Published online May 26, 2019. doi: 10.12998/wjcc.v7.i10.1221
Peer-review started: January 4, 2019
First decision: January 27, 2019
Revised: February 19, 2019
Accepted: March 16, 2019
Article in press: March 16, 2019
Published online: May 26, 2019
Lung squamous cell cancer (LSCC) rarely harbors epidermal growth factor receptor (EGFR) mutations, even much rarer for acquired T790M mutation. Although clinical trials of AURA series illustrated that non-small cell lung cancer (NSCLC) with EGFR T790M mutation can benefit from osimertinib, only five LSCC patients were enrolled in total; moreover, the efficacy for LSCC was not shown in the results. Therefore, the response of LSCC to osimertinib is still unclear to date.
We report an LSCC case with T790M-related acquired resistance after treatments with first-generation EGFR-tyrosine kinase inhibitors (EGFR-TKIs) and benefited from osimertinib significantly. A 63-year-old Chinese man was diagnosed with stage IV (cT2N2M1b) LSCC harboring an EGFR exon 19-deletion mutation. Following disease progression after gefitinib and multi-line chemotherapy, re-biopsy was conducted. Molecular testing of EGFR by amplification refractory mutation system-polymerase chain reaction detected the exon 19-deletion without T790M mutation. Therefore, the patient was given erlotinib, but progression developed only 3 mo later. Then the frozen re-biopsy tissue was tested by next-generation sequencing (NGS), which detected an EGFR T790M mutation. However, he was very weak with symptoms of dysphagia and cachexia. Fortunately, osimertinib was started, leading to alleviation from the symptoms. Four months later, normal deglutition was restored and partial response was achieved. Finally, the patient achieved an overall survival time period of 29 mo.
Our findings highlight that EGFR T790M mutation may also be an important acquired drug resistance mechanism for LSCC and offer direct evidence of the efficacy of osimertinib in LSCC with T790M mutation. NGS and better preservation conditions may contribute to higher sensitivity of EGFR T790M detection.
Core tip: This is a case report of T790M-related acquired drug resistant lung squamous cell cancer (LSCC) patient with good response to osimertinib, which indicated that T790M is also an important mechanism for acquired resistance in LSCC. In this case, the secondary T790M mutation of epidermal growth factor receptor (EGFR) was detected by next-generation sequencing (NGS) for frozen tissue but not detected by amplification refractory mutation system-polymerase chain reaction for formalin-fixed and paraffin-embedded sample, which suggests that NGS and better preservation conditions may contribute to higher sensitivity of EGFR T790M detection.
- Citation: Zhang Y, Chen HM, Liu YM, Peng F, Yu M, Wang WY, Xu H, Wang YS, Lu Y. Significant benefits of osimertinib in treating acquired resistance to first-generation EGFR-TKIs in lung squamous cell cancer: A case report. World J Clin Cases 2019; 7(10): 1221-1229
- URL: https://www.wjgnet.com/2307-8960/full/v7/i10/1221.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i10.1221
The oncogenic driver profile of lung squamous cell lung cancer (LSCC) is significantly different from that of lung adenocarcinoma[1]. Epidermal growth factor receptor (EGFR) is the most important driver gene in lung adenocarcinoma; therefore, LSCC rarely harbours EGFR mutations[2,3].
Although lung adenocarcinoma can benefit from EGFR-tyrosine kinase inhibitors (TKIs) and the acquired resistance mechanism has been widely researched[4], the data for LSCC are very limited due to the rare incidence of EGFR-positive LSCC. We previously performed a multicentre retrospective study of EGFR-positive LSCC patients treated with EGFR-TKI[5], which showed that the progression-free survival (PFS) for LSCC is only 5.1 mo[6], significantly inferior to lung adenocarcinoma, which is about 9.7 to 13.1 mo[7-9]. This indicates that the EGFR signalling pathway in LSCC may not be identical to that in adenocarcinoma.
Osimertinib, an oral, potent, irreversible EGFR-TKI, has been reported to be highly effective in patients with EGFR T790M mutation-positive non-small-cell lung cancer (NSCLC) in previous three clinical trials of the AURA series. Although 882 NSCLC patients were enrolled in the three clinical trials, only five LSCC patients were included (3 from AURA, 2 from AURA2, and 0 from AURA3); moreover, the efficacy of osimertinib for LSCC was not shown in the results[10-12]. T790M-positive LSCC is rarely reported. Only 14 additional cases were reported previously in addition to the cases in the AURA series clinical trials; however, none of these patients were treated with osimertinib[13-20]. Although one patient with a T790M mutation was administered with another third-generation EGFR-TKI, rociletinib, this was an LSCC transformation from adenocarcinoma, rather than acquired resistance to first-generation TKIs[20]. The response of LSCC to osimertinib is still unclear to date. More clinical evidence is needed for the management of LSCC with T790M after treatment with first-generation EGFR-TKIs.
Here, we report an LSCC patient with T790M-related acquired drug resistance after treatments with first-generation EGFR-TKIs who benefited from the third-generation EGFR-TKI osimertinib.
A 62-year-old male patient was initially admitted to our hospital due to cough and sputum for one month and hemoptysis for ten days.
One month ago, the patient developed symptoms of cough, expectorated white phlegm, but did not take any medicine. Then, he began suffering hemoptysis then days ago.
Unremarkable.
The patient had a long-term history of smoking for about 40 years (10 cigarettes per day) without personal or family history of other diseases.
At admission, he was conscious with a regular heart rate of 75 bpm and a blood pressure of 128/75 mmHg. He had lost 4 kg weight in the past two months. Left lower lung breath sounds weakened. The other physical examinations were normal.
Results of laboratory routine examinations including complete blood count, fecal occult blood, blood biochemistry, and urine were within normal limits. But his carcinoembryonic antigen was 6.93 ng/mL (reference, <3.4 ng/mL) and cytokeratin 19 fragment antigen 21-1 was 14.63 ng/mL (reference, <3.0 ng/mL).
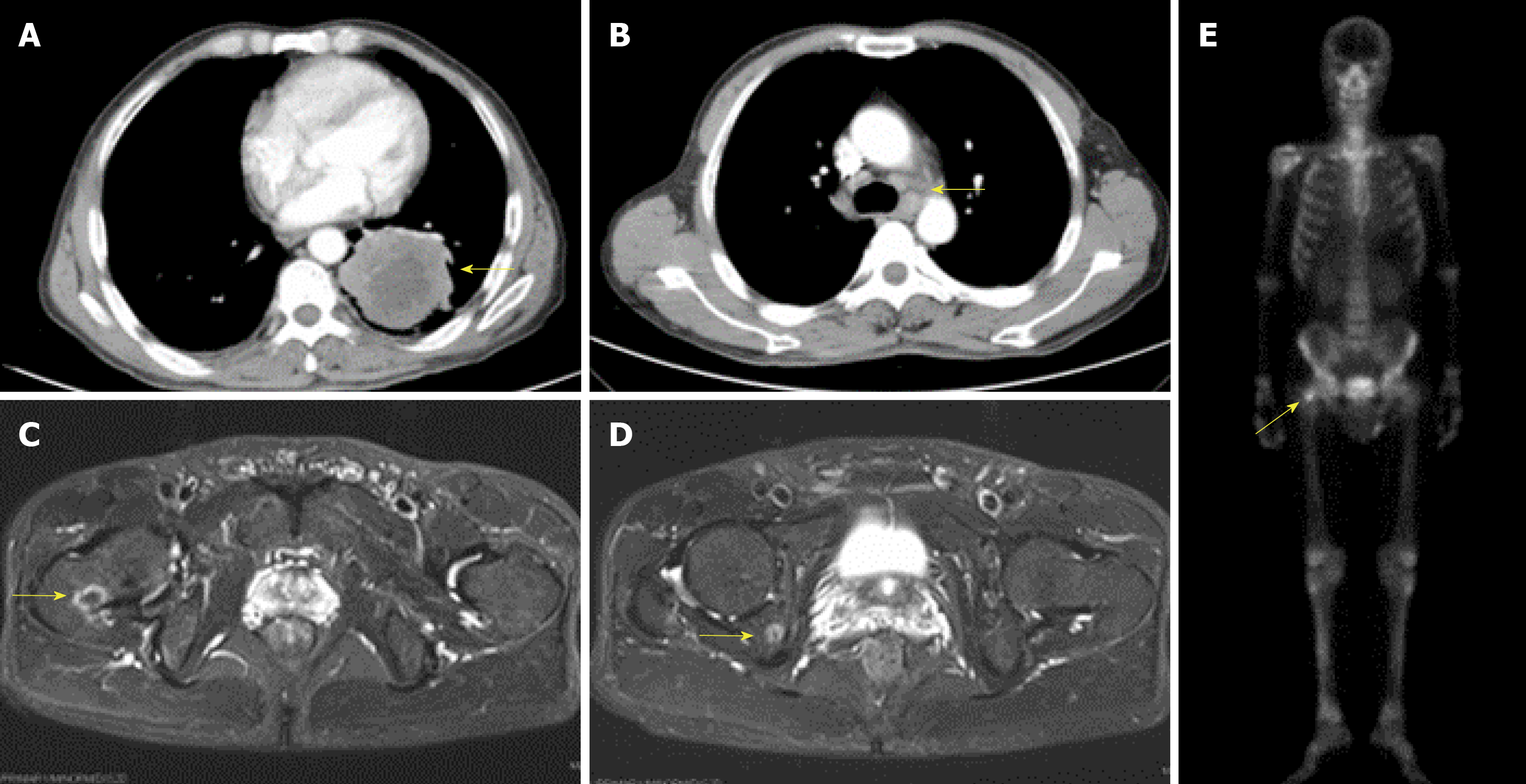
Computed tomography of the chest revealed an occupying lesion in the inferior lobe of the left lung (Figure 1A) with hilar and mediastinal lymphadenectasis (Figure 1B). Magnetic resonance imaging showed abnormal long T1 and T2 signals at the right femoral neck and ischium and radionuclide bone imaging revealed increased bone uptake on TC-99m (Figure 1C-E).
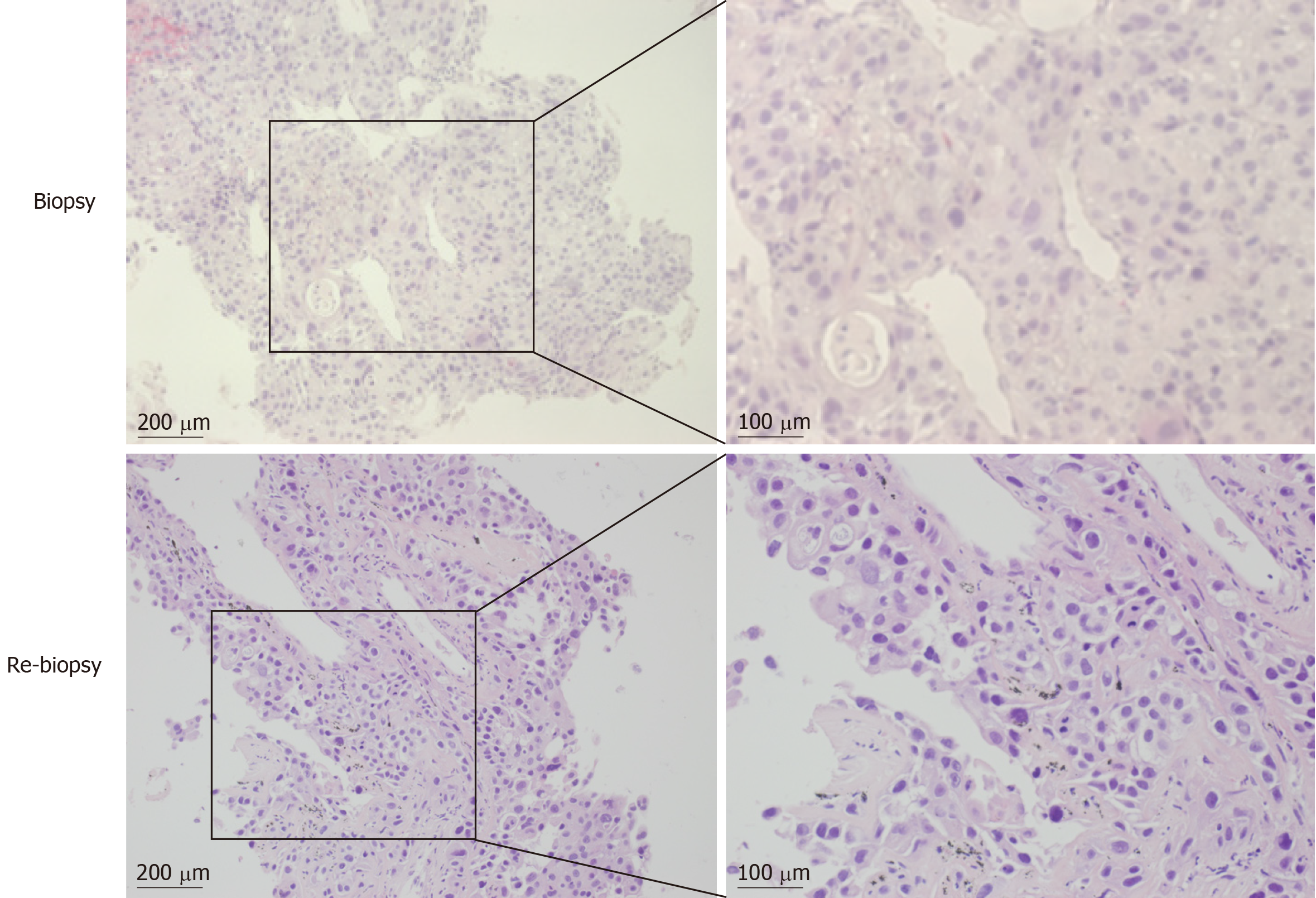
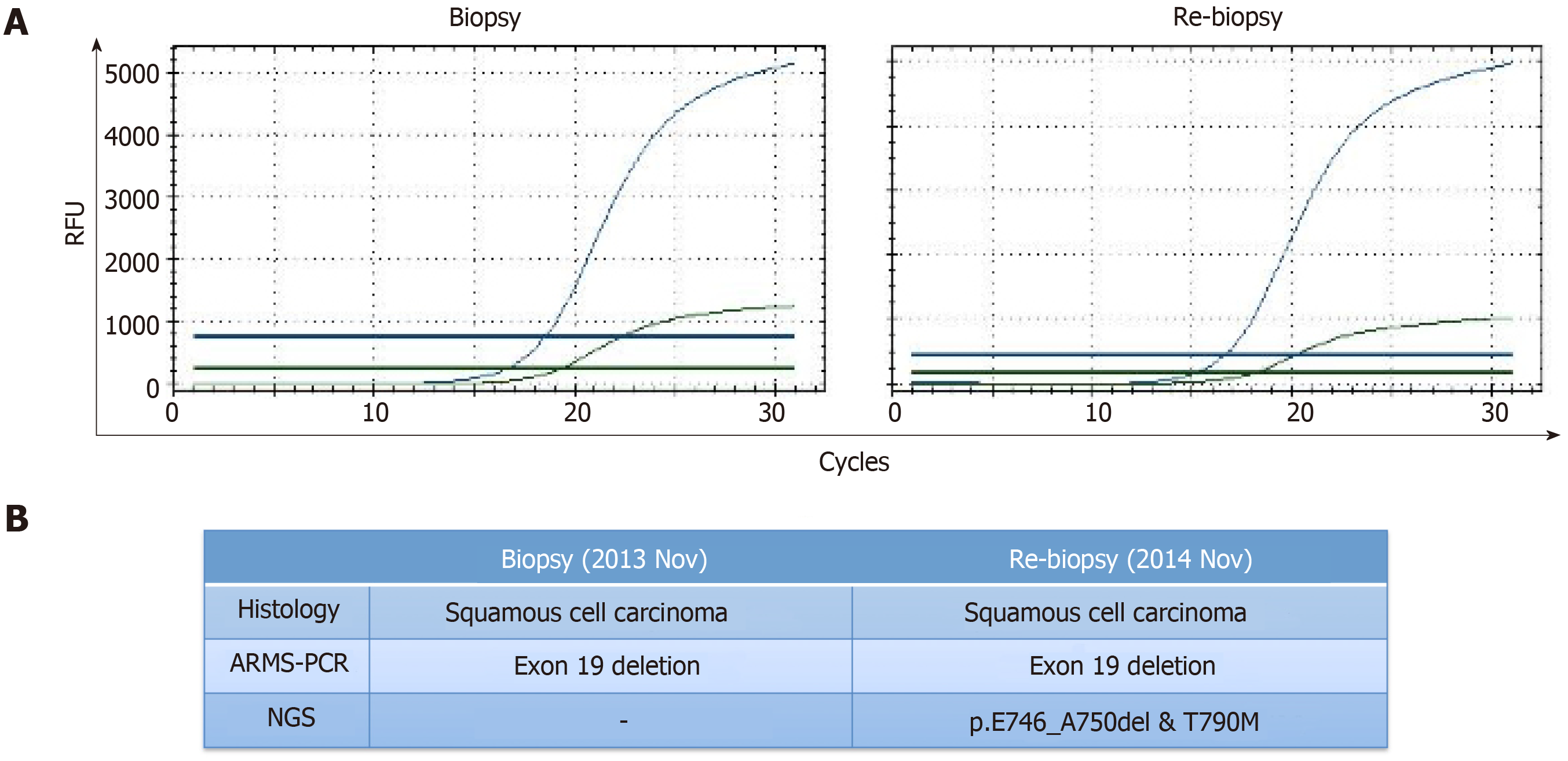
Histological examination of a transbronchial lung biopsy and a cytological examination of the bronchus and sputum confirmed LSCC, without adenosquamous carcinoma or mixture of other components. The final diagnosis was stage IV (cT2N2M1b) LSCC. We also tested for EGFR mutations by amplification refractory mutation system-polymerase chain reaction (ARMS-PCR; AmoyDx, Xiamen, China) using a small biopsy specimen. We found that this patient had an EGFR exon 19 deletion mutation.
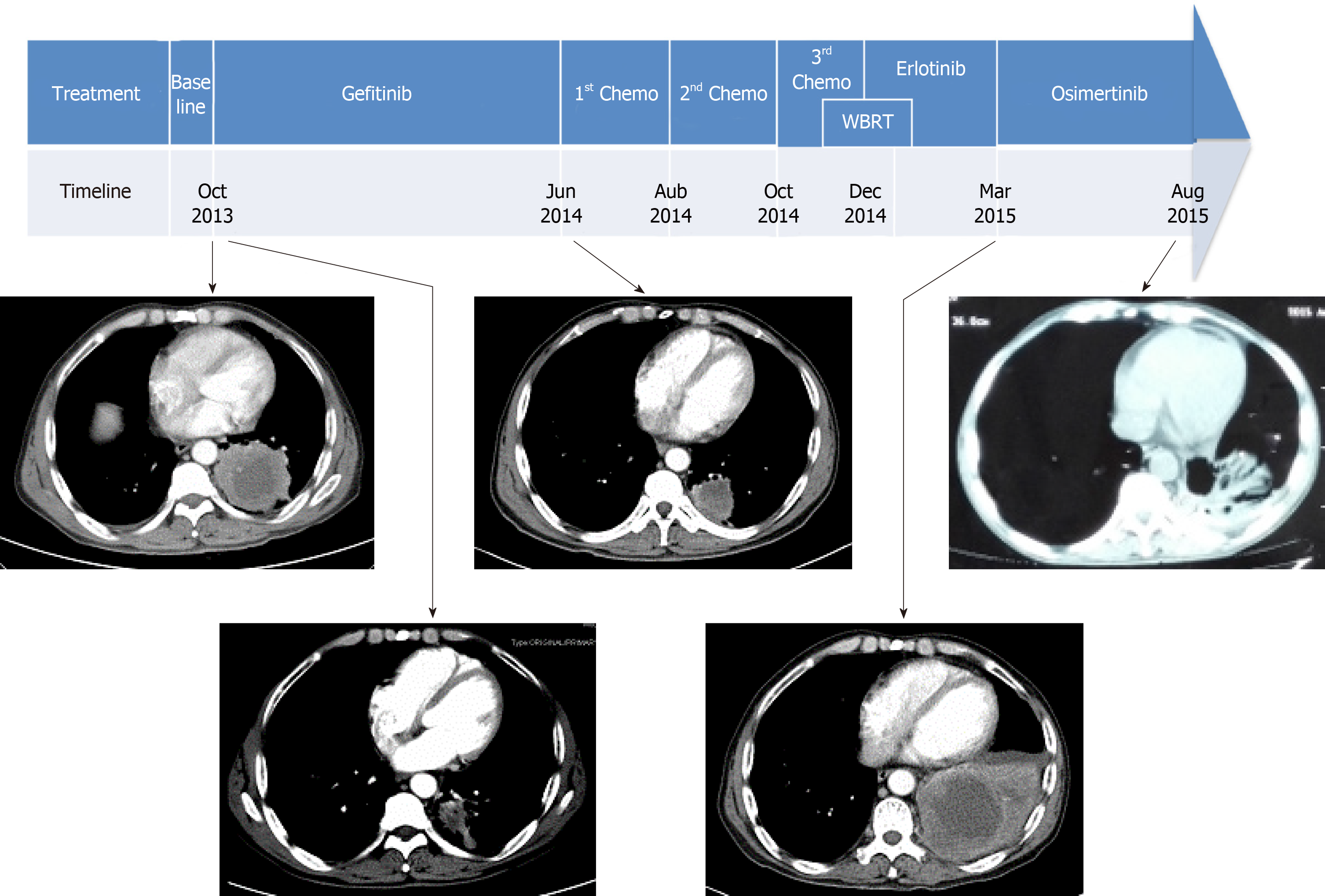
Systemic treatments were subsequently administered (Figure 2). The patient began initial gefitinib 250 mg per day from November 2013 and had a partial response until June 2014, when CT scans showed disease progression in the left lung and new metastases in the rib and abdominal lymph nodes. He subsequently stopped gefitinib and started combination chemotherapy with gemcitabine and cisplatin for two cycles. Unfortunately, he again developed disease progression in the lung and T11 costotransverse joint. Then, second-line docetaxel and cisplatin were administered for two cycles. After treatment, he complained of headaches, and brain MRI showed disease progression with multiple new lesions in the left cerebellum. Subsequently, he was treated with whole brain radiotherapy (WBRT, 37.5 Gy/2.5 Gy/15 f) and chemotherapy with vinorelbine alone (20 mg/m2 intravenously days 1, 8, 15, once every 4 wk). However, chemotherapy scheduled on day 15 was discontinued due to severe bone marrow suppression.
The patient underwent re-biopsy of the left lung mass through CT-guided percutaneous puncture, and two specimens were obtained. One specimen was formalin-fixed and paraffin-embedded for pathological and gene alteration tests, and the other was stored in liquid nitrogen. Pathological testing showed identical LSCC (Figure 3), and molecular testing of EGFR by ARMS-PCR quantified the exon 19 deletion without the T790M mutation, which remained unchanged from the baseline status (Figure 4A). Then, he began to receive treatment with erlotinib from December 2014. Unfortunately, after 3 mo, the disease progressed to the liver, and the patient developed dysphagia due to compression by enlarged mediastinal lymph nodes. He felt increasingly weak in the following days and developed cachexia.
Then, the frozen tissue was subjected to molecular testing by next-generation sequencing (NGS; NextSeq, Illumina), which confirmed the presence of an EGFR T790M mutation (allele frequency of 9.2%) in addition to the baseline exon 19 deletion mutation with an allele frequency of 70.2% (Figure 4B). From March 2015, the patient was administered with osimertinib at 80 mg PO QD. It is comforting that his dysphagia and Eastern Cooperative Oncology Group (ECOG) status gradually improved over the period of two weeks. Four months later, deglutition was restored to normal, and a partial response was achieved based on evaluation by chest computed tomography.
The patient’s ECOG status significantly deteriorated from January 2016, and 1 mo later, the patient died from disease progression in February 2016. The PFS was no more than 10 mo and the overall survival time was 29 mo. The patient did not receive CT scan from August 2015 to February 2016.
LSCC harbouring activating EGFR mutations are rare and even rarer for the coexistence of T790M mutations. This is a rare case of LSCC with coexistence of the EGFR exon 19 deletion and T790M mutation. Moreover, the patient benefited from osimertinib with a partial response. The overall survival time was 29 months.
LSCC rarely harbours EGFR mutations, not to mention an acquired T790M mutation. We review the previous literature that reports LSCC harbouring the T790M mutation (Table 1)[13-20]. To date, only 14 patients were reported in addition to the five LSCC patients enrolled in the clinical trials of the AURA series. Detailed TKI treatment information was available for only nine patients, of which five had LSCC transformation from adenocarcinoma[14,15,17,20]. The remaining three patients were acquired resistance cases after first-generation EGFR-TKI, but none were treated with third-generation EGFR-TKI[13,16]. It is worth noting that patient 5 received another third-generation EGFR-TKI, rociletinib[20]. However, this patient had an LSCC transformation derived from adenocarcinoma with de novo T790M detected at baseline. Furthermore, osimertinib has been proven by the FDA and is probably more potent than rociletinib[21]. As far as we are aware, this is the first reported T790M-related acquired resistant LSCC case with response to osimertinib, which serves as direct evidence of the effectiveness of osimertinib in LSCC.
| Case ID | Baseline | Targeted therapy | Treatment response | Progression | Ref. | |||||||||||
| Age / sex | Smoker | Stage | Morphology | Sampling method | Anatomic site | EGFR mutation | Progression time (mo) | Sampling method | Anatomic site | Morphology | EGFR mutation | 3rd generation TKI | ||||
| 1 | 63/F | Never | IV | ADC | PE | RUL | WT1 | Erlotinib | PR | 22 | B | RUL | SCC | L858R + T790M | No | Bugano et al[11] |
| 2 | NA | NA | IV | SCC | B | NA | Exon 19 deletion | Erlotinib | NA | 10 | B | NA | SCC | Exon19-deletion + T790M | NA | Masago et al[10] |
| 3 | 48/F | Never | IV | SCC | B | RUL | p.L747_P753>S | Gefitinib | PD | 2 | B | RUL | SCC | Exon19-deletion + T790M | No | Graziano et al[13] |
| 4 | 70/F | Never | IV | SCC | B | LUL | L858R | Gefitinib | SD / PR2 | 4 | B | Liver | SCC | L858R + T790M | No | Graziano et al[13] |
| 5 | 64/F | Never | IV | ADC | B | RL | L858R+T790M | Gefitinib | SD | 10 | B | RL | SCC | L858R + T790M | Rociletinib | Haratani et al[17] |
| 6 | 74/F | Former | IV | ADC | B | LL | L858R | Gefitinib | PR | 10 | B | LL | SCC | L858R + T790M | No | Jukna et al[14] |
| 7 | 79/F | Never | IV | ADC | PE | RLL | p.E746_A750del | Gefitinib | PR | 15 | B | RL | SCC | p.E746_A750del + T790M | No | Jukna et al[14] |
| 8 | 52/M | Former | IA | ADC | EB | LUL | L858R | Gefitinib | SD | 12 | B | Pleura | SCC | L858R + T790M | No | Ding et al[12] |
| 9 | 53/M | Former | IIIA | SCC | B | NA | T790M | NA | NA | NA | NA | NA | NA | NA | NA | Lai et al[15] |
| 10 | 65/M | Never | IB | SCC | B | NA | T790M | NA | NA | NA | NA | NA | NA | NA | NA | Lai et al[15] |
| 11 | 50/F | Never | IIA | SCC | B | NA | T790M | NA | NA | NA | NA | NA | NA | NA | NA | Lai et al[15] |
| 12 | 71/F | Current | NA | SCC | EB | NA | T790M | NA | NA | NA | NA | NA | NA | NA | NA | Ou et al[16] |
| 13 | 60/F | Current | NA | SCC | EB | NA | T790M | NA | NA | NA | NA | NA | NA | NA | NA | Ou et al[16] |
| 14 | 72/M | Current | NA | SCC | EB | NA | T790M | NA | NA | NA | NA | NA | NA | NA | NA | Ou et al[16] |
| 15 | 63/M | Former | IV | SCC | B | RLL | Exon 19 deletion | Gefitinib/erlotinib | PR / SD3 | 8 | B | RLL | SCC | p.E746_A750del + T790M | Osimertinib | Current article |
In this case of LSCC, we observed a secondary T790M mutation of EGFR, contributing to the acquired resistance to first-generation EGFR inhibitors. This means that T790M is also an important mechanism for acquired resistance in LSCC. However, it is a key issue if this was a pure LSCC or not. Sometimes adenosquamous carcinoma or cancer with a mixture of other components may be mistakenly diagnosed as LSCC. It was reported that tests of multiple biopsies are helpful for accurate pathological and molecular diagnosis[22]. In this study, two biopsies of separate sites at different times and subsequent multiple serial sections were examined. Both of the results supported an identical diagnosis of LSCC with an EGFR exon 19 deletion mutation (Figure 3). Moreover, diagnosis by cytological examination of the bronchus and sputum also supported the LSCC diagnosis. In addition, imaging characteristics and long-term smoking history also supported this diagnosis. There was no evidence of coexistence with other components in multiple biopsies that were collected at multiple time points using multi-detection methods, so we consider this patient to have pure LSCC.
Previous research has suggested that the EGFR pathway in LSCC may be different from that in adenocarcinoma[5]. The PFS of patients receiving first-line gefitinib is about 8 mo. Although it is higher than the median PFS of our previous study, it is still obviously lower than that in adenocarcinoma[9]. Our case suggests that the EGFR pathway may be different between lung adenocarcinoma and LSCC. But it still warrants investigation in large clinical trials.
Previous reports revealed that the threshold of ARMS-PCR was at least 1% for detecting a mutant allele fraction, whereas that for NGS was as low as 0.04%[23]. In this case, the second biopsy specimen was analysed for EGFR mutation by ARMS-PCR and NGS separately; however, the EGFR T790M mutation was only detected by NGS, which was attributed to the higher sensitivity of NGS[24] and lower degradation rate of DNA stored in liquid nitrogen. We foresee that NGS will play a more important role in EGFR T790M detection in the future.
In summary, our findings highlighted that EGFR T790M is also an important mechanism of acquired resistance for LSCC and offered direct evidence of the effectiveness of osimertinib in LSCC patients with the T790M mutation. Novel detection methods, such as NGS and better preservation conditions, hold promise for the more sensitive detection of the EGFR T790M mutation.
The authors are very grateful to Dr. Yi-Xi Chen for helpful comments on language editing, and Jing Zhang from Burning Rock Medical Examination Institute Co., Ltd for her technical help with genomics.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tsuji T S-Editor: Gong ZM L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Gandara DR, Hammerman PS, Sos ML, Lara PN, Hirsch FR. Squamous cell lung cancer: from tumor genomics to cancer therapeutics. Clin Cancer Res. 2015;21:2236-2243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 2. | Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 822] [Cited by in F6Publishing: 861] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 3. | Tang Y, Wang WY, Zheng K, Jiang L, Zou Y, Su XY, Chen J, Zhang WY, Liu WP. EGFR mutations in non-small cell lung cancer: an audit from West China Hospital. Expert Rev Mol Diagn. 2016;16:915-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M, Riely GJ. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240-2247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1585] [Cited by in F6Publishing: 1852] [Article Influence: 168.4] [Reference Citation Analysis (0)] |
| 5. | Liu Y, Zhang Y, Zhang L, Liu B, Wang Y, Zhou X, Li Y, Zhao Q, Gong Y, Zhou L, Zhu J, Ding Z, Wang J, Peng F, Huang M, Li L, Ren L, Lu Y. Efficacy of epidermal growth factor receptor-tyrosine kinase inhibitors for lung squamous carcinomas harboring EGFR mutation: A multicenter study and pooled analysis of published reports. Oncotarget. 2017;8:49680-49688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ, Hughes G, Rahi A, Jacobs VN, Red Brewer M, Ichihara E, Sun J, Jin H, Ballard P, Al-Kadhimi K, Rowlinson R, Klinowska T, Richmond GH, Cantarini M, Kim DW, Ranson MR, Pao W. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046-1061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1265] [Cited by in F6Publishing: 1486] [Article Influence: 148.6] [Reference Citation Analysis (0)] |
| 7. | Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, De Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Muñoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L; Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3670] [Cited by in F6Publishing: 4169] [Article Influence: 347.4] [Reference Citation Analysis (0)] |
| 8. | Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5906] [Cited by in F6Publishing: 6238] [Article Influence: 415.9] [Reference Citation Analysis (0)] |
| 9. | Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Hu C, Luo Y, Chen L, Ye M, Huang J, Zhi X, Zhang Y, Xiu Q, Ma J, Zhang L, You C. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2700] [Cited by in F6Publishing: 3124] [Article Influence: 240.3] [Reference Citation Analysis (0)] |
| 10. | Goss G, Tsai CM, Shepherd FA, Bazhenova L, Lee JS, Chang GC, Crino L, Satouchi M, Chu Q, Hida T, Han JY, Juan O, Dunphy F, Nishio M, Kang JH, Majem M, Mann H, Cantarini M, Ghiorghiu S, Mitsudomi T. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016;17:1643-1652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 463] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 11. | Jänne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, Haggstrom D, Felip E, Kim JH, Frewer P, Cantarini M, Brown KH, Dickinson PA, Ghiorghiu S, Ranson M. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689-1699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1435] [Cited by in F6Publishing: 1562] [Article Influence: 173.6] [Reference Citation Analysis (0)] |
| 12. | Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, Lee CK, Sebastian M, Templeton A, Mann H, Marotti M, Ghiorghiu S, Papadimitrakopoulou VA; AURA3 Investigators. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med. 2017;376:629-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2206] [Cited by in F6Publishing: 2271] [Article Influence: 324.4] [Reference Citation Analysis (0)] |
| 13. | Masago K, Fujita S, Muraki M, Hata A, Okuda C, Otsuka K, Kaji R, Takeshita J, Kato R, Katakami N, Hirata Y. Next-generation sequencing of tyrosine kinase inhibitor-resistant non-small-cell lung cancers in patients harboring epidermal growth factor-activating mutations. BMC Cancer. 2015;15:908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Bugano DDG, Kalhor N, Zhang J, Neskey M, William WN. Squamous-cell transformation in a patient with lung adenocarcinoma receiving erlotinib: Co-occurrence with T790M mutation. Cancer Treat Commun. 2015;4:34-36. [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Ding X, Wang L, Liu X, Sun X, Yu J, Meng X. Genetic characterization drives personalized therapy for early-stage non-small-cell lung cancer (NSCLC) patients and survivors with metachronous second primary tumor (MST): A case report. Medicine (Baltimore). 2017;96:e6221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Graziano P, de Marinis F, Gori B, Gasbarra R, Migliorino R, De Santis S, Pelosi G, Leone A. EGFR-Driven Behavior and Intrapatient T790M Mutation Heterogeneity of Non-Small-Cell Carcinoma With Squamous Histology. J Clin Oncol. 2015;33:e115-e118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Jukna A, Montanari G, Mengoli MC, Cavazza A, Covi M, Barbieri F, Bertolini F, Rossi G. Squamous Cell Carcinoma "Transformation" Concurrent with Secondary T790M Mutation in Resistant EGFR-Mutated Adenocarcinomas. J Thorac Oncol. 2016;11:e49-e51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Lai Y, Zhang Z, Li J, Sun D, Zhou Y, Jiang T, Han Y, Huang L, Zhu Y, Li X, Yan X. EGFR mutations in surgically resected fresh specimens from 697 consecutive Chinese patients with non-small cell lung cancer and their relationships with clinical features. Int J Mol Sci. 2013;14:24549-24559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Oh JE, An CH, Yoo NJ, Lee SH. Detection of low-level EGFR T790M mutation in lung cancer tissues. APMIS. 2011;119:403-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Haratani K, Hayashi H, Watanabe S, Kaneda H, Yoshida T, Takeda M, Shimizu T, Nakagawa K. Two cases of EGFR mutation-positive lung adenocarcinoma that transformed into squamous cell carcinoma: successful treatment of one case with rociletinib. Ann Oncol. 2016;27:200-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Sequist LV, Soria JC, Camidge DR. Update to Rociletinib Data with the RECIST Confirmed Response Rate. N Engl J Med. 2016;374:2296-2297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Paik PK, Varghese AM, Sima CS, Moreira AL, Ladanyi M, Kris MG, Rekhtman N. Response to erlotinib in patients with EGFR mutant advanced non-small cell lung cancers with a squamous or squamous-like component. Mol Cancer Ther. 2012;11:2535-2540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Sorber L, Zwaenepoel K, Deschoolmeester V, Van Schil PE, Van Meerbeeck J, Lardon F, Rolfo C, Pauwels P. Circulating cell-free nucleic acids and platelets as a liquid biopsy in the provision of personalized therapy for lung cancer patients. Lung Cancer. 2017;107:100-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 24. | Tuononen K, Mäki-Nevala S, Sarhadi VK, Wirtanen A, Rönty M, Salmenkivi K, Andrews JM, Telaranta-Keerie AI, Hannula S, Lagström S, Ellonen P, Knuuttila A, Knuutila S. Comparison of targeted next-generation sequencing (NGS) and real-time PCR in the detection of EGFR, KRAS, and BRAF mutations on formalin-fixed, paraffin-embedded tumor material of non-small cell lung carcinoma-superiority of NGS. Genes Chromosomes Cancer. 2013;52:503-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |