Published online Nov 24, 2018. doi: 10.5527/wjn.v7.i7.143
Peer-review started: August 17, 2018
First decision: August 31, 2018
Revised: September 6, 2018
Accepted: October 10, 2018
Article in press: October 10, 2018
Published online: November 24, 2018
Renal artery stenosis is a common cause of secondary hypertension and chronic kidney disease. We present here a case of fibromuscular dysplasia that was treated with surgical revascularization, resulting in recovery of kidney function with eventual cessation of chronic dialysis. The case involves a 25-year-old female with coincidentally discovered hypertension, who underwent further investigations revealing a diagnosis of renal artery stenosis due to fibromuscular dysplasia. She subsequently developed two episodes of malignant hypertension, with flash pulmonary oedema and worsening renal failure that resulted in dialysis dependence. After evidence was obtained that the right kidney was still viable, a revascularization procedure was performed, improving blood pressure control and restoring kidney function, thereby allowing dialysis to be stopped. This case highlights the importance of evaluating patients with renal artery stenosis for revascularization before committing them to a life of chronic dialysis.
Core tip: Renal failure requiring dialysis support is a rare complication of renal artery stenosis due to fibromuscular dysplasia. We present a 25-year-old woman with fibromuscular dysplasia who developed dialysis dependence following acute loss of kidney function after suspected renal arterial dissection. Surgical revascularization resulted in dialysis cessation and improved blood pressure control. This case illustrates that in well-selected dialysis-dependent patients with renal artery stenosis secondary to fibromuscular dysplasia, surgical revascularization may not only improve the control of blood pressure but also restore enough kidney function for dialysis cessation.
- Citation: Chothia MY, Davids MR, Bhikoo R. Awakening the sleeping kidney in a dialysis-dependent patient with fibromuscular dysplasia: A case report and review of literature. World J Nephrol 2018; 7(7): 143-147
- URL: https://www.wjgnet.com/2220-6124/full/v7/i7/143.htm
- DOI: https://dx.doi.org/10.5527/wjn.v7.i7.143
Renal artery stenosis (RAS) is a common cause of secondary hypertension and chronic kidney disease. The most common cause of RAS is atherosclerosis, while fibromuscular dysplasia (FMD) is regarded as a rare aetiology. The latter is regarded as a non-atherosclerotic, non-inflammatory vascular disease, most frequently affecting the renal arteries (60%-75%) followed by the carotid arteries (25%-30%); however, many other vascular beds have been described[1].
The pathological classification of FMD is based on the arterial layer primarily affected. The most common pathological type is medial fibroplasia, which has a characteristic “string-of-beads” appearance on renal angiography. The cause of FMD remains unknown but may have a genetic component since the disease tends to affect first-degree relatives of affected individuals[2]. The disease is frequently asymptomatic and may only be identified coincidentally. Studies have reported that FMD represents < 10% of cases of renovascular hypertension[3]. Even more uncommon is renal failure due to FMD, despite studies reporting that up to 63% of patients have loss of kidney volume[4].
We present herein a case of FMD that resulted in severe dialysis-dependent renal failure, where there was recovery of kidney function following revascularization and eventual cessation of chronic dialysis.
A 25-year-old female was seen in August 2015 at our medical outpatient clinic after hypertension was diagnosed following surgery for a distal tibia-fibula fracture. During hospitalisation, the patient’s systolic and diastolic blood pressure (BP) was noted to be in the range of 180-225 mmHg and 110-130 mmHg, respectively, despite anti-hypertensive therapy that included hydrochlorothiazide 25 mg daily and amlodipine 10 mg daily.
The patient showed no symptoms related to her hypertension. On clinical examination, she had a normal body habitus with a body mass index of 23 kg/m2 and no syndromic features for an endocrine cause of hypertension. All pulses were palpable and equal in volume, with no radio-femoral delay. There were no differences in BP between the right and left upper limbs, or between the upper and lower limbs. No renal or carotid arterial bruits were audible. There was a prominent, pressure- loaded apical impulse. Her serum creatinine concentration was 67 µmol/L.
Since parenchymal kidney disease and renovascular disease are the two most common causes of secondary hypertension, a radiological examination of these systems was performed. Ultrasound measurements of the patient’s left and right kidney showed a discrepancy in sizes of 72 mm and 117 mm, respectively. Resistive index could not be determined for the left kidney, due to very poor perfusion; however, the right kidney had normal perfusion, with a resistive index of 60%. Computed tomography angiography (CTA) revealed normal cortico-medullary enhancement in the right kidney, while the left kidney had cortical infarcts and fibrous tissue that encased the left renal artery, originating approximately 8 mm from the ostium up to the level of the renal hilum with near-complete occlusion. The right renal artery had nearly 50% stenosis that originated 17 mm from the ostium. The rest of the aorta and its branches were otherwise normal in configuration.
A diagnosis of FMD was made, since the CTA was most consistent with this condition (as opposed to Takayasu’s arteritis). The lumina of the aorta and its branches were not narrowed and had a smooth wall, and no post-stenotic dilatations were noted. Also, the origins of the stenosis in the renal arteries were not at the ostia but rather at the mid-vessel level.
During this time, the patient’s serum creatinine had increased slightly to 87 µmol/L and she developed resistant hypertension. Her antihypertensive regimen included atenolol at 50 mg daily, furosemide at 160 mg daily, amlodipine at 10 mg daily and minoxidil 5 mg daily. Antagonists of the renin-angiotensin system were avoided due to the concern of effects that this class of drugs may have on renal function.
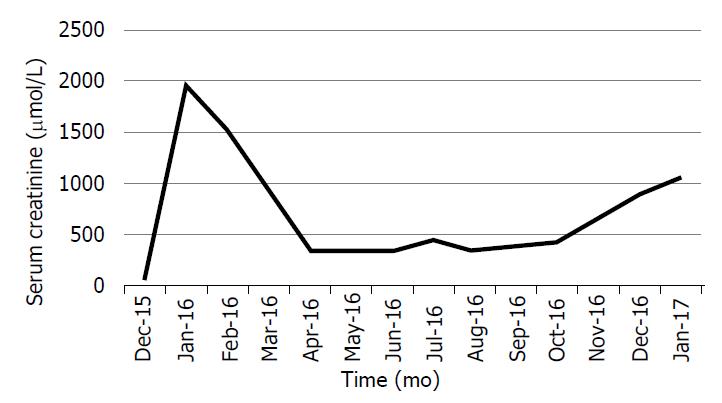
In January 2016, the patient presented with malignant hypertension, flash pulmonary oedema and a serum creatinine level that had risen to 1807 µmol/L. Despite this, her urine volumes ranged from 700 mL to 1000 mL daily. Repeat ultrasound showed that the right kidney size was now 92 mm and that the resistive index had increased to 70%. A diuresis renogram revealed a differential function of 80% in the right kidney and 20% in the left, with poor global function. The patient was initiated on haemodialysis and her serum creatinine improved to 650 µmol/L. Dialysis was stopped. However, 3 wk later, she re-presented with a second episode of malignant hypertension and flash pulmonary oedema and her serum creatinine had again increased to 1509 µmol/L. Haemodialysis was then re-initiated.
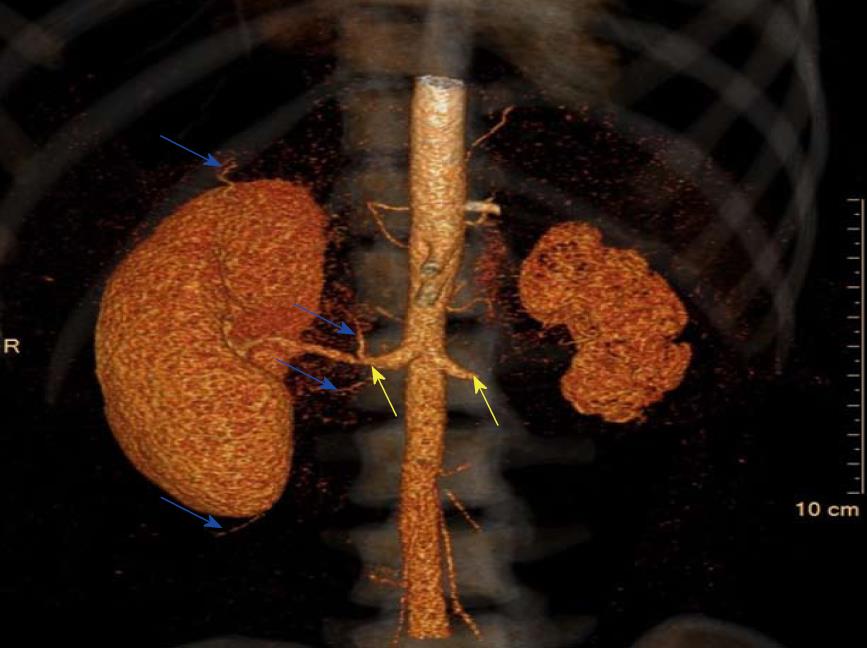
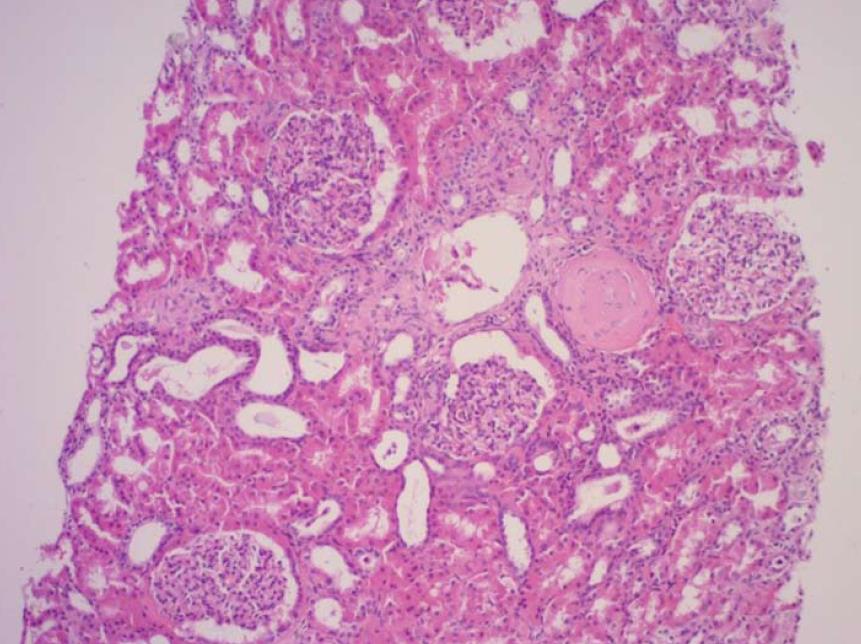
Notably, the patient’s urine volumes throughout this entire period remained at nearly 1000 mL daily, which was an indication of ongoing right kidney perfusion, possibly via collateral blood vessels. This was confirmed on a repeat CTA (Figure 1). A kidney biopsy of the right kidney was performed and revealed viable, normal- appearing tissue (Figure 2).
Due to the abrupt loss of kidney function, arterial dissection and/or thrombosis involving the right renal artery was suspected. Because of limited experience with the endovascular treatment of complicated renal arterial lesions at our centre, a decision was made to attempt primary surgical revascularization. Due to the negligible contribution to overall kidney function, no interventions were planned for the left kidney.
In April 2016, the patient underwent ex vivo reconstruction of the right renal artery using a saphenous venous graft from the left leg. A long segment of arterial dissection up to the branch vessels was identified. The occluded arterial segment was resected, and the venous graft was interposed with end-to-side anastomoses. Post- operative Doppler ultrasound showed that the kidney size had increased to 132 mm and excellent renal perfusion was noted.
Approximately 1 wk post-operatively, the patient complained of right flank pain. An ultrasound examination revealed a right perinephric haematoma and suspected kinking of the ureter, causing hydronephrosis. A pigtail catheter was placed to drain the haematoma, and a double-J-stent was inserted to relieve the obstruction. There was a dramatic improvement of both kidney function and BP control. Haemodialysis was tapered and eventually discontinued. Her serum creatinine settled at 350 µmol/L, nearly 2 mo following surgery. BP control was achieved with a single agent. The patient remained dialysis-free for 9 mo before her chronic kidney disease progressed and she eventually returned to chronic dialysis (Figure 3).
The “sleeping” kidney is a term used to describe a non-functioning but viable kidney, usually due to RAS. Most of the reported cases have involved those with atherosclerotic RAS, with fewer cases involving FMD.
The main blood supply to the kidneys is via the renal arteries; however, they also have a rich collateral blood supply. These pre-formed collateral vessels originate from branches of the renal capsular, peri-pelvic and peri-ureteric systems[5]. We suspect that our patient must have had an extensive collateral blood supply to the right kidney since she maintained good urine volumes of up to 1000 mL per day despite the demonstration on CTA of extremely poor perfusion via the main right renal artery.
Renal arterial dissection may cause renal infarction and subsequent chronic kidney disease; however, FMD as a cause of chronic kidney disease is very uncommon and cases that progress to end-stage kidney disease are rare[6,7]. A large registry of FMD patients showed that out of a total of 447 vascular events, 4.3% of patients had renal arterial dissection and that only 1.6% and 0.9% of patients had renal failure and renal infarction, respectively[7]. In our case, renal arterial dissection and/or thrombosis was suspected as a cause of acute kidney injury due to the unexplained, abrupt loss of kidney function.
The indications for renal revascularization, as recommended by the American Heart Association (commonly known as the AHA), include resistant hypertension, new-onset hypertension, branch disease, dissection and aneurysm, and the preservation of kidney function[7]. Regarding the latter, the AHA recommends revascularization when there is progressive decline in kidney function and/or reduction in kidney mass. We decided to offer our patient a revascularization procedure because she had resistant hypertension that was complicated by episodes of malignant hypertension and flash pulmonary oedema. Also, the on-going urine production despite the negligible perfusion seen on radioisotope renogram and CTA suggested a potentially viable right kidney.
There are no guidelines regarding revascularization of RAS in dialysis-dependent patients. A study that investigated 15 hypertensive patients with non-functioning kidneys due to either complete or segmental renal arterial occlusion found that the presence of viable glomeruli on kidney biopsy, angiographic evidence of collateral blood supply, and the presence of a patent distal renal artery were associated with successful revascularization and salvage of non-functioning kidneys[8]. In addition, others have recommended kidney length of at least 90 mm and renal vein renin sampling as an additional measure of nephron viability[9-11]. Our case demonstrated three of these criteria: the right kidney size being 92 mm; detection of collateral perfusion on CTA as well as on Doppler ultrasound; and, kidney biopsy showing normal glomeruli without tubular atrophy or significant interstitial fibrosis.
Guidelines recommend percutaneous transluminal angioplasty (PTA) as the primary revascularization procedure for renal FMD, with surgery as a secondary procedure if PTA is unsuccessful[7]. However, these studies predominantly focussed on the effect of PTA on the rates of hypertension cure and/or control. Evidence for PTA to restore or preserve renal function is less robust, with many studies, albeit their representing a small number, reporting unclear outcomes with none of the patients established on dialysis[12-18]. One study demonstrated that 12 of 14 patients had improved renal function following PTA, with a mean serum creatinine concentration of 212 µmol/L at baseline that improved to 150 µmol/L after a mean follow-up period of 33 mo[19]. Again, none of these patients were dialysis-dependent. There have been few documented case reports of successful renal revascularization in dialysis- dependent patients. In a case of presumed FMD that was dialysis-dependent for 6 mo, the patient became dialysis-independent following surgical correction that involved a spleno-renal bypass procedure[20].
In our case, the decision to surgically repair the right renal artery instead of attempting PTA was largely influenced by the abrupt loss of kidney function, which suggested arterial dissection and/or thrombosis. Also, the lack of local experience with regards to renal arterial stenting was an additional factor that influenced our decision. Because our patient was dialysis-dependent and had a potentially salvageable solitary kidney, we believed that surgical repair would offer the greatest chance for successful revascularization.
The outcomes of revascularization in dialysis-dependent patients are not well known. In the case mentioned above, the patient remained dialysis-free for 17 mo, with a serum creatinine of 203 µmol/L. In a study that included 6 patients with FMD and required a secondary revascularization procedure after a failed primary procedure, all cases were dialysis-free after an average follow-up of 58 mo[21]; however, none of those patients were dialysis-dependent prior to surgery. The initial indication for revascularization in all cases was for BP control rather than improvement of kidney function. Our patient had improvement of both hypertension as well as kidney function but eventually returned to dialysis after 9 mo.
In conclusion, the case presented herein illustrates that in well-selected dialysis-dependent patients with RAS, revascularization may not only improve BP control but also restore kidney function. Patients with RAS should be evaluated for revascularization before fully committing them to a life of chronic dialysis.
We report the case of a young woman with renal artery stenosis (RAS) due to fibromuscular dysplasia who became dialysis dependent following arterial dissection; after surgical revascularization, the patient was able to stop dialysis.
Renal artery stenosis secondary to fibromuscular dysplasia complicated by arterial dissection and subsequent dialysis dependence.
Renal artery stenosis secondary to Takayasu’s arteritis.
Three-dimensional computed tomography reconstruction indicating a rich collateral renal blood supply and confirming the origins and extent of the renal artery stenosis.
Kidney biopsy confirming viable tissue.
Surgical revascularization by ex vivo repair of the renal artery using a saphenous venous graft.
The paper by Libertino et al is important for readers to appreciate how to identify those patients that are likely to have a good response to revascularization.
The “sleeping” kidney refers to a non-functional but potentially viable kidney that may recover function following revascularization.
Patients with RAS should be evaluated for revascularisation before fully committing them to a life of chronic dialysis.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Urology and nephrology
Country of origin: South Africa
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Friedman EA, Mahmoud KM, Robles NR, Yong D S- Editor: Ma RY L- Editor: A E- Editor: Wu YXJ
| 1. | Slovut DP, Olin JW. Fibromuscular dysplasia. N Engl J Med. 2004;350:1862-1871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 628] [Cited by in F6Publishing: 478] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 2. | Pannier-Moreau I, Grimbert P, Fiquet-Kempf B, Vuagnat A, Jeunemaitre X, Corvol P, Plouin PF. Possible familial origin of multifocal renal artery fibromuscular dysplasia. J Hypertens. 1997;15:1797-1801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 111] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 750] [Cited by in F6Publishing: 610] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 4. | Goncharenko V, Gerlock AJ Jr, Shaff MI, Hollifield JW. Progression of renal artery fibromuscular dysplasia in 42 patients as seen on angiography. Radiology. 1981;139:45-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 102] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Love L, Bush IM. Early demonstration of renal collateral arterial supply. Am J Roentgenol Radium Ther Nucl Med. 1968;104:296-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Kadian-Dodov D, Gornik HL, Gu X, Froehlich J, Bacharach JM, Chi YW, Gray BH, Jaff MR, Kim ES, Mace P. Dissection and Aneurysm in Patients With Fibromuscular Dysplasia: Findings From the U.S. Registry for FMD. J Am Coll Cardiol. 2016;68:176-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 7. | Olin JW, Gornik HL, Bacharach JM, Biller J, Fine LJ, Gray BH, Gray WA, Gupta R, Hamburg NM, Katzen BT. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation. 2014;129:1048-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 280] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 8. | Libertino JA, Zinman L, Breslin DJ, Swinton NW Jr, Legg MA. Renal artery revascularization. Restoration of renal function. JAMA. 1980;244:1340-1342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Schefft P, Novick AC, Stewart BH, Straffon RA. Renal revascularization in patients with total occlusion of the renal artery. J Urol. 1980;124:184-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Lawrie GM, Morris GC Jr, DeBakey ME. Long-term results of treatment of the totally occluded renal artery in forty patients with renovascular hypertension. Surgery. 1980;88:753-759. [PubMed] [Cited in This Article: ] |
| 11. | Whitehouse WM Jr, Kazmers A, Zelenock GB, Erlandson EE, Cronenwett JL, Lindenauer SM, Stanley JC. Chronic total renal artery occlusion: effects of treatment on secondary hypertension and renal function. Surgery. 1981;89:753-763. [PubMed] [Cited in This Article: ] |
| 12. | Trinquart L, Mounier-Vehier C, Sapoval M, Gagnon N, Plouin PF. Efficacy of revascularization for renal artery stenosis caused by fibromuscular dysplasia: a systematic review and meta-analysis. Hypertension. 2010;56:525-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Millan VG, McCauley J, Kopelman RI, Madias NE. Percutaneous transluminal renal angioplasty in nonatherosclerotic renovascular hypertension. Long-term results. Hypertension. 1985;7:668-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Bell GM, Reid J, Buist TA. Percutaneous transluminal angioplasty improves blood pressure and renal function in renovascular hypertension. Q J Med. 1987;63:393-403. [PubMed] [Cited in This Article: ] |
| 15. | Greminger P, Steiner A, Schneider E, Kuhlmann U, Steurer J, Siegenthaler W, Vetter W. Cure and improvement of renovascular hypertension after percutaneous transluminal angioplasty of renal artery stenosis. Nephron. 1989;51:362-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Bonelli FS, McKusick MA, Textor SC, Kos PB, Stanson AW, Johnson CM, Sheedy PF 2nd, Welch TJ, Schirger A. Renal artery angioplasty: technical results and clinical outcome in 320 patients. Mayo Clin Proc. 1995;70:1041-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 153] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Rodríguez Pérez JC, Maynar Moliner M, Pérez Borges P, Plaza Toledano C, Reyes Pérez R, Pulido Duque JM, Palop Cubillo L, Rodríguez Pérez A. [The long-term results on arterial pressure and kidney function after the percutaneous transluminal dilatation of renal artery stenosis]. Med Clin (Barc). 1997;108:366-372. [PubMed] [Cited in This Article: ] |
| 18. | Mounier-Vehier C, Lions C, Jaboureck O, Devos P, Haulon S, Wibaux M, Carré A, Beregi JP. Parenchymal consequences of fibromuscular dysplasia renal artery stenosis. Am J Kidney Dis. 2002;40:1138-1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Tegtmeyer CJ, Selby JB, Hartwell GD, Ayers C, Tegtmeyer V. Results and complications of angioplasty in fibromuscular disease. Circulation. 1991;83:I155-I161. [PubMed] [Cited in This Article: ] |
| 20. | Agraharkar M, Nair V, Patlovany M. Recovery of renal function in dialysis patients. BMC Nephrol. 2003;4:9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Hansen KJ, Deitch JS, Oskin TC, Ligush J Jr, Craven TE, Dean RH. Renal artery repair: consequence of operative failures. Ann Surg. 1998;227:678-689; discussion 689-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |