ruh T, Ohsam J, Pasternack R, Yokoyama K, Kumazawa Y, Hils M. Microbial transglutaminase treatment in pasta-production does not affect the immunoreactivity of gliadin with celiac disease patients' sera. J Agric Food Chem. 2014;62:7604-7611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Heil A, Ohsam J, van Genugten B, Diez O, Yokoyama K, Kumazawa Y, Pasternack R, Hils M. Microbial Transglutaminase Used in Bread Preparation at Standard Bakery Concentrations Does Not Increase Immunodetectable Amounts of Deamidated Gliadin. J Agric Food Chem. 2017;65:6982-6990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Zhou L, Kooy-Winkelaar YMC, Cordfunke RA, Dragan I, Thompson A, Drijfhout JW, van Veelen PA, Chen H, Koning F. Abrogation of Immunogenic Properties of Gliadin Peptides through Transamidation by Microbial Transglutaminase Is Acyl-Acceptor Dependent. J Agric Food Chem. 2017;65:7542-7552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Lombardi E, Bergamo P, Maurano F, Bozzella G, Luongo D, Mazzarella G, Rotondi Aufiero V, Iaquinto G, Rossi M. Selective inhibition of the gliadin-specific, cell-mediated immune response by transamidation with microbial transglutaminase. J Leukoc Biol. 2013;93:479-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Elli L, Roncoroni L, Hils M, Pasternack R, Barisani D, Terrani C, Vaira V, Ferrero S, Bardella MT. Immunological effects of transglutaminase-treated gluten in coeliac disease. Hum Immunol. 2012;73:992-997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Mazzarella G, Salvati VM, Iaquinto G, Stefanile R, Capobianco F, Luongo D, Bergamo P, Maurano F, Giardullo N, Malamisura B, Rossi M. Reintroduction of gluten following flour transamidation in adult celiac patients: a randomized, controlled clinical study. Clin Dev Immunol. 2012;2012:329150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Heredia-Sandoval NG, Islas-Rubio AR, Cabrera-Chávez F, Calderón de la Barca AM. Transamidation of gluten proteins during the bread-making process of wheat flour to produce breads with less immunoreactive gluten. Food Funct. 2014;5:1813-1818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Ribeiro M, Nunes FM, Guedes S, Domingues P, Silva AM, Carrillo JM, Rodriguez-Quijano M, Branlard G, Igrejas G. Efficient chemo-enzymatic gluten detoxification: reducing toxic epitopes for celiac patients improving functional properties. Sci Rep. 2015;5:18041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Gulfam M, Kim JE, Lee JM, Ku B, Chung BH, Chung BG. Anticancer drug-loaded gliadin nanoparticles induce apoptosis in breast cancer cells. Langmuir. 2012;28:8216-8223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | El-Wakil NA, Hassan EA, Abou-Zeid RE, Dufresne A. Development of wheat gluten/nanocellulose/titanium dioxide nanocomposites for active food packaging. Carbohydr Polym. 2015;124:337-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 27. | Groh KJ, Geueke B, Muncke J. Food contact materials and gut health: Implications for toxicity assessment and relevance of high molecular weight migrants. Food Chem Toxicol. 2017;109:1-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Yang FM, Li HM, Li F, Xin ZH, Zhao LY, Zheng YH, Hu QH. Effect of nano-packing on preservation quality of fresh strawberry (Fragaria ananassa Duch. cv Fengxiang) during storage at 4 degrees C. J Food Sci. 2010;75:C236-C240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 29. | Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3231] [Cited by in F6Publishing: 2415] [Article Influence: 142.1] [Reference Citation Analysis (0)] |
| 30. | Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella MF, Rejeski D, Hull MS. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol. 2015;6:1769-1780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1332] [Cited by in F6Publishing: 944] [Article Influence: 104.9] [Reference Citation Analysis (0)] |
| 31. | Arora S, Sharma P, Kumar S, Nayan R, Khanna PK, Zaidi MGH. Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Regul. 2012;66:303-310. [DOI] [Cited in This Article: ] |
| 32. | Kumar V, Guleria P, Kumar V, Yadav SK. Gold nanoparticle exposure induces growth and yield enhancement in Arabidopsis thaliana. Sci Total Environ. 2013;461-462:462-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 33. | Yao M, He L, McClements DJ, Xiao H. Uptake of Gold Nanoparticles by Intestinal Epithelial Cells: Impact of Particle Size on Their Absorption, Accumulation, and Toxicity. J Agric Food Chem. 2015;63:8044-8049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Hanley C, Thurber A, Hanna C, Punnoose A, Zhang J, Wingett DG. The Influences of Cell Type and ZnO Nanoparticle Size on Immune Cell Cytotoxicity and Cytokine Induction. Nanoscale Res Lett. 2009;4:1409-1420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 229] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 35. | Imai S, Morishita Y, Hata T, Kondoh M, Yagi K, Gao JQ, Nagano K, Higashisaka K, Yoshioka Y, Tsutsumi Y. Cellular internalization, transcellular transport, and cellular effects of silver nanoparticles in polarized Caco-2 cells following apical or basolateral exposure. Biochem Biophys Res Commun. 2017;484:543-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Karlsson HL, Cronholm P, Hedberg Y, Tornberg M, De Battice L, Svedhem S, Wallinder IO. Cell membrane damage and protein interaction induced by copper containing nanoparticles--importance of the metal release process. Toxicology. 2013;313:59-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 37. | McShan D, Ray PC, Yu H. Molecular toxicity mechanism of nanosilver. J Food Drug Anal. 2014;22:116-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 486] [Cited by in F6Publishing: 410] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 38. | Lichtenstein D, Ebmeyer J, Meyer T, Behr AC, Kästner C, Böhmert L, Juling S, Niemann B, Fahrenson C, Selve S, Thünemann AF, Meijer J, Estrela-Lopis I, Braeuning A, Lampen A. It takes more than a coating to get nanoparticles through the intestinal barrier in vitro. Eur J Pharm Biopharm. 2017;118:21-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Yang D, Liu D, Qin M, Chen B, Song S, Dai W, Zhang H, Wang X, Wang Y, He B, Tang X, Zhang Q. Intestinal Mucin Induces More Endocytosis but Less Transcytosis of Nanoparticles across Enterocytes by Triggering Nanoclustering and Strengthening the Retrograde Pathway. ACS Appl Mater Interfaces. 2018;10:11443-11456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Sokolov SV, Tschulik K, Batchelor-McAuley C, Jurkschat K, Compton RG. Reversible or not? Distinguishing agglomeration and aggregation at the nanoscale. Anal Chem. 2015;87:10033-10039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 41. | Peters R, Kramer E, Oomen AG, Rivera ZE, Oegema G, Tromp PC, Fokkink R, Rietveld A, Marvin HJ, Weigel S, Peijnenburg AA, Bouwmeester H. Presence of nano-sized silica during in vitro digestion of foods containing silica as a food additive. ACS Nano. 2012;6:2441-2451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 207] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 42. | Walczak AP, Fokkink R, Peters R, Tromp P, Herrera Rivera ZE, Rietjens IM, Hendriksen PJ, Bouwmeester H. Behaviour of silver nanoparticles and silver ions in an in vitro human gastrointestinal digestion model. Nanotoxicology. 2013;7:1198-1210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 43. | Sieg H, Kästner C, Krause B, Meyer T, Burel A, Böhmert L, Lichtenstein D, Jungnickel H, Tentschert J, Laux P, Braeuning A, Estrela-Lopis I, Gauffre F, Fessard V, Meijer J, Luch A, Thünemann AF, Lampen A. Impact of an Artificial Digestion Procedure on Aluminum-Containing Nanomaterials. Langmuir. 2017;33:10726-10735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 44. | McCracken C, Zane A, Knight DA, Dutta PK, Waldman WJ. Minimal intestinal epithelial cell toxicity in response to short- and long-term food-relevant inorganic nanoparticle exposure. Chem Res Toxicol. 2013;26:1514-1525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 45. | Albanese A, Chan WC. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano. 2011;5:5478-5489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 641] [Cited by in F6Publishing: 554] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 46. | Bellmann S, Carlander D, Fasano A, Momcilovic D, Scimeca JA, Waldman WJ, Gombau L, Tsytsikova L, Canady R, Pereira DI, Lefebvre DE. Mammalian gastrointestinal tract parameters modulating the integrity, surface properties, and absorption of food-relevant nanomaterials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:609-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 47. | Walczak AP, Kramer E, Hendriksen PJ, Helsdingen R, van der Zande M, Rietjens IM, Bouwmeester H. In vitro gastrointestinal digestion increases the translocation of polystyrene nanoparticles in an in vitro intestinal co-culture model. Nanotoxicology. 2015;9:886-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 48. | Wang Y, Yuan L, Yao C, Ding L, Li C, Fang J, Sui K, Liu Y, Wu M. A combined toxicity study of zinc oxide nanoparticles and vitamin C in food additives. Nanoscale. 2014;6:15333-15342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 49. | Cao Y, Roursgaard M, Kermanizadeh A, Loft S, Møller P. Synergistic effects of zinc oxide nanoparticles and Fatty acids on toxicity to caco-2 cells. Int J Toxicol. 2015;34:67-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 50. | Martirosyan A, Bazes A, Schneider YJ. In vitro toxicity assessment of silver nanoparticles in the presence of phenolic compounds--preventive agents against the harmful effect? Nanotoxicology. 2014;8:573-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 51. | Martirosyan A, Grintzalis K, Polet M, Laloux L, Schneider YJ. Tuning the inflammatory response to silver nanoparticles via quercetin in Caco-2 (co-)cultures as model of the human intestinal mucosa. Toxicol Lett. 2016;253:36-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 52. | Ministry of Agriculture Fish and Food. Dietary intake of food additives in the UK: initial surveillance (food surveillance paper 37). London, UK: MAFF; 1993; . [Cited in This Article: ] |
| 53. | Fröhlich E, Roblegg E. Models for oral uptake of nanoparticles in consumer products. Toxicology. 2012;291:10-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 54. | Powell JJ, Faria N, Thomas-McKay E, Pele LC. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J Autoimmun. 2010;34:J226-J233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 290] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 55. | Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol. 2012;46:2242-2250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1542] [Cited by in F6Publishing: 1183] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 56. | EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food). Re-evaluation of titanium dioxide (E 171) as a food additive. EFSA Journal. 2016;14:e04545. [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 57. | SCCS (Scientific Committee on Consumer Safety). Opinion on titanium dioxide (nano form), 22 July 2013, revision of 22 April 2014. Available from: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_136.pdf. [Cited in This Article: ] |
| 58. | Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2121] [Cited by in F6Publishing: 2385] [Article Influence: 159.0] [Reference Citation Analysis (0)] |
| 59. | Schumann M, Siegmund B, Schulzke JD, Fromm M. Celiac Disease: Role of the Epithelial Barrier. Cell Mol Gastroenterol Hepatol. 2017;3:150-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 60. | Wapenaar MC, Monsuur AJ, van Bodegraven AA, Weersma RK, Bevova MR, Linskens RK, Howdle P, Holmes G, Mulder CJ, Dijkstra G, van Heel DA, Wijmenga C. Associations with tight junction genes PARD3 and MAGI2 in Dutch patients point to a common barrier defect for coeliac disease and ulcerative colitis. Gut. 2008;57:463-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 61. | Lerner A, Matthias T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun Rev. 2015;14:479-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 277] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 62. | Brun E, Barreau F, Veronesi G, Fayard B, Sorieul S, Chanéac C, Carapito C, Rabilloud T, Mabondzo A, Herlin-Boime N, Carrière M. Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia. Part Fibre Toxicol. 2014;11:13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 189] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 63. | Williams KM, Gokulan K, Cerniglia CE, Khare S. Size and dose dependent effects of silver nanoparticle exposure on intestinal permeability in an in vitro model of the human gut epithelium. J Nanobiotechnology. 2016;14:62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 64. | Koeneman BA, Zhang Y, Westerhoff P, Chen Y, Crittenden JC, Capco DG. Toxicity and cellular responses of intestinal cells exposed to titanium dioxide. Cell Biol Toxicol. 2010;26:225-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 65. | Sheth P, Basuroy S, Li C, Naren AP, Rao RK. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem. 2003;278:49239-49245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 66. | Ruiz PA, Morón B, Becker HM, Lang S, Atrott K, Spalinger MR, Scharl M, Wojtal KA, Fischbeck-Terhalle A, Frey-Wagner I, Hausmann M, Kraemer T, Rogler G. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: role of the NLRP3 inflammasome. Gut. 2017;66:1216-1224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 67. | Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:71-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 68. | Howe SE, Lickteig DJ, Plunkett KN, Ryerse JS, Konjufca V. The uptake of soluble and particulate antigens by epithelial cells in the mouse small intestine. PLoS One. 2014;9:e86656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 69. | Lichtenstein D, Ebmeyer J, Knappe P, Juling S, Böhmert L, Selve S, Niemann B, Braeuning A, Thünemann AF, Lampen A. Impact of food components during in vitro digestion of silver nanoparticles on cellular uptake and cytotoxicity in intestinal cells. Biol Chem. 2015;396:1255-1264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 70. | Bajka BH, Rigby NM, Cross KL, Macierzanka A, Mackie AR. The influence of small intestinal mucus structure on particle transport ex vivo. Colloids Surf B Biointerfaces. 2015;135:73-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 71. | Lomer MC, Thompson RP, Powell JJ. Fine and ultrafine particles of the diet: influence on the mucosal immune response and association with Crohn's disease. Proc Nutr Soc. 2002;61:123-130. [PubMed] [Cited in This Article: ] |
| 72. | Powell JJ, Harvey RS, Ashwood P, Wolstencroft R, Gershwin ME, Thompson RP. Immune potentiation of ultrafine dietary particles in normal subjects and patients with inflammatory bowel disease. J Autoimmun. 2000;14:99-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 73. | Evans SM, Ashwood P, Warley A, Berisha F, Thompson RP, Powell JJ. The role of dietary microparticles and calcium in apoptosis and interleukin-1beta release of intestinal macrophages. Gastroenterology. 2002;123:1543-1553. [PubMed] [Cited in This Article: ] |
| 74. | Cui Y, Liu H, Zhou M, Duan Y, Li N, Gong X, Hu R, Hong M, Hong F. Signaling pathway of inflammatory responses in the mouse liver caused by TiO2 nanoparticles. J Biomed Mater Res A. 2011;96:221-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 75. | Khan HA, Abdelhalim MA, Alhomida AS, Al Ayed MS. Transient increase in IL-1β, IL-6 and TNF-α gene expression in rat liver exposed to gold nanoparticles. Genet Mol Res. 2013;12:5851-5857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 76. | Chang H, Ho CC, Yang CS, Chang WH, Tsai MH, Tsai HT, Lin P. Involvement of MyD88 in zinc oxide nanoparticle-induced lung inflammation. Exp Toxicol Pathol. 2013;65:887-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 77. | Dhupal M, Oh JM, Tripathy DR, Kim SK, Koh SB, Park KS. Immunotoxicity of titanium dioxide nanoparticles via simultaneous induction of apoptosis and multiple toll-like receptors signaling through ROS-dependent SAPK/JNK and p38 MAPK activation. Int J Nanomedicine. 2018;13:6735-6750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 78. | Park J, Lim DH, Lim HJ, Kwon T, Choi JS, Jeong S, Choi IH, Cheon J. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem Commun (Camb). 2011;47:4382-4384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 79. | Kim S, Choi IH. Phagocytosis and endocytosis of silver nanoparticles induce interleukin-8 production in human macrophages. Yonsei Med J. 2012;53:654-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Triboulet S, Aude-Garcia C, Armand L, Collin-Faure V, Chevallet M, Diemer H, Gerdil A, Proamer F, Strub JM, Habert A, Herlin N, Van Dorsselaer A, Carrière M, Rabilloud T. Comparative proteomic analysis of the molecular responses of mouse macrophages to titanium dioxide and copper oxide nanoparticles unravels some toxic mechanisms for copper oxide nanoparticles in macrophages. PLoS One. 2015;10:e0124496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 81. | Bancos S, Stevens DL, Tyner KM. Effect of silica and gold nanoparticles on macrophage proliferation, activation markers, cytokine production, and phagocytosis in vitro. Int J Nanomedicine. 2014;10:183-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 82. | Chen Q, Wang N, Zhu M, Lu J, Zhong H, Xue X, Guo S, Li M, Wei X, Tao Y, Yin H. TiO<sub>2</sub> nanoparticles cause mitochondrial dysfunction, activate inflammatory responses, and attenuate phagocytosis in macrophages: A proteomic and metabolomic insight. Redox Biol. 2018;15:266-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 83. | Poon WL, Alenius H, Ndika J, Fortino V, Kolhinen V, Meščeriakovas A, Wang M, Greco D, Lähde A, Jokiniemi J, Lee JC, El-Nezami H, Karisola P. Nano-sized zinc oxide and silver, but not titanium dioxide, induce innate and adaptive immunity and antiviral response in differentiated THP-1 cells. Nanotoxicology. 2017;11:936-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 84. | Yang EJ, Kim S, Kim JS, Choi IH. Inflammasome formation and IL-1β release by human blood monocytes in response to silver nanoparticles. Biomaterials. 2012;33:6858-6867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 85. | Lozano-Fernández T, Ballester-Antxordoki L, Pérez-Temprano N, Rojas E, Sanz D, Iglesias-Gaspar M, Moya S, González-Fernández Á, Rey M. Potential impact of metal oxide nanoparticles on the immune system: The role of integrins, L-selectin and the chemokine receptor CXCR4. Nanomedicine. 2014;10:1301-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 86. | Zhu R, Zhu Y, Zhang M, Xiao Y, Du X, Liu H, Wang S. The induction of maturation on dendritic cells by TiO2 and Fe(3)O(4)@TiO(2) nanoparticles via NF-κB signaling pathway. Mater Sci Eng C Mater Biol Appl. 2014;39:305-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 87. | Olivares M, Neef A, Castillejo G, Palma GD, Varea V, Capilla A, Palau F, Nova E, Marcos A, Polanco I, Ribes-Koninckx C, Ortigosa L, Izquierdo L, Sanz Y. The HLA-DQ2 genotype selects for early intestinal microbiota composition in infants at high risk of developing coeliac disease. Gut. 2015;64:406-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 198] [Article Influence: 22.0] [Reference Citation Analysis (1)] |
| 88. | Caminero A, Galipeau HJ, McCarville JL, Johnston CW, Bernier SP, Russell AK, Jury J, Herran AR, Casqueiro J, Tye-Din JA, Surette MG, Magarvey NA, Schuppan D, Verdu EF. Duodenal Bacteria From Patients With Celiac Disease and Healthy Subjects Distinctly Affect Gluten Breakdown and Immunogenicity. Gastroenterology. 2016;151:670-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 89. | D'Argenio V, Casaburi G, Precone V, Pagliuca C, Colicchio R, Sarnataro D, Discepolo V, Kim SM, Russo I, Del Vecchio Blanco G, Horner DS, Chiara M, Pesole G, Salvatore P, Monteleone G, Ciacci C, Caporaso GJ, Jabrì B, Salvatore F, Sacchetti L. Metagenomics Reveals Dysbiosis and a Potentially Pathogenic N. flavescens Strain in Duodenum of Adult Celiac Patients. Am J Gastroenterol. 2016;111:879-890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 90. | Dudefoi W, Moniz K, Allen-Vercoe E, Ropers MH, Walker VK. Impact of food grade and nano-TiO<sub>2</sub> particles on a human intestinal community. Food Chem Toxicol. 2017;106:242-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 91. | Li J, Yang S, Lei R, Gu W, Qin Y, Ma S, Chen K, Chang Y, Bai X, Xia S, Wu C, Xing G. Oral administration of rutile and anatase TiO<sub>2</sub> nanoparticles shifts mouse gut microbiota structure. Nanoscale. 2018;10:7736-7745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 92. | van den Brule S, Ambroise J, Lecloux H, Levard C, Soulas R, De Temmerman PJ, Palmai-Pallag M, Marbaix E, Lison D. Dietary silver nanoparticles can disturb the gut microbiota in mice. Part Fibre Toxicol. 2016;13:38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 93. | Javurek AB, Suresh D, Spollen WG, Hart ML, Hansen SA, Ellersieck MR, Bivens NJ, Givan SA, Upendran A, Kannan R, Rosenfeld CS. Gut Dysbiosis and Neurobehavioral Alterations in Rats Exposed to Silver Nanoparticles. Sci Rep. 2017;7:2822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 94. | Gokulan K, Bekele AZ, Drake KL, Khare S. Responses of intestinal virome to silver nanoparticles: safety assessment by classical virology, whole-genome sequencing and bioinformatics approaches. Int J Nanomedicine. 2018;13:2857-2867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |