Published online Feb 26, 2019. doi: 10.12998/wjcc.v7.i4.508
Peer-review started: November 12, 2018
First decision: November 27, 2018
Revised: December 23, 2018
Accepted: January 3, 2019
Article in press: January 3, 2019
Published online: February 26, 2019
To report on the use of percutaneous hydrochloric acid (HCl) enhanced radiofrequency ablation (HRFA) for the treatment of large (maximum diameter ≥ 5 cm) hepatocellular carcinoma (HCC) in the caudate lobe.
Between August 2013 and June 2016, three patients with a large HCC (maximum diameter: 5.0, 5.7, and 8.1 cm) in the caudate lobe were treated by transarterial chemoembolization followed by computer tomography (CT) guided RFA using a monopolar perfusion RF electrode, which was enhanced by local infusion of 10% HCl at 0.2 mL/min (total volume, 3 to 12 mL). The output power of HRFA reached 100 W, and the average ablation time was 39 min (range, 15 to 60 min). Two patients each underwent one session of HRFA and one patient two sessions. After treatment, CT/magnetic resonance imaging showed that all the three lesions were completely ablated. There was no major complication. Two patients had asymptomatic bile duct dilatation. One patient died of tongue cancer 24 mo after ablation. The remaining two patients were alive and no area of enhancement is detected in the caudate lobe at 28 and 60 mo after ablation, respectively.
Percutaneous CT-guided HRFA is safe and efficacious in treating large HCC in the caudate lobe.
Core tip: Caudate lobe hepatocellular carcinoma (HCC) was considered highly technically difficult by surgeons and the outcome of interventional therapies, including transarterial chemoembolization and conventional radiofrequency ablation (RFA), according to previous studies was unsatisfied. Hydrochloric acid enhanced RFA, an innovative technique, can create an ablation zone larger than 5 cm by a single perfusate electrode without major complications, which is promising to treat large caudate lobe HCC patient.
- Citation: Deng HX, Huang JH, Lau WY, Ai F, Chen MS, Huang ZM, Zhang TQ, Zuo MX. Hydrochloric acid enhanced radiofrequency ablation for treatment of large hepatocellular carcinoma in the caudate lobe: Report of three cases. World J Clin Cases 2019; 7(4): 508-515
- URL: https://www.wjgnet.com/2307-8960/full/v7/i4/508.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i4.508
Hepatocellular carcinoma (HCC) arising from the caudate lobe is rare[1]. The caudate lobe is situated deep between the hepatic hilum and the inferior vena cava. Caudate lobectomy is considered to be technically difficult even for small tumors, with high risks of local recurrence and poor overall survival[2,3]. For large tumors in the caudate lobe, resection is challenging even in the hands of experienced liver surgeons[4,5]. Interventional therapies, including various intravascular and extravascular procedures, have been reported to treat caudate lobe HCC[6-14]. However, most of those focused on treating small HCC (maximum diameter < 5 cm). This is a retrospective study on three patients with HCC ≥ 5 cm in the caudate lobe treated by hydrochloric acid (HCl) enhanced radiofrequency ablation (HRFA).
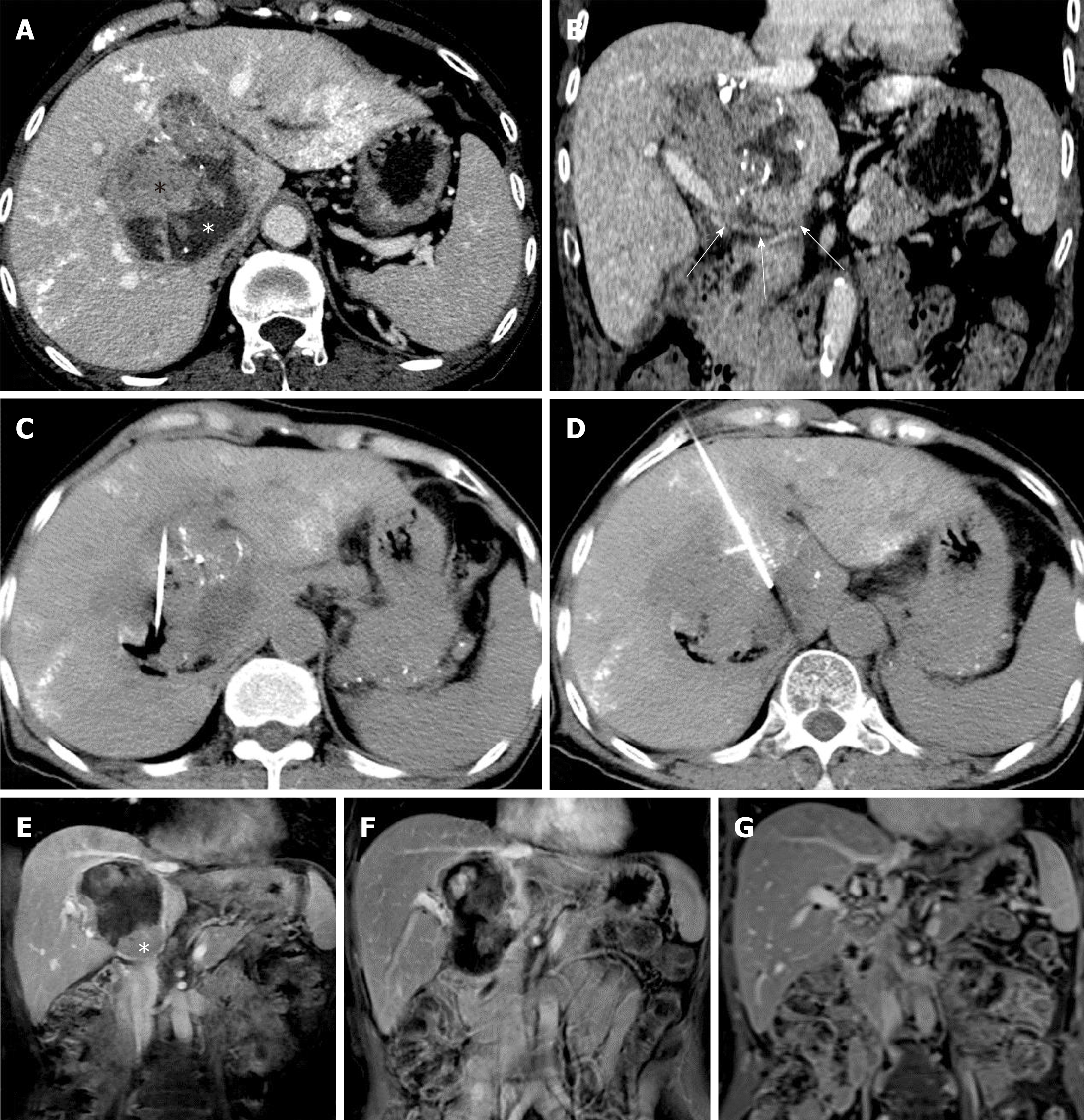
A 61-year-old woman was found to have a caudate lobe lesion (55 mm × 57 mm × 63 mm) on magnetic resonance imaging (MRI). The patient had a history of chronic hepatic B virus (HBV) infection, with Child-Pugh A liver function and a negative serum alpha-fetoprotein (AFP) concentration (0.71 ng/mL). A biopsy confirmed HCC. Transarterial chemoembolization (TACE) using an emulsion of tetrahydropalmatine (THP) 50 mg, lobaplatin 50 mg, and lipiodol 10 mL was performed in August 2013. One month later, computed tomography (CT) showed that the tumor had enlarged (72 mm × 75 mm × 81 mm), and was separated into the superior and inferior parts by a fibrous septum.
Since the tumor enlarged after TACE, the patient was suggested to undergo ablation therapy. Conventional RFA was insufficient to ablate such a huge tumor. Thus, the patient received two sessions of HRFA in September and December 2013, respectively, to ablate the superior and inferior parts of the tumor. HRFA was applied for 60 min in the first and 30 min in the second session. No discomfort during ablation and no complications such as fever, pain, or hemorrhage after HRFA were observed.
One month after the first HRFA, a peripheral hyper-metabolic nodule was detected by PET-CT. Thus, the patient underwent two more sessions of COOL-TIP RFA in December 2013 and March 2014, respectively. After that, the hypermetabolic lesion was no longer visible. The last follow-up CT in July 2018 showed that the tumor had decreased to an inactive fibrous tissue mass of about 2 cm in diameter. During the course of treatment and follow-up, there were no major complications. A minor complication was asymptomatic slight dilation (total bilirubin concentration once elevated to 72 μmol/L 18 mo after HRFA and returned normal without any treat-ment) of bile ducts (Figure 1).
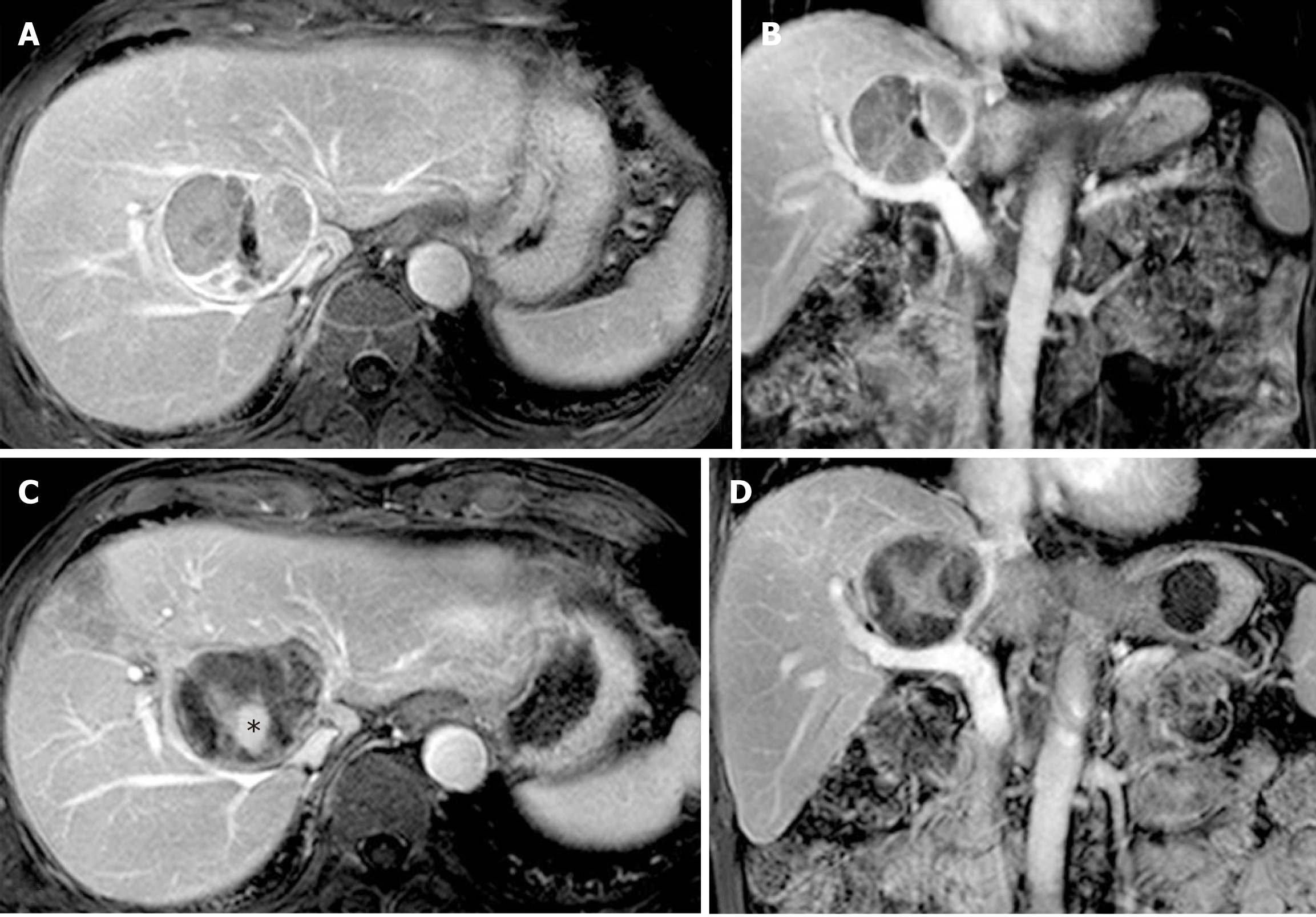
A 69-year-old man was found on health checkup to have a caudate lobe tumor, 17 mm × 25 mm on PET-CT in June 2013. He had a history of chronic HBV infection. The AFP concentration was negative, and he had Child-Pugh A liver function. A biopsy confirmed the diagnosis of HCC. The patient received two sessions of TACE in August and November 2013, respectively. CT performed in March 2014 showed that the lesion had enlarged to 47 mm × 57 mm, with poor lipiodol deposition. Besides, the patient had a history of tongue cancer and received radiotherapy 4 years ago.
In March 2014, HRFA through an anterior approach was applied for 60 min. There was no acute adverse effect occurring in the peri-ablation and post-ablation periods, and an MRI scan one month later showed no areas of enhancement.
Ten months after HRFA, in January 2015, MRI showed the margin of the lesion to be suspiciously enhanced by contrast. He underwent one session of COOL-TIP RFA. No visible active lesion was detected in the next MRI scan. The last MRI scan in December 2015 found no active lesion in the liver. Twenty-four months after HRFA, the patient died of recurrent tongue cancer (Figure 2).
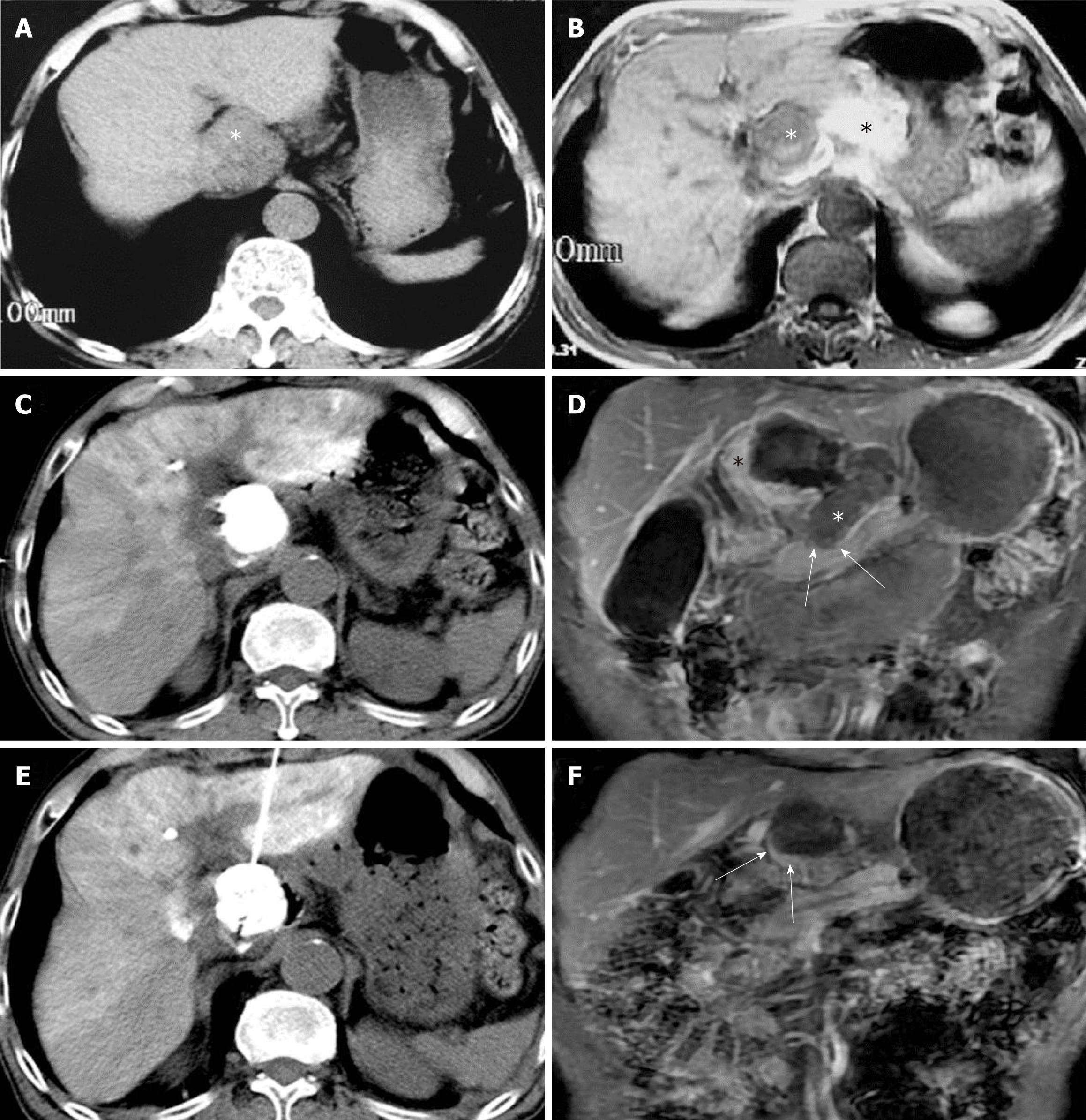
A 73-year-old man presented with chest and abdomen pain in February 2016. CT showed a mass in the pancreatic neck and a low density lesion, 35 × 50 mm, in the caudate lobe. The patient had a history of chronic HBV infection, Child-Pugh A disease, and a negative serum AFP concentration. The PIVKA level was 20.266 AU/m. An exploratory laparotomy showed a primary tumor in the caudate lobe and that the lesion in pancreatic neck was a spontaneous hematoma. Frozen section analysis of an enlarged portal lymph node showed metastatic HCC. TACE was performed in April 2016, with an emulsion of THP 30 mg, lobaplatin 30 mg, and lipiodol 10 mL. CT after TACE showed good lipiodol deposition.
Three days later, HRFA was applied for 15 min through an anterior approach. No major complications occurred. One month later, the PIVKA concentration dropped to 2.566 AU/mL and no visible contrast enhanced areas on MRI. Follow-up at 28 mo after HRFA showed no signs of relapse or metastasis. (Figure 3)
The summary of the three patients is shown in Table 1.
| Case 1 | Case 2 | Case 3 | |
| Sex | Female | Male | Male |
| Age | 61 | 69 | 73 |
| Tumor size | 75 × 81 | 47 × 57 | 35 × 50 |
| Session(s) of HRFA | 2 | 1 | 1 |
| Complementary RFA | Yes | Yes | None |
| Survival (mo) | 60 | 48 | 28 |
| Complications | Asymptomatic bile duct dilatation | None | None |
| Alive | Alive | Died of tongue cancer recurrence | Alive |
Surgical resection of a caudate lobe tumor is technically challenging as the caudate lobe is situated deep between the hepatic hilum and the inferior vena cava[1]. In a report on 12 patients, the median operative time was 568 min, the median intraoperative blood loss was 550 mL, and five patients developed postoperative bile leak with problems in renal function[4]. Caudate lobectomy is commonly combined with major or extended hepatectomy with sacrifice of a large amount of non-tumorous liver parenchyma which increases the risk of postoperative liver failure.
Compared to surgery, interventional therapies such as TACE or RFA have a lower risk of treatment morbidities. In 1986, Takayasu et al[6] reported five patients who underwent transcatheter arterial infusion (TAI) or transcatheter arterial embolization (TAE) for treating advanced-stage caudate lobe HCC. Unfortunately, four of these patients died during a mean of 5.5 mo. With advances in interventional technology and a better understanding of arterial blood supply of caudate lobe HCC[8,15,16], the success rate of selective subsegmental TACE in treating caudate lobe HCC has been greatly improved. However, long-term survival after treatment remains a problem. Kim et al[9] performed selective TACE to treat 34 patients with caudate lobe HCC with a diameter of less than 3 cm. The 5-year overall survival and progression-free rates were 72% and 21%, respectively. TACE cannot completely block the feeding arteries and gain a complete tumor necrosis, which causes recurrence[10]. For a large caudate lobe HCC, the results of TACE are even worse.
Percutaneous ablation therapies, including percutaneous ethanol injection (PEI), RFA, and MWA, are well-established and widely used treatments for HCC. In 2002, Shibata et al[11] first introduced PEI with or without TAE to treat 25 patients with caudate lobe HCC (average diameter, 27 mm). Peng et al[13] reported on 17 patients who underwent RFA treatment for caudate lobe HCC (average diameter, 31 mm). However, most of these studies focused on treating small caudate lobe tumors. Nevertheless, incomplete ablation and recurrence still happened. Nishigaki et al[14] compared the recurrence rates in patients with caudate lobe HCC or HCC located in other liver segments. They found that the caudate lobe patients had a higher risk of developing tumor recurrence. Caudate lobe HCCs are more difficult to be completely ablated than those in other liver segments due to the restricted approach through which an RFA electrode can be introduced, and the heat sink effect of the inferior vena cava.
In the last decade, several new techniques, such as normal saline perfused radiofrequency ablation (NSRFA) and multi-electrode applications had been developed, aiming to create a large ablative zone[17]. Our previous experiments showed that infusing diluted HCl instead of normal saline during RFA could enlarge the diameter of ablation zone from a mean (SD) of 3.52 cm (0.07) to 6.85 cm (0.32) at 30 W-30 min[18]. This is because the conductivity of HCl is about three times higher than that of saline, greatly increasing the conductivity around the RF electrode[19]. In in vivo experiments, HRFA also exhibited a larger ablative zone with favorable safety[21-22]. Based on these studies, we have reported performing HRFA on a patient with spontaneously ruptured HCC, which successfully controlled bleeding and achieved complete necrosis after ablation without any complications[19]. In a word, HRFA, a technique that can create a large ablation volume by using a monopolar electrode, is promising in treating large caudate lobe HCCs. In the present study, all three patients had unresectable large caudate lobe HCC. One patient underwent two sessions of HRFA and the other two patients underwent one session each. After HRFA and followed complementary COOL-TIP RFA, all the three caudate lobe tumors showed complete necrosis.
Among four sessions of HRFA, three were performed through an anterior approach and the remaining one was through a lateral approach (case 1, the 2nd session) in order to protect peripheral vessels and the biliary system. HRFA also avoids repeated punctures because one session of HRFA is sufficient to achieve complete necrosis. Besides, the electrode in HRFA could be placed at the center of the lesion whereas in other RFA techniques, it must reach the tumor margin, which would induce damage to the structure nearby. There was no major complication and asymptomatic bile duct dilatation as a minor complication occurred in patient 1 18 mo after HRFA. It was hypothesized that the non-active lesion, which had shrunk from 7 cm to less than 3 cm, stretched its peripheral liver tissue and induced bile duct dilatation.
HRFA can be an efficacious and safe choice for patients with a large caudate lobe HCC. However, further research is necessary to determine the appropriate role of HRFA in treating caudate lobe HCCs. Combination of HRFA with TACE or other systemic therapies is expected to further improve the prognosis of patients.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bramhall S, Jani K, Ekpenyong CE S- Editor: Dou Y L- Editor: Wang TQ E- Editor: Tan WW
| 1. | Kumon M. Anatomy of the caudate lobe with special reference to portal vein and bile duct. Acta Hepatol Jpn. 1985;26:1193-1199. [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 108] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Tanaka S, Shimada M, Shirabe K, Maehara S, Tsujita E, Taketomi A, Maehara Y. Surgical outcome of patients with hepatocellular carcinoma originating in the caudate lobe. Am J Surg. 2005;190:451-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Kumon M. Anatomical Study of the Caudate Lobe with Special Reference to Portal Venous and Biliary Branches Using Corrosion Liver Casts and Clinical Application. Liver Cancer. 2017;6:161-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Viganò L, Costa G, Procopio F, Donadon M, Cimino M, Del Fabbro D, Gatti A, Torzilli G. Parenchyma-Sparing Liver Surgery for Large Segment 1 Tumors: Ultrasound-Guided Lateral and Superior Approaches as Safe Alternatives to Major Hepatectomy. J Am Coll Surg. 2015;221:e65-e73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Wang ZG, Lau W, Fu SY, Liu H, Pan ZY, Yang Y, Zhang J, Wu MC, Zhou WP. Anterior hepatic parenchymal transection for complete caudate lobectomy to treat liver cancer situated in or involving the paracaval portion of the caudate lobe. J Gastrointest Surg. 2015;19:880-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Takayasu K, Muramatsu Y, Shima Y, Goto H, Moriyama N, Yamada T, Makuuchi M, Kaneko A, Itabashi M, Shimamura Y. Clinical and radiologic features of hepatocellular carcinoma originating in the caudate lobe. Cancer. 1986;58:1557-1562. [PubMed] [Cited in This Article: ] |
| 7. | Terayama N, Miyayama S, Tatsu H, Yamamoto T, Toya D, Tanaka N, Mitsui T, Miura S, Fujisawa M, Kifune K, Matsui O, Takashima T. Subsegmental transcatheter arterial embolization for hepatocellular carcinoma in the caudate lobe. J Vasc Interv Radiol. 1998;9:501-508. [PubMed] [Cited in This Article: ] |
| 8. | Woo S, Kim HC, Chung JW, Jung HS, Hur S, Lee M, Jae HJ. Chemoembolization of extrahepatic collateral arteries for treatment of hepatocellular carcinoma in the caudate lobe of the liver. Cardiovasc Intervent Radiol. 2015;38:389-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Kim HC, Chung JW, Jae HJ, Yoon JH, Lee JH, Kim YJ, Lee HS, Yoon CJ, Park JH. Caudate lobe hepatocellular carcinoma treated with selective chemoembolization. Radiology. 2010;257:278-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Goldberg SN, Ahmed M. Minimally invasive image-guided therapies for hepatocellular carcinoma. J Clin Gastroenterol. 2002;35:S115-S129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Shibata T, Maetani Y, Ametani F, Kubo T, Itoh K, Konishi J. Efficacy of nonsurgical treatments for hepatocellular carcinoma in the caudate lobe. Cardiovasc Intervent Radiol. 2002;25:186-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Yamakado K, Nakatsuka A, Akeboshi M, Takaki H, Takeda K. Percutaneous radiofrequency ablation for the treatment of liver neoplasms in the caudate lobe left of the vena cava: electrode placement through the left lobe of the liver under CT-fluoroscopic guidance. Cardiovasc Intervent Radiol. 2005;28:638-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Peng ZW, Liang HH, Chen MS, Zhang YJ, Li JQ, Zhang YQ, Lau WY. Percutaneous radiofrequency ablation for the treatment of hepatocellular carcinoma in the caudate lobe. Eur J Surg Oncol. 2008;34:166-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Nishigaki Y, Tomita E, Hayashi H, Suzuki Y, Iritani S, Kato T, Yamada T. Efficacy and safety of radiofrequency ablation for hepatocellular carcinoma in the caudate lobe of the liver. Hepatol Res. 2013;43:467-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Yoon CJ, Chung JW, Cho BH, Jae HJ, Kang SG, Kim HC, Choi YH, Jeon UB, Park JH. Hepatocellular carcinoma in the caudate lobe of the liver: angiographic analysis of tumor-feeding arteries according to subsegmental location. J Vasc Interv Radiol. 2008;19:1543-50; quiz 1550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Miyayama S, Yamashiro M, Yoshie Y, Nakashima Y, Ikeno H, Orito N, Yoshida M, Matsui O. Hepatocellular carcinoma in the caudate lobe of the liver: variations of its feeding branches on arteriography. Jpn J Radiol. 2010;28:555-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Kang TW, Rhim H. Recent Advances in Tumor Ablation for Hepatocellular Carcinoma. Liver Cancer. 2015;4:176-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Jiang XY, Gu YK, Huang JH, Gao F, Zou RH, Zhang TQ. Ex Vivo Liver Experiment of Hydrochloric Acid-Infused and Saline-Infused Monopolar Radiofrequency Ablation: Better Outcomes in Temperature, Energy, and Coagulation. Cardiovasc Intervent Radiol. 2016;39:600-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Huang JH, Morelli JN, Ai F, Zou RH, Gu YK, Gao F, Zhang TQ, Yao W, Jiang XY, Zhang YY. Hydrochloric acid-enhanced radiofrequency ablation for treating a large hepatocellular carcinoma with spontaneous rapture: a case report. Chin J Cancer. 2017;36:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Weijian F, Zan L, Suhong H, Hongmei Z, Lei Z, Yanjie Z, Yi C, Ni J. Destructive effect of percutaneous hydrochloric acid injection therapy for liver cancer--a preliminary experimental and clinical study. Gan To Kagaku Ryoho. 2006;33:1852-1856. [PubMed] [Cited in This Article: ] |
| 21. | Yao W, Gu YK, Wang J, Gao F, Liu WL, Huang JH. Safety evaluation of a potential ablation agent-hydrochloric acid in the rabbits' model. Ann Palliat Med. 2014;3:250-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 22. | Zhang TQ, Huang SM, Gu YK, Gao F, Huang ZM, Jiang XY, Liu DX, Huang JH. Safety and effect on ablation size of hydrochloric acid-perfused radiofrequency ablation in animal livers. Int J Hyperthermia. 2018;34:925-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |