Published online Mar 26, 2019. doi: 10.12998/wjcc.v7.i6.765
Peer-review started: November 23, 2018
First decision: January 12, 2019
Revised: January 27, 2019
Accepted: February 26, 2019
Article in press: February 26, 2019
Published online: March 26, 2019
Sinonasal undifferentiated carcinoma (SNUC) is a rare aggressive tumor that is often unresectable. Optimal treatment for patients with unresectable, locally advanced SNUC (LA-SNUC) has not been established, and the patient outcome remains poor. We report two cases of unresectable LA-SNUC in which induction chemotherapy with docetaxel, cisplatin and fluorouracil (TPF) followed by radiotherapy with concurrent cisplatin (CCRT), a standard treatment option for locally advanced head and neck cancer, demonstrated promising outcomes.
A 39-year-old man presented with tearing and pain in the right eye. A biopsy of the tumor invading the sinonasal cavities, right orbit and cranial base confirmed the diagnosis of LA-SNUC. Induction TPF chemotherapy induced remarkable tumor shrinkage and rapidly improved the symptoms. He subsequently received CCRT and achieved complete remission of the disease. The other case is a 21-year-old man who presented with worsening vision. The unresectable tumor involving the nasal septum and cranial base was pathologically diagnosed as SNUC. TPF chemotherapy followed by CCRT yielded complete remission of the disease with preserved visual function. Both patients have been disease-free for 44 mo.
Induction TPF chemotherapy followed by CCRT may remarkably improve the outcomes in LA-SNUC patients.
Core tip: Sinonasal undifferentiated carcinoma easily invades adjacent organs and is often diagnosed in unresectable disease. Though multimodality treatment has been developed, there is no standard of care for locally advanced Sinonasal undifferentiated carcinoma (LA-SNUC) and the patient outcome remains dismal. We report two cases of unresectable LA-SNUC in which induction chemotherapy with docetaxel, cisplatin and fluorouracil (TPF) followed by cisplatin-based concurrent chemoradiotherapy (CCRT) rapidly improved symptoms and yielded durable complete remission. Together with review of literature, our cases show that TPF chemotherapy followed by CCRT, a standard treatment for locally advanced head and neck cancer, should be more encouraged for unresectable LA-SNUC.
- Citation: Watanabe S, Honma Y, Murakami N, Igaki H, Mori T, Hirano H, Okita N, Shoji H, Iwasa S, Takashima A, Kato K, Kobayashi K, Matsumoto F, Yoshimoto S, Itami J, Boku N. Induction chemotherapy with docetaxel, cisplatin and fluorouracil followed by concurrent chemoradiotherapy for unresectable sinonasal undifferentiated carcinoma: Two cases of report. World J Clin Cases 2019; 7(6): 765-772
- URL: https://www.wjgnet.com/2307-8960/full/v7/i6/765.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i6.765
Sinonasal undifferentiated carcinoma (SNUC) is an uncommon neoplasm of uncertain pathogenesis that originates in the nasal cavity or paranasal sinuses. SNUC is diagnosed as a locally advanced disease at the T4 stage in 71%-100% of patients[1,2] and with neck nodal involvement in 12%-24%[1,3]. Hematogenous metastasis is rarely found on presentation. Though the use of surgery provided the largest survival benefit, a poor disease-free survival rate of 26.3% was reported with a median follow-up of 15 mo[4]. A substantial number of SNUC patients do not have surgery because of the wide extension of the tumor[5] and receive only chemotherapy and/or radiotherapy, which provided limited efficacy with a two-year survival rate of 25%-40%[4]. Therefore, there has long been a need for intensive multimodality therapy, but no consensus has been reached on the optimal treatment regimen or sequence.
We describe here two cases of locally advanced SNUC (LA-SNUC) in which induction chemotherapy with docetaxel, cisplatin and fluorouracil (TPF) and subsequent intensity-modulated radiotherapy (IMRT) with concurrent high-dose cisplatin led to a durable remission of the disease with manageable toxicities.
In case 1, a 39-year-old man presented with tearing and pain in the right eye. The chief complaint of case 2 was visual disturbance.
The patient in case 1 reported that the symptoms had been worsening for the last two months. In case 2, the patient had felt worsening visual disturbance for one week.
Both patients had no remarkable history of illness. They were current smokers.
No remarkable personal or family history of illness was found in either case.
In case 1, on physical examination, edema was found in the right eyelid. In case 2, a visual acuity test showed totally impaired vision in the right eye.
In both cases, no remarkable findings were discovered in the laboratory tests.
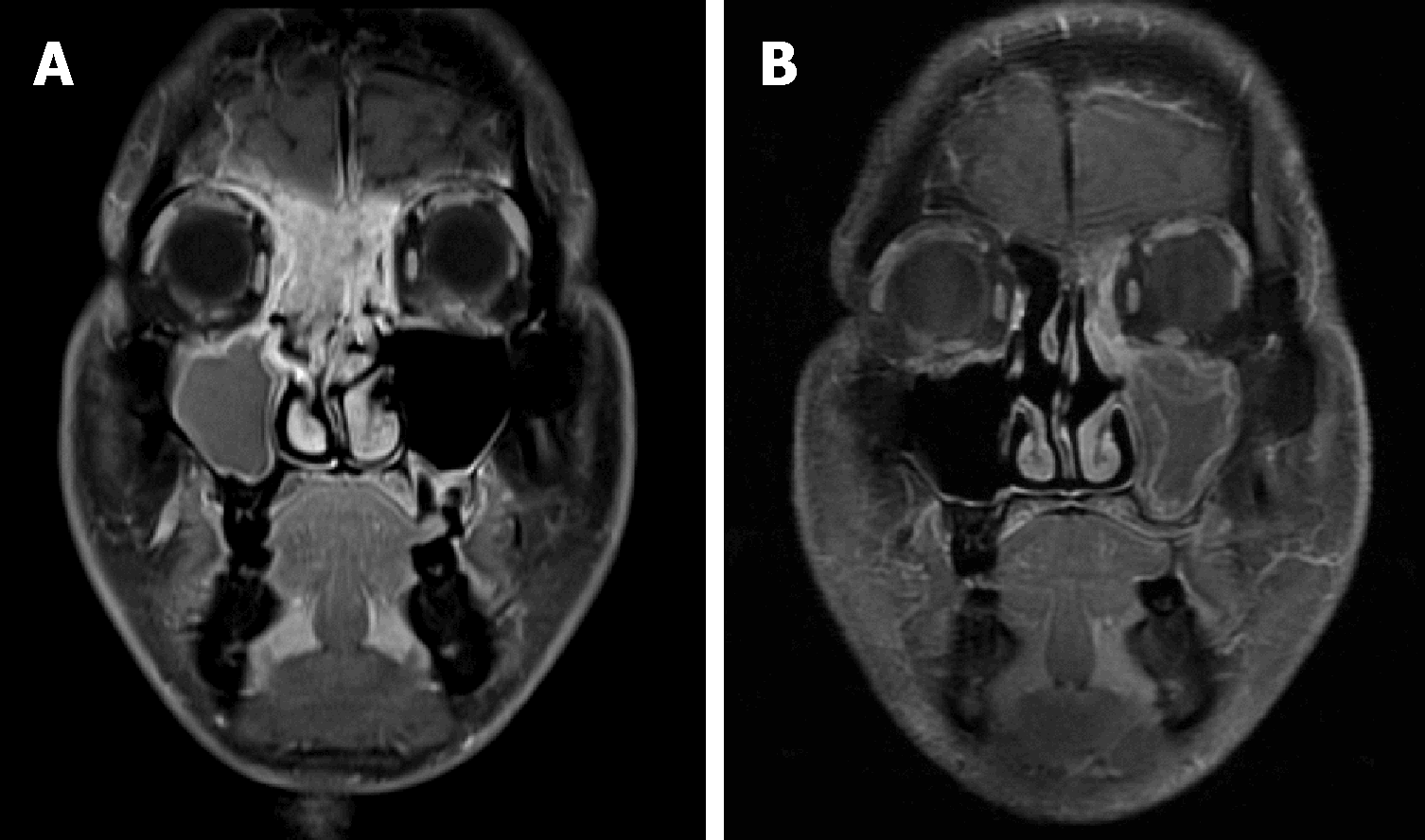
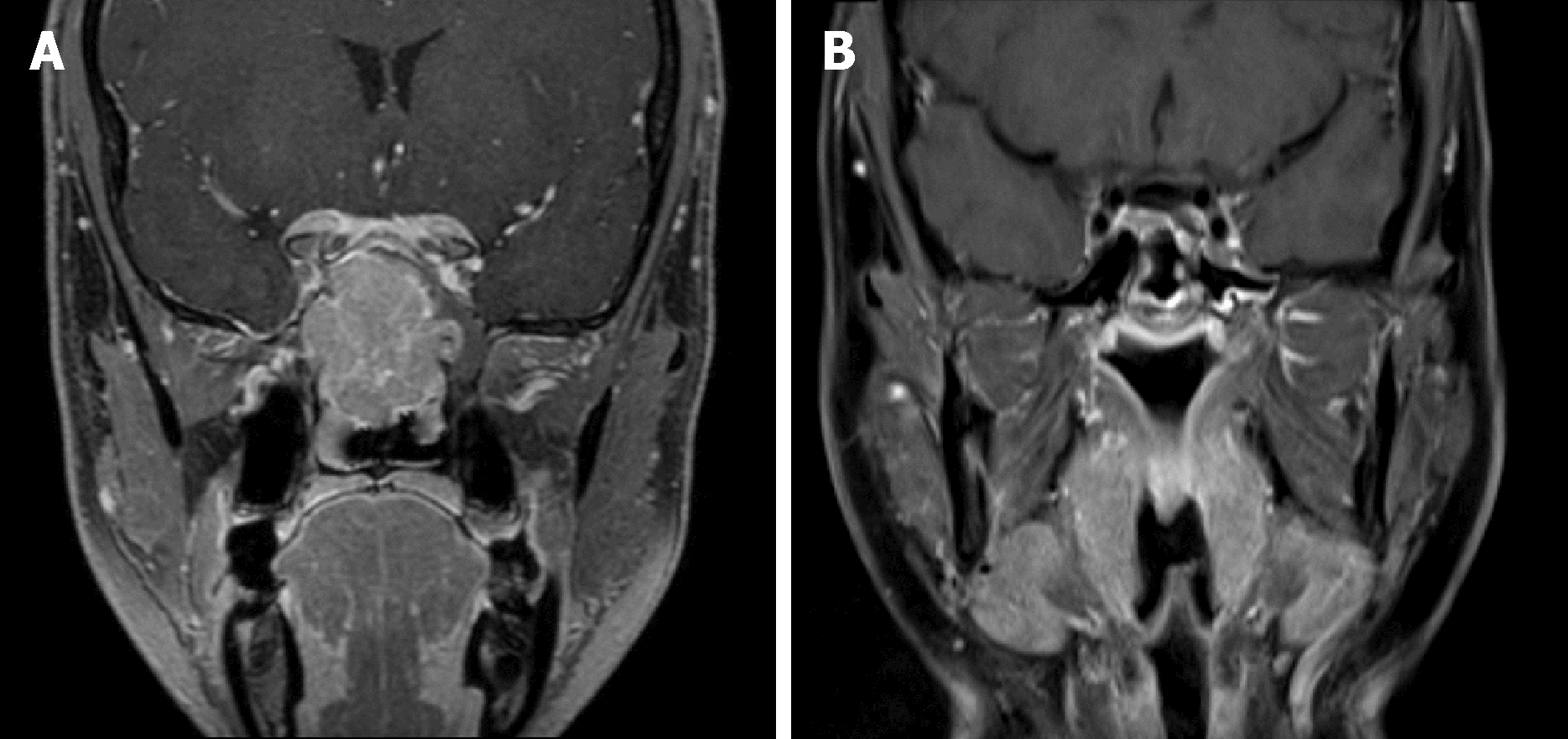
In case 1, a contrast-enhanced magnetic resonance imaging (MRI) of the head showed a tumor involving the paranasal sinuses, nasal cavities, right orbit and cranial base (Figure 1A). Cervical lymph nodes were not affected. In case 2, a contrast-enhanced MRI of the head revealed an intranasal tumor invading the nasal septum, orbital apex and cranial base (Figure 2A).
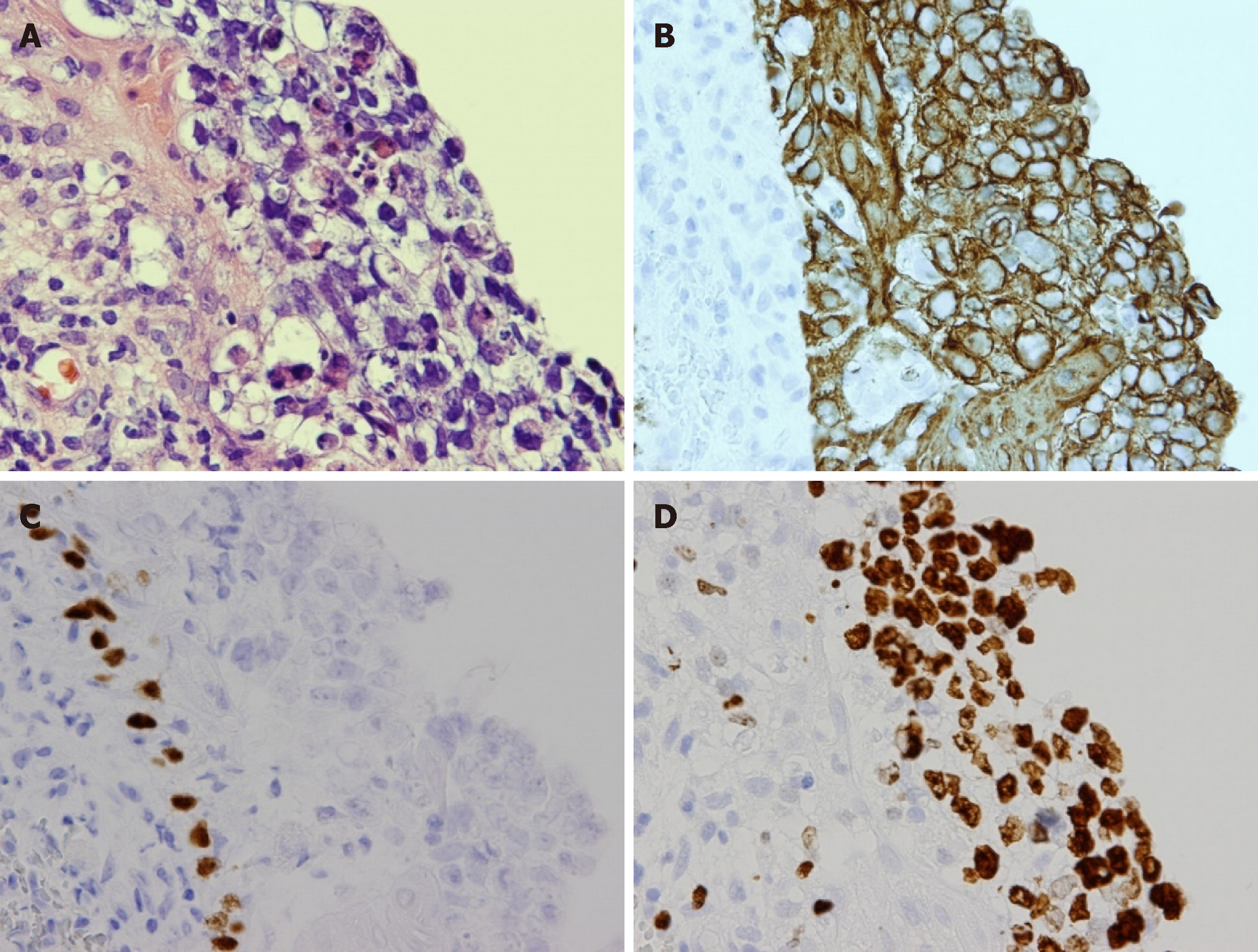
In case 1, a transnasal biopsy revealed pleomorphic tumor cells in the nasal epithelium. Immunohistochemically, the tumor cells were positive for AE1/3. Staining of the tumor cells for p40, thyroid transcription factor-1, neuron-specific enolase, S100, CD56, and nuclear protein in testis were negative (Figure 3). The tumor was diagnosed as SNUC. In case 2, a diagnosis of SNUC was also made based on the pathological features and immunophenotype of transnasal biopsy samples. The imaging features confirmed the locally advanced stages of the tumors in both cases (cT4bN0M0, stage IV, Union for International Cancer Control, the 7th edition of TNM classification[6]).
In case 1, the tumor was considered unresectable due to its wide invasion to the dura mater. Three cycles of TPF chemotherapy (docetaxel, 70 mg/m2, day 1; cisplatin, 70 mg/m2, day 1; fluorouracil, 750 mg/m2, day 1-5) was initiated with three-week intervals. On the first cycle of chemotherapy, a significant tumor reduction was obtained with marked improvement of the symptoms. His treatment was complicated with mild dysgeusia and anorexia. After achieving a partial response (Response Evaluation Criteria in Solid Tumours, version. 1.1), he was treated with definitive IMRT of 70 Gy. The intensity-modulating technique helped minimize radiation exposure of the optic nerves. Three cycles of cisplatin was concurrently administered every three weeks with a dose reduced to 80% (80 mg/m2) due to the prolonged anorexia caused by the prior TPF chemotherapy. In case 2, because of the invasion to the sphenoid sinus, surgical resection was not appropriate for the patient. He received three cycles of induction TPF chemotherapy, which led to a partial response with a substantial improvement of vision. The adverse events included mild nausea and transient hearing impairment. He was subsequently treated with a combination of IMRT of 70 Gy and three cycles of cisplatin (100 mg/m2, day 1). The third dose of cisplatin was reduced to 80% because of severe nausea.
A complete response was achieved (Figure 1B) in case 1 and the patient has been alive and well without disease for 44 mo. In case 2, a complete remission of the disease was confirmed (Figure 2B), and he remains recurrence-free with recovered vision for 44 mo.
SNUC is a high-grade uncommon tumor possibly arising from the schneiderian epithelium, which lines the nasal cavity and paranasal sinuses. The pathological diagnosis of SNUC is difficult because of the rarity of the disease and the varying morphology of the tumor cells. Immunohistochemical profiling of the tumor cells is an essential part of the diagnosis, but no consistent immunoreactive markers, except pankeratins, have been reported. Lack of p40 expression has been reported to be a robust marker[7], which was consistent with the findings in our cases.
Patients often present with non-specific symptoms, including nasal obstruction, epistaxis, facial swelling or pain, headache and cranial nerve deficits, which also make diagnosis challenging. SNUC rapidly invades the neighboring structures, which leads to the high prevalence of advanced disease at diagnosis and the poor outcome[5].
Since improved disease-free survival was shown in patients receiving a combination of chemotherapy, radiotherapy and surgery[8], a multimodal approach was adopted. The frequent presentation of patients with unresectable disease, the poor outcome with initial surgery, and the chemo-sensitive nature of SNUC cells motivated the strategy of induction therapy. A favorable two-year survival rate of 64% was reported in Kadish B or C[9] SNUC patients who had good performance status and received the induction treatment followed by craniofacial resection. The use of induction chemotherapy was associated with an improved two-year recurrence-free survival of 73% in a multicenter study[10]. However, the optimal regimen of induction chemotherapy has not been established. A poor response to chemotherapy with cyclophosphamide, doxorubicin and vincristine warranted a change in the regimen[11]. A platinum-based regimen has been preferred since a response rate of 60% was obtained in patients treated with the induction che-motherapy of platinum and fluorouracil[12]. Induction regimens comprising taxane, cisplatin and fluorouracil significantly improved local control, distant failure and overall survival in locally advanced head and neck cancer patients, compared with induction chemotherapy with cisplatin and fluorouracil[13]. Based on these results, TPF chemotherapy has been applied as an induction regimen and shown to provide durable local control in a few LA-SNUC cases, although no details were provided[14,15]. In our cases, TPF induced rapid responses, which helped in the radiographic planning of IMRT, and did not compromise patients’ compliance in the subsequent chemoradiotherapy, all of which supported the use of the TPF regimen in this setting. As the chemotherapeutic regimens differ among institutions and the choice of regimen is often made by the treating physicians, more research is needed in order to establish the optimal induction regimen.
Although surgery remains the preferable treatment for LA-SNUC, its use has been limited by some issues: selection criteria for surgery varied across institutions, and 10%-75% of patients failed to receive surgery[5]. In the surgical cases, pathologically complete resection of the tumor has often been unachievable because of tumor invasion to the adjacent structures. In fact, approximately half of patients receiving surgery with or without neoadjuvant chemotherapy had close or positive surgical margins, which might result in local recurrence[11]. Additionally, the external incisions potentially compromise the cosmetic outcome and quality of life of patients.
In unresectable LA-SNUC cases, definitive chemoradiotherapy alone has been widely used[3], but it has shown less promising efficacy than the multimodality treatment that includes surgery[5]. The case series showed a poor median overall survival, ranging from 7 to 19 mo, in patients receiving chemoradiotherapy only[2,16,17], which demanded more intensive treatment. A small number of LA-SNUC patients received induction chemotherapy prior to chemoradiotherapy, which is recom-mended for locally advanced nasopharyngeal carcinoma patients[18]. Chemotherapy with platinum and fluorouracil and subsequent chemoradiotherapy with concomitant cisplatin showed a two-year survival rate of 64% in LA-SNUC patients[12]. Considering that most patients had more advanced diseases that were not resectable, the two-year survival rate appeared to be better than that in patients who underwent surgery[11]. In order to investigate the efficacy of induction chemotherapy plus chemoradiotherapy, we undertook a review of the literature using PubMed (Table 1). The search terms used were “sinonasal undifferentiated carcinoma” and “SNUC”. All SNUC cases published until November 2018 were included in the search. Then, the data of patients receiving induction chemotherapy plus chemoradiotherapy were extracted. Cases in which treatment options or outcomes were not individually described were excluded from the review. Our review highlighted a survival benefit of adding induction chemotherapy to chemoradiotherapy. Compared with the previous cases, our cases showed improved survival. In addition to the effect of the TPF induction chemotherapy, IMRT may contribute to the outcome. IMRT enabled an achievement of steep dose gradients near target volumes, which greatly impacted on a dose-response relationship in SNUC, and led to a lower rate of radiation-induced toxicity. Retrospective studies indicated the crucial role of IMRT, showing that the use of IMRT was related to longer survival of SNUC patients[10,19]. A recently published study also demonstrated the intriguing finding that in patients who responded to induction chemotherapy, definitive chemoradiotherapy provided a better chance of disease control and improved survival than did surgical resection[20], suggesting the possibility that definitive chemoradiotherapy can replace surgery even in resectable LA-SNUC cases.
| Ref. | Year | n | T Stage(T3/4) | N Stage(N0/1/2) | Induction chemotherapy (cycle, regimen) | Chemoradiotherapy(radiation dose, regimen) | ENI | MST1 (mo, range) |
| Musy et al[11] | 2002 | 5 | NS2 | NS | 3, CAV/EP | 55-63 Gy, NS | - | 9 (4-114) |
| Rischin et al[12] | 2004 | 7 | 0/7 | 4/1/2 | 3, FC/FP | 50-60 Gy, CBDCA/CDDP | + | 21 (5-51) |
| Yoshida et al[16] | 2008 | 1 | 0/1 | 0/0/1 | NS, EP | 72 Gy, CDDP | + | 41 |
| Mourad et al[14] | 2013 | 3 | 0/3 | 3/0/0 | 2-3, CDDP/FP/TPF | 3DCRT/IMRT of 60-70 Gy, CDDP3 | + | 27 (16-28) |
| Ansari et al[22] | 2013 | 1 | 0/1 | 0/0/1 | 3, EAP | 60 Gy, CBDCA | - | 16 |
| Gray et al[2] | 2015 | 3 | 0/3 | 2/0/1 | NS, EC/EP | PB of 70 CGE, EC/EP | + | 34 (32-58) |
| Zielinski et al[15] | 2016 | 1 | 0/1 | 0/1/0 | 6, TPF | 60 Gy, CDDP | NS | 17 |
| Bhasker et al[21] | 2017 | 5 | NS | NS | 2, EP/FP/TC | 3DCRT of 70 Gy, CDDP | + | 6 (2.4-34.2) |
| Sienna et al[23] | 2018 | 1 | 0/1 | 0/0/1 | 3, CDDP | 50 Gy, CDDP | - | 17 |
| Our cases | 2 | 0/2 | 0/0/0 | 3, TPF | IMRT of 70 Gy, CDDP | - | 44 | |
The prognostic factors for LA-SNUC remain unclear. UICC TNM staging of head and neck cancer has long been applied to SNUC, but the T staging on admission was not prognostic[10]. Our cases with T4 disease showed improved survival, possibly because they were down-staged by induction TPF chemotherapy and received subsequent chemoradiotherapy. The impact of local tumor spread on survival should be further investigated. Metastatic lymph nodes of the neck were occasionally found at presentation of SNUC[1]. The dissection or elective neck node irradiation (ENI) for metastatic cervical nodes led to excellent local control[17,21], but the prognosis for cervical lymph node metastasis still remains controversial. Two retrospective studies reported conflicting results: the neck involvement was associated with lower survival in Kadish C patients in a meta-analysis[4]. In contrast, no significant difference in survival was shown between N0 and N1-2 patients in a multicenter study[10]. For the node-negative SNUC, the survival benefit of prophylactic ENI has not been clarified, and the comprehensive ENI may worsen the long-term quality of life[1]. Patients with relapsed lymph nodes who do not receive ENI might be successfully salvaged with surgery or radiotherapy. Our patients with node-negative disease did not receive the ENI and remained disease-free. Thus, the role of prophylactic ENI for the N0 SNUC patients can still be debated.
In conclusion, our cases, along with our review of the literature, highlighted the promising efficacy and safety of intensive treatment with induction TPF chemotherapy followed by chemoradiotherapy with cisplatin in LA-SNUC. This treatment option should be a curative alternative to surgery for LA-SNUC patients.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Alkan A, Yamagata M, Hashimoto N, Cerwenka H S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ
| 1. | Ahn PH, Mitra N, Alonso-Basanta M, Adappa ND, Palmer JN, O'Malley BW, Rassekh CH, Chalian A, Cohen RB, Lin A. Nodal metastasis and elective nodal level treatment in sinonasal small-cell and sinonasal undifferentiated carcinoma: a surveillance, epidemiology and end results analysis. Br J Radiol. 2016;89:20150488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Gray ST, Herr MW, Sethi RK, Diercks G, Lee L, Curry W, Chan A, Clark J, Holbrook EH, Rocco J, Sadow PM, Lin DT. Treatment outcomes and prognostic factors, including human papillomavirus, for sinonasal undifferentiated carcinoma: a retrospective review. Head Neck. 2015;37:366-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Lopez F, Suárez V, Vivanco B, Suárez C, Llorente JL. Current management of sinonasal undifferentiated carcinoma. Rhinology. 2015;53:212-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Reiersen DA, Pahilan ME, Devaiah AK. Meta-analysis of treatment outcomes for sinonasal undifferentiated carcinoma. Otolaryngol Head Neck Surg. 2012;147:7-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Christopherson K, Werning JW, Malyapa RS, Morris CG, Mendenhall WM. Radiotherapy for sinonasal undifferentiated carcinoma. Am J Otolaryngol. 2014;35:141-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Sobin LH, Gospodarowicz MK, and Wittekind C. International Union Against Cancer. UICC TNM classification of malignant tumours. Wiley-Blackwell: A John Wiley Sons, Ltd., Pub (UK) 2009; 46-50. [Cited in This Article: ] |
| 7. | Singh L, Ranjan R, Arava S, Singh MK. Role of p40 and cytokeratin 5/6 in the differential diagnosis of sinonasal undifferentiated carcinoma. Ann Diagn Pathol. 2014;18:261-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Deutsch BD, Levine PA, Stewart FM, Frierson HF, Cantrell RW. Sinonasal undifferentiated carcinoma: a ray of hope. Otolaryngol Head Neck Surg. 1993;108:697-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Kadish S, Goodman M, Wang CC. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer. 1976;37:1571-1576. [PubMed] [Cited in This Article: ] |
| 10. | de Bonnecaze G, Verillaud B, Chaltiel L, Fierens S, Chapelier M, Rumeau C, Malard O, Gavid M, Dufour X, Righini C, Uro-Coste E, Rives M, Bach C, Baujat B, Janot F, de Gabory L, Vergez S. Clinical characteristics and prognostic factors of sinonasal undifferentiated carcinoma: a multicenter study. Int Forum Allergy Rhinol. 2018;8:1065-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Musy PY, Reibel JF, Levine PA. Sinonasal undifferentiated carcinoma: the search for a better outcome. Laryngoscope. 2002;112:1450-1455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Rischin D, Porceddu S, Peters L, Martin J, Corry J, Weih L. Promising results with chemoradiation in patients with sinonasal undifferentiated carcinoma. Head Neck. 2004;26:435-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Blanchard P, Bourhis J, Lacas B, Posner MR, Vermorken JB, Cruz Hernandez JJ, Bourredjem A, Calais G, Paccagnella A, Hitt R, Pignon JP; Meta-Analysis of Chemotherapy in Head and Neck Cancer, Induction Project, Collaborative Group. Taxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: an individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J Clin Oncol. 2013;31:2854-2860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 195] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 14. | Mourad WF, Hauerstock D, Shourbaji RA, Hu KS, Culliney B, Li Z, Jacobson A, Tran T, Manolidis S, Schantz S, Urken M, Persky M, Harrison LB. Trimodality management of sinonasal undifferentiated carcinoma and review of the literature. Am J Clin Oncol. 2013;36:584-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Zielinski V, Laban S, Tribius S, Schafhausen P, Veldhoen S, Knecht R, Clauditz T, Muenscher A. Management of sinonasal undifferentiated carcinoma with intracerebral invasion: Clinical experience at a single institution and review of the literature. Ear Nose Throat J. 2016;95:23-28. [PubMed] [Cited in This Article: ] |
| 16. | Yoshida E, Aouad R, Fragoso R, Farwell DG, Gandour-Edwards R, Donald PJ, Chen AM. Improved clinical outcomes with multi-modality therapy for sinonasal undifferentiated carcinoma of the head and neck. Am J Otolaryngol. 2013;34:658-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Tanzler ED, Morris CG, Orlando CA, Werning JW, Mendenhall WM. Management of sinonasal undifferentiated carcinoma. Head Neck. 2008;30:595-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Colevas AD, Yom SS, Pfister DG, Spencer S, Adelstein D, Adkins D, Brizel DM, Burtness B, Busse PM, Caudell JJ, Cmelak AJ, Eisele DW, Fenton M, Foote RL, Gilbert J, Gillison ML, Haddad RI, Hicks WL, Hitchcock YJ, Jimeno A, Leizman D, Maghami E, Mell LK, Mittal BB, Pinto HA, Ridge JA, Rocco J, Rodriguez CP, Shah JP, Weber RS, Witek M, Worden F, Zhen W, Burns JL, Darlow SD. NCCN Guidelines Insights: Head and Neck Cancers, Version 1.2018. J Natl Compr Canc Netw. 2018;16:479-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 371] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 19. | Gamez ME, Lal D, Halyard MY, Wong WW, Vargas C, Ma D, Ko SJ, Foote RL, Patel SH. Outcomes and patterns of failure for sinonasal undifferentiated carcinoma (SNUC): The Mayo Clinic Experience. Head Neck. 2017;39:1819-1824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Amit M, Abdelmeguid AS, Watcherporn T, Takahashi H, Tam S, Bell D, Ferrarotto R, Glisson B, Kupferman ME, Roberts DB, Su SY, Raza SM, DeMonte F, Hanna EY. Induction Chemotherapy Response as a Guide for Treatment Optimization in Sinonasal Undifferentiated Carcinoma. J Clin Oncol. 2019;37:504-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 21. | Bhasker S, Mallick S, Benson R, Bhanuprasad V, Sharma A, Thakar A. A multimodality approach to sinonasal undifferentiated carcinoma: a single institute experience. J Laryngol Otol. 2017;131:19-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Ansari M, Guo S, Fakhri S, Citardi MJ, Blanco A, Patino M, Buryanek J, Amato R, Karni R, Brown RE. Sinonasal undifferentiated carcinoma (SNUC): morphoproteomic-guided treatment paradigm with clinical efficacy. Ann Clin Lab Sci. 2013;43:45-53. [PubMed] [Cited in This Article: ] |
| 23. | Sienna J, Nguyen NT, Arsenault J, Hodson I, Meyers B. A Case of Sinonasal Undifferentiated Carcinoma with Brain Metastases. Cureus. 2018;10:e2320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |