Peer-review started: March 25, 2018
First decision: April 10, 2018
Revised: July 11, 2018
Accepted: August 11, 2018
Article in press: August 11, 2018
Published online: September 7, 2018
To evaluate the novel platelet-derived growth factor receptor and vascular endothelial growth factor receptor dual kinase inhibitor ANG3070 in a polycystic kidney disease-congenital hepatic fibrosis model.
At 6 wk of age, PCK rats were randomized to vehicle or ANG3070 for 4 wk. At 10 wk, 24 h urine and left kidneys were collected and rats were continued on treatment for 4 wk. At 14 wk, 24 h urine was collected, rats were sacrificed, and liver and right kidneys were collected for histological evaluation. For Western blot studies, PCK rats were treated with vehicle or ANG3070 for 7 d and sacrificed approximately 30 min after the last treatments.
Compared to the wild-type cohort, the PCK kidney (Vehicle cohort) exhibited a marked increase in kidney and liver mass, hepato-renal cystic volume, hepato-renal fibrosis and hepato-renal injury biomarkers. Intervention with ANG3070 in PCK rats decreased kidney weight, reduced renal cystic volume and reduced total kidney hydroxyproline, indicating significantly reduced rental interstitial fibrosis compared to the PCK-Vehicle cohort. ANG3070 treatment also mitigated several markers of kidney injury, including urinary neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, cystatin C and interleukin-18 levels. In addition, this treatment attenuated key indices of renal dysfunction, including proteinuria, albuminuria and serum blood urea nitrogen and creatinine, and significantly improved renal function compared to the PCK-Vehicle cohort. ANG3070 treatment also significantly decreased liver enlargement, hepatic lesions, and liver fibrosis, and mitigated liver dysfunction compared to the PCK-Vehicle cohort.
These results suggest that ANG3070 has the potential to slow disease, and may serve as a bridge toward hepato-renal transplantation in patients with fibropolycystic disease.
Core tip: In autosomal recessive polycystic kidney disease (ARPKD)-congenital hepatic fibrosis (CHF), a genetically acquired and congenital disease, approximately 20-30% of affected patients succumb within the first 1-2 mo of life, with pulmonary insufficiency secondary to renal enlargement as the primary cause of death. For children, nephrectomy and dialysis or kidney liver transplant is often warranted by approximately ten years of age. Other than transplantation, there is no cure for ARPKD-CHF. We report that platelet-derived growth factor and vascular endothelial growth factor are the intermediaries between the cystic and fibrotic components of progressive fibropolycystic disease and ANG3070, a novel dual kinase inhibitor therapy that may serve as an interesting bridge toward hepato-renal transplantation in patients with ARPKD-CHF.
- Citation: Paka P, Huang B, Duan B, Li JS, Zhou P, Paka L, Yamin MA, Friedman SL, Goldberg ID, Narayan P. A small molecule fibrokinase inhibitor in a model of fibropolycystic hepatorenal disease. World J Nephrol 2018; 7(5): 96-107
- URL: https://www.wjgnet.com/2220-6124/full/v7/i5/96.htm
- DOI: https://dx.doi.org/10.5527/wjn.v7.i5.96
The highly aggressive fibropolycystic disease, autosomal recessive polycystic kidney disease (ARPKD) - congenital hepatic fibrosis (CHF), is characterized by the formation and expansion of fluid-filled cysts in the kidneys, enlargement of the kidneys and progressive fibrosis of both the kidney and the liver[1,2]. Caroli’s disease, which manifests as cystic dilatation of the intrahepatic ducts, often accompanies ARPKD-CHF[3]. Afflicted children that survive past two years of age more often than not require renal and/or hepatic transplantation by age ten. The need for transplantation is driven as much by progressive organ dysfunction as by significant enlargement of the diseased organ(s) accompanied by severe pain[4].
Aberrant signaling by tyrosine kinases, including platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) and their receptors (R), PDGFR and VEGFR/KDR, respectively, has been implicated in the formation and expansion of renal cysts. A PDGF-driven ciliopathy and/or overexpression of PDGF in the cyst lining and adjacent tubules are thought to, in part, drive renal cystic disease[5-7]. Cowley et al[8] posited that elevated and abnormal c-myc proto-oncogene expression drives ARPKD; c-myc expression is controlled by PDGF[9].
VEGF-driven angiogenesis is also thought to contribute to the growth of renal cysts, and inhibition of VEGFR/KDR signaling is associated with decreased tubule cell proliferation, decreased cystogenesis, and blunted renal enlargement[10,11]. Nevertheless, the role of VEGF in fibropolycystic disease is more controversial, with at least two reports suggesting that this growth factor might be associated with disease mitigation[12]. Aside from their roles in renal cyst formation and expansion, it is being recognized in ARPKD-CHF that aberrant PDGF and VEGF signaling are also associated with extracellular matrix deposition in the liver and kidney[13-15].
The PCK rat model is a well-established and well-characterized model that resembles human polycystic kidney and liver disease[16]. In the present study, we employed the PCK rat model to evaluate the therapeutic effects of ANG3070 on hepato-renal fibropolycystic disease. ANG3070 is an orally bioavailable, highly water-soluble, small molecule PDGFR and VEGFR/KDR inhibitor that binds its target receptors with nanomolar affinity but exhibits limited interaction with other receptor tyrosine kinases as described by Panicker et al[17] and Narayan et al[18].
All studies relating to animals were approved by the Angion Biomedica animal use and care committee. Four week old male PCK/CrljCrl-Pkhd1pck/Crl rats and age-matched male Sprague-Dawley (wild-type) rats were purchased from Charles River Labs (Wilmington, MA) and acclimatized for a week with a standard laboratory diet and water ad libitum at Angion prior to starting experiments. The PCK rat model includes ARPKD and autosomal dominant polycystic kidney disease (ADPKD). Many of the biochemical and morphological changes using these PCK rat models closely resemble human hepato-renal fibro polycystic disease[16]. We will use the best-characterized PCK rat model to establish therapeutic efficacy and the time window of our novel dual kinase inhibitor, ANG3070. PCK rats exhibit renal pathology starting from 4 wk of age with continuous progression of hepato-renal fibropolycystic disease with aging[16,17].
Upon consultation with a biostatistician and based on formal power analysis, we expect a 30-50% reduction in hepato-renal injury and pathology with ANG3070 treatment vs vehicle cohort, with a 30% standard deviation in each group and 80% power required to observe P < 0.05. Ten to 14 PCK rats were required for each group.
The total rats used in three separate experiments = approximately 18 wild type SD rats and 68 PCK rats. Some animals (PCK, n = 2; wild-type, n = 4) were sacrificed at 6 wk of age to confirm disease pathology. The PCK rats were then randomized to vehicle (water, n = 14) or ANG3070 (25 mg/kg, PO, BID; n = 14). Drug dose and dosing schedule was based on data (not shown) from previous studies in models of chronic kidney disease. At 10 wk of age
(i.e., after 4 wk of drug treatment), 24 h urine was collected, animals were anesthetized with isoflurane (2%), a midline incision was made and the left kidney was removed for analysis. Animals were then returned to their cages and allowed to recover. At 14 wk of age (i.e., after 8 wk of drug dosing), 24 h urine was collected, animals were anesthetized, and the right kidney and liver were removed. For Western blot studies, 10 wk old male PCK rats were treated with vehicle or ANG3070 (25 mg/kg, PO, BID) for 7 d and sacrificed approximately 30 min after the last vehicle/drug administration. Kidneys were collected and stored in formalin and liquid N2.
Cystic index (i.e., the percentage of renal parenchyma occupied by cysts) was quantified using two independent blinded observers in hematoxylin and eosin (H and E) - stained kidney sections using digital planimetry (NIS Elements Viewer) as described previously[18]. The data from the two observers were averaged for each kidney.
Body weight, kidney and liver masses were determined. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN) and serum creatinine (SCr) were measured by Northwell laboratory (New Hyde Park, NY). Proteinuria was measured using a modified Bradford and Lowry Bio-Rad protein assay and expressed as mg/24 h urine. Microalbuminuria (Abcam ELISA) was expressed as μg/24 h urine. Levels of neutrophil gelatinase-associated lipocalin (NGAL) (BioPorto Diagnostics, http://www.bioporto.com), cystatin C (R and D Systems, http://www.rndsystems.com), interleukin-18 (IL-18) (Biomedical Assay, http://www.biotechist.com) and kidney injury molecule-1 (KIM-1) (BioTrend, http://www.biotrend.com) were determined in urine samples using an enzyme-linked immunosorbent assay. Kidney and liver hydroxyproline, markers of tissue fibrosis, were measured from tissue homogenates[19] and expressed as μg/kidney or μg/liver.
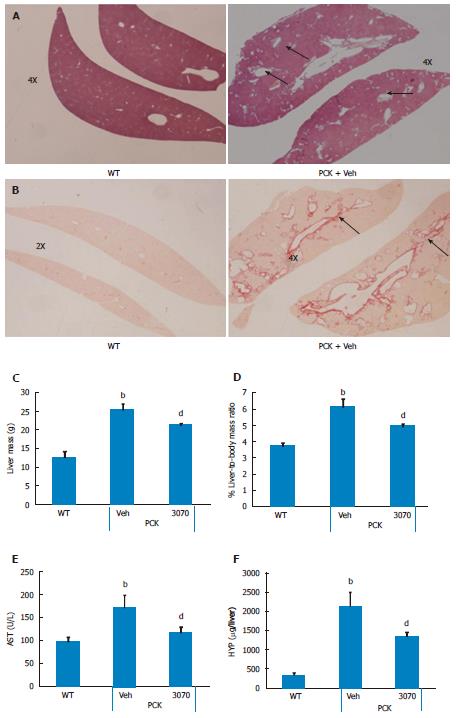
Formalin-fixed kidney and liver sections from PCK and/or wild-type rats were stained with Masson’s trichrome or Picrosirius red to visualize collagen deposition in these tissues. The presence of multiple large and small cysts in the kidney and highly dilated irregular-shaped ducts in the liver, characteristic of this model of fibropolycystic kidney and liver disease, made it difficult to quantify fibrosis using histochemical stains. These stains therefore acted instead as a visual aid for the presence and location of matrix deposition in this model. Kidneys were also stained with anti-phospho PDGFR antibody (Antibody #3161, Cell Signaling) conjugated to horseradish peroxidase to visualize the presence and location of the activated receptor in this model of renal disease.
As described by Takikita-Suzuki et al[20], the frozen kidney tissue was homogenized on ice in buffer containing Tris-HCl (20 mmol/L; pH 7.5), ethylenediamine tetraacetic acid (1 mmol/L), NaCl (140 mmol/L), Nonidet P-40 (1%), aprotinin (50 μg/mL), NaF (50 mmol/L), sodium orthovanadate (1 mmol/L) and phenylmethyl sulfonyl fluoride (1 mmol/L). Homogenized tissue was centrifuged for 30 min at 15000 rpm at 4 °C. Supernatant was collected and protein concentrations were measured using the method of Bradford. After 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the protein was transferred to a nylon membrane (Amersham Pharmacia Biotech, Buckinghamshire, England, United Kingdom). Blocking was performed for 2 h in phosphate-buffered saline (PBS) solution containing 5% (w/v) skim milk, followed with incubation with anti-α smooth muscle actin (α-SMA; Abcam) or anti-pPDGFRβ (Antibody #3161, Cell Signaling) antibodies, followed by an appropriate HRP-conjugated secondary antibody (Cell Signaling). Proteins were detected using an enhanced chemiluminescence kit (Amersham/GE Healthcare, United Kingdom). Densitometric analysis for pPDGFRβ was performed with normalization to GAPDH.
Data are presented as mean ± SD. Between-group effects were analyzed by one-way analysis of variance followed by Tukey’s post-hoc test. A P-value < 0.05 is considered significant.
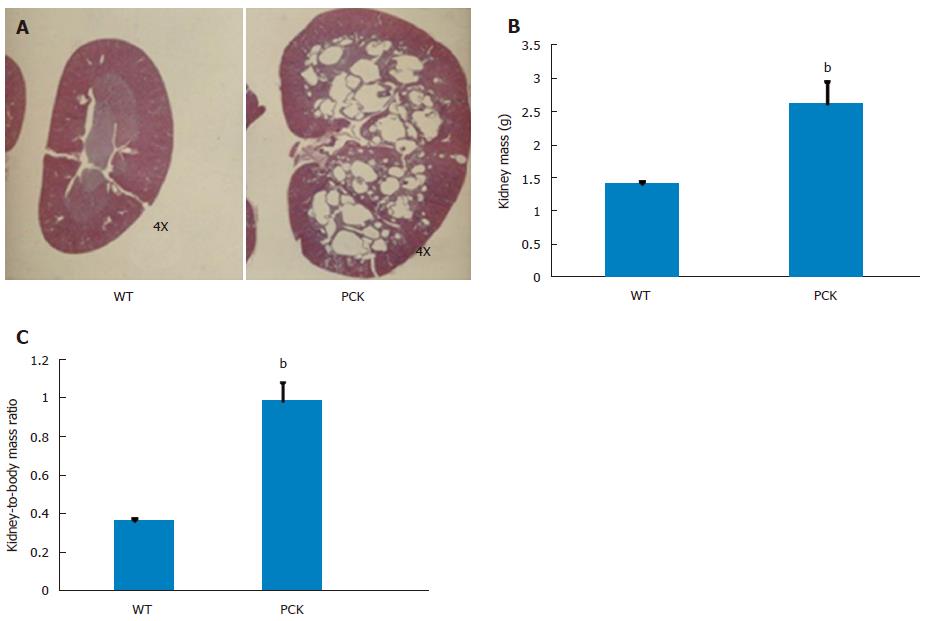
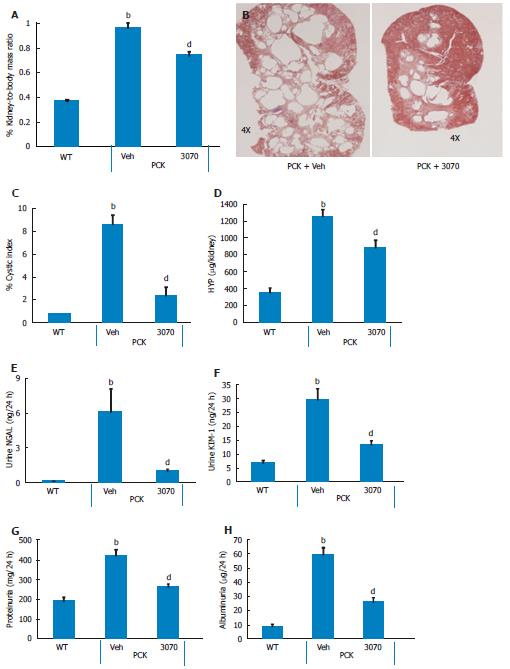
Four-week treatment study: By approximately 6 wk of age, kidneys from PCK rats were enlarged and filled with numerous cysts (Figure 1). Both renal mass and renal-to-body mass ratio in PCK rats were significantly greater compared to the wild-type, age-matched cohort (Figure 1). By 10 wk of age, PCK rats (treated with vehicle, i.e., 0.5 mL water, PO, BID) exhibited significant renomegaly compared to the wild-type cohort. Intervention with ANG3070 from weeks 6-10 was associated with a reduction in renal mass, renal-to-body mass ratio and renal cystic index (Figure 2). Compared to kidneys from wild-type animals, kidneys from PCK rats exhibited increased fibrosis, evidenced by increased tissue hydroxyproline content with ANG3070 treatment of the PCK rat associated with a reduction in renal hydroxyproline content (Figure 2). In addition to its effects on renal morphology, ANG3070 therapy was associated with the mitigation of kidney injury demonstrated by a reduction in 24-h urine NGAL and urine KIM-1 and amelioration of renal dysfunction, evidenced by reduced proteinuria and reduced albuminuria (Figure 2).
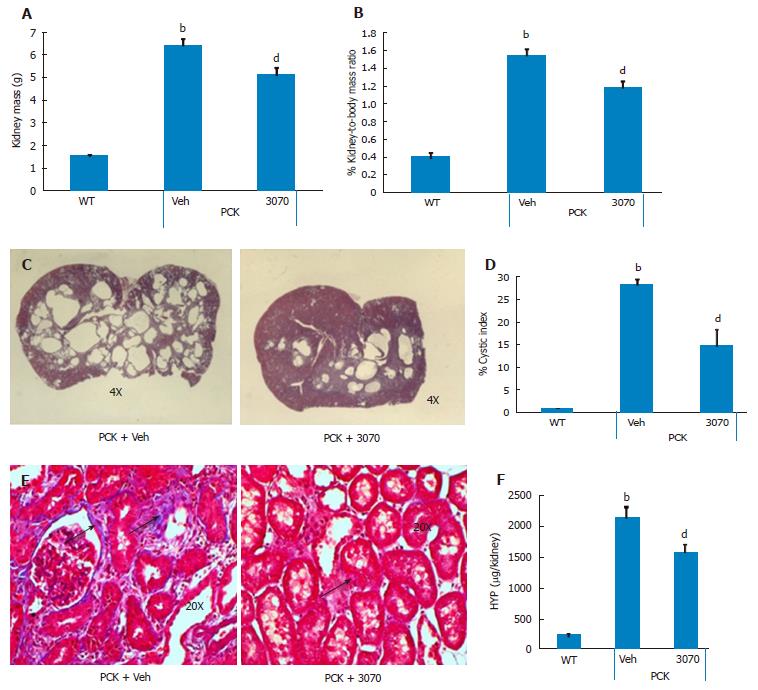
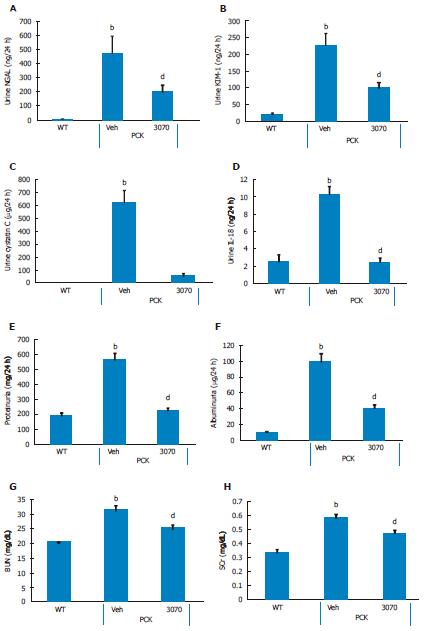
Eight-week treatment study: The effects of 8 wk of ANG3070 treatment were obvious in both kidneys and livers harvested from 14 wk old animals. At this time, the average kidney mass in the PCK rat (vehicle cohort) was several fold that of its wild-type counterpart and cystic index was approximately 30%. Nevertheless, a reduction in renal mass, renal-to-body mass ratio and cystic index was observed with drug treatment. At 14 wk of age, in addition to severe renal cyst formation, kidneys from PCK rats exhibited interstitial fibrosis evidenced by trichrome staining. The dominating presence of large cysts made analysis of the trichrome-stained area impractical, and the extent of renal extracellular matrix deposition was therefore estimated biochemically in renal homogenates. Compared to the wild-type cohort, the PCK kidney (vehicle cohort) exhibited a marked increase in hydroxyproline content. Intervention with ANG3070 reduced renal fibrosis, evidenced by a decrease in total PCK kidney hydroxyproline content (Figure 3). Equally important, treatment with drug reduced renal injury, as evidenced by decreased 24-h urine - NGAL, KIM-1, cystatin C and IL-18 levels and attenuated key indices of renal dysfunction including proteinuria, albuminuria, BUN and SCr (Figure 4).
ANG3070 mitigates liver lesions: In addition to fibropolycystic kidney disease, PCK rats exhibit features of CHF and Caroli’s disease, including hepatomegaly and ductal fibrosis with highly dilated intrahepatic ducts. Upon sacrifice (i.e., approximately 14 wk of age), these characteristics of CHF and Caroli’s disease were seen in PCK rats. Livers showed dilated intrahepatic ducts which were surrounded by matrix deposition, visible using H and E and Picrosirius red stains (Figure 5). Furthermore, frank hepatomegaly was evident in the PCK (vehicle) cohort. Although there was no increase in ALT (PCK vs wild-type, data not shown), AST was elevated. Treatment with ANG3070 reduced liver mass, liver-to-body mass ratio, AST and total liver hydroxyproline content (Figure 5).
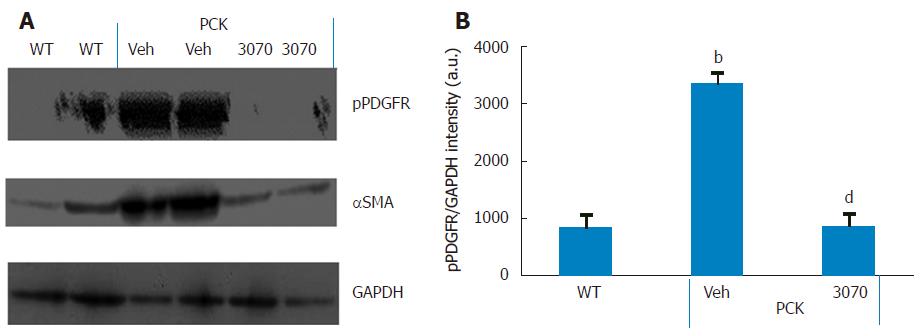
ANG3070 pharmacodynamics: It has been reported that the cystic milieu is enriched in growth factors such as PDGF[20]. The presence of multiple large and extensive cysts made immuno-histochemical quantification of pPDGFR impractical. Therefore, to determine whether the salutary effects of ANG3070 are associated with inhibition of this target, we evaluated the levels of pPDGFR together with the fibrotic marker α-SMA in renal homogenates from wild-type and vehicle- or ANG3070-treated PCK rats. As seen in Figure 6, compared to kidneys from wild-type animals, kidneys from the vehicle-treated PCK animals exhibited increased levels of both pPDGFR and α-SMA. In comparison to the PCK + Veh cohort, kidneys from the PCK + ANG3070 cohort exhibited decreased pPDGFR and α-SMA levels. Densitometric analysis of Western blots of renal homogenates from wild-type vs vehicle-treated PCK rats exhibited increased pPDGFR intensity, an effect that was reduced with ANG3070 treatment (Figure 6).
ANG3070 safety/toxicology profile: ANG3070 was efficacious and no potential toxic effects were observed in several rodent models. As detailed below, to date, there is no evidence of toxicity (liver or renal) in mice or rats dosed repeatedly over several weeks with ANG3070. In hundreds of mice and rats dosed several weeks with 150 mg/kg ANG3070 (PO, QD), there were no excursions in BUN (Veh 73 mg/dL; ANG3070 69 mg/dL) or SCr (veh 0.36 mg/dL; ANG3070 0.35 mg/dL) with ANG3070. In other rats dosed for 3 wk with ANG3070 (25 mg/kg, PO, BID × 3 wk), no excursions were seen in liver enzymes vs vehicle-dosed rats (ALT - veh: 92 IU; ANG3070: 61 IU; AST - Veh 158 IU; ANG3070 136 IU). There were no adverse events reported in 14 d toxicology studies in rats and dogs at nine-fold higher doses (450 mg/d) (data not shown) than the efficaceous dose of ANG3070 (50 mg/d) at which antifibrotic efficacy was observed in fibropolycystic kidney disease-CHF. These studies indicate that ANG3070 was safe and well-tolerated without any potential toxic effects. In PCK rats, treatment of ANG3070 for several weeks did not increase sCR, BUN, AST and ALT. In fact, sCR and AST were reduced with 3070 treatment. Overall ANG3070 was efficacious in decreasing kidney and liver fibrosis with no potential toxic effects in other organs.
We herein report that intervention with the orally bioavailable small molecule PAGFR and VEGFR dual kinase inhibitor ANG3070 ameliorates fibropolycystic disease progression in the PCK rat model of ARPKD-CHF. Intervention with this drug mitigated renomegaly, renal injury and renal dysfunction and fibrosis, effects associated with reduced renal PDGFR phosphorylation. Treatment with ANG3070 also reduced hepatic enlargement and fibrosis while improving liver function.
The formation and expansion of fluid-filled cysts drive kidney enlargement, with both an increasing cystic index and progressive extracellular matrix deposition driving functional insufficiency in fibropolycystic ARPKD-CHF[21,22]. A genetically-acquired and congenital disease, approximately 20-30% of affected patients succumb within the first 1-2 mo of life, with pulmonary insufficiency secondary to renal enlargement as the primary cause of death. For children making it past that stage, nephrectomy and dialysis or kidney transplant is often warranted by approximately ten years of age[23,24]. Intervention at this age is driven both by renal insufficiency and the need for a reduction in severe flank pain due to highly enlarged kidneys. The hepatic lesion in ARPKD-CHF is CHF resulting from a malformation of the ductal plate secondary biliary strictures and periportal fibrosis, with the majority of patients also presenting with hepatomegaly[25,26]. Clinical studies in ARPKD-CHF reveal that a subset of patients progress to hepatocellular carcinoma[27]. Other than transplantation, there is no cure for ARPKD-CHF.
Mutations in the human PKHD1 gene or mutations in PKHD1 orthologs in rats and mice are required for development of ARPKD-CHF. However, experimental studies have identified that epithelial cells drive changes in the renal interstitium, with alterations in the cystic epithelia followed by changes in the interstitial fibroblasts and progressive accumulation of extracellular matrix. This ultimately leads to the development of renal fibrosis within that organ[28,29]. Furthermore, data from a number of studies suggest that growth factors, including PDGF and VEGF, are the intermediaries between the cystic and fibrotic components of progressive fibropolycystic disease[5,11]. A study[5] in the DBA/2FG-pcy mouse model of PKD suggests that increased expression of PDGF-A and PDGF-B chains may contribute to the progression of renal cystic lesions. Qin et al[30] reported impaired degradation of PDGFR in renal cells from PKD mice. In the ORPK murine model of PKD, responses to PDGF by fibroblasts, in which ciliary assembly is defective, are abnormal. In fact, Norman et al[31] identified a paracrine, PDGF-mediated regulatory loop between inner medullary collecting duct epithelial cells and medullary fibroblasts, highlighting the importance of tubular epithelial-interstitial fibroblast interactions in PKD. Compared to age-matched normal fibroblasts, PKD fibroblasts demonstrate an enhanced proliferative response to PDGF, synthesize more fibroblast growth factor, and elicit more rapid and persistent tyrosine phosphorylation of intracellular proteins. Consistent with these reports, pPDGFR signaling in our hands appeared to be increased in PCK rats compared to the wild-type cohort. PDGF activation was accompanied by upregulation of the fibrotic marker αSMA and matrix deposition in the renal interstitium. ANG3070 treatment decreased pPDGFR signaling and αSMA expression, indicating a decrease in fibrosis.
In the kidney, VEGF expression is most prominent in glomerular podocytes and in tubular epithelial cells, while VEGF receptors are mainly found on pre-glomerular, glomerular, and peritubular endothelial cells. Raina et al[32] reported that anti-VEGF therapy in the Han: SPRD rat, a model of ADPKD, was associated with an exaggerated cystic response of the proximal tubules and severe kidney injury. Huang et al[33] reported that VEGF therapy in the Pkd1nl/nl mouse model of ADPKD was associated with robust benefits across a spectrum of endpoints, with far more modest benefits evident in the Cys1cpk/cpk mouse, a model of ARPKD. On the other hand, it has been postulated that VEGF-driven angiogenesis drives cyst cells to grow, and may be responsible for increased vascular permeability and fluid secretion into the cysts. In fact, data from clinical trials point to a relationship between circulating VEGF and renal structural disease, including total renal volume, cyst volume and cyst number, and are indicative of a potential role for upregulated angiogenesis in early renal cyst progression[10-13]. Finally, Jiang et al[14] postulated that a pathogenic triumvirate, comprised by hyperproliferation of cyst wall growth, pericystic fibrosis, and inflammation, drives CHF/ARPKD progression.
ANG3070 is a proprietary, highly water-soluble, orally bioavailable potent inhibitor of PDGF and VEGF/KDR, which binds its targets with a Kd of approximately 5 nmol/L. In the PCK rat model of ARPKD-CHF, ANG3070 efficacy was observed across a spectrum of clinically-relevant endpoints, including a decrease in renomegaly, renal cystic index, renal injury markers, renal fibrosis and improvement in kidney function. Importantly, intervention with ANG3070, even after established renal disease at both 10 and 14 wk (a time when renal pathology is fairly advanced in the PCK rat), proved efficacious. Taken together, these data indicate that ANG3070 has therapeutic efficacy and slows a hallmark indicator of disease progression in ARPKD-CHF via cystic expansion of the kidney.
Another salient finding of this study was the effect of ANG3070 in mitigating renal injury in this model of ARPKD-CHF. Urinary markers of renal injury, including NGAL, KIM-1, cystatin C and IL-18, were increased in the PCK rat compared to wild-type controls. Previous studies have described the elevation of such markers in models of ARPKD-CHF. In fact, work by Nieto et al[34] indicates that BUN, SCr and 24-h urine IL-18 levels are biomarkers for increasing cystic index and increasing renal mass in the PCK rat. The fact that ANG3070 treatment was associated with a reduction of these biomarkers, including BUN, SCr and urine IL-18, not only suggests that this drug attenuates kidney injury but also suggests that it mitigates cystogenesis and renal expansion.
A pharmacodynamic exercise to confirm the mechanism of action of ANG3070 was undertaken in renal homogenates from the wild-type and PCK cohorts. Consistent with data from the aforementioned studies, PDGFR signaling was enhanced in the PCK rat kidney, evidenced by increased levels of pPDGFR. Administration of ANG3070 to the PCK rat reduced renal phosphorylated PDGFR levels, suggesting that the drug is indeed engaging its target and that the salutary effects of ANG3070 in the kidney are associated with inhibition of PDGFR signaling.
In addition to its effects on the kidney, ANG3070 exhibited activity against the hepatic lesions that accompany this disease. While hepatomegaly, elevated serum AST and increased hepatic collagen content were observed in the PCK rat at 14 wk of age, 8 wk treatment with ANG3070 resulted in significant amelioration of both liver pathology and liver dysfunction. This is an important finding, in that not only can hepatomegaly and hepatic fibrosis necessitate liver transplantation in ARPKD-CHF patients, but also that a cytokine-driven feedback mechanism might exist between hepatic and renal lesions in this disease. Needless to say, there were some clear limitations to this study. Given the historical challenges associated with demonstrating a pharmacodynamic signature of VEGFR/KDR phosphorylation inhibition, we did not attempt to evaluate this signaling mechanism in the kidney or liver. Finally, the PCK rat is one model of ARPKD-CHF and it remains to be determined whether ANG3070 exerts similar effects in other models of this disease. In summary, intervention with ANG3070 favorably impacted both the renal and hepatic components in the PCK rat model of fibropolycystic disease. These data suggest that ANG3070 has the potential to slow ARPKD-CHF and may serve as a bridge toward hepato-renal transplantation in patients with fibropolycystic disease.
In autosomal recessive polycystic kidney disease (ARPKD)-congenital hepatic fibrosis (CHF), a genetically acquired and congenital disease, mutations in the human PKHD1 gene or mutations in PKHD1 orthologs in rats and mice are required for the development of ARPKD-CHF. Nevertheless, experimental studies have identified that epithelial cells drive changes in the renal interstitium, with alterations made to the cystic epithelia followed by changes in the interstitial fibroblasts and progressive accumulation of extracellular matrix. This leads to the development of renal fibrosis within the kidney and/or liver. For children, nephrectomy and dialysis or kidney or liver transplant is often warranted by approximately ten years of age. Other than transplantation, there is no cure for ARPKD-CHF. We report that platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) are the intermediaries between the cystic and fibrotic components of progressive fibropolycystic disease, and that a PDGFR + VEGFR dual inhibitor can be a novel therapeutic approach.
The development of new therapies that prevent the transition from cystogenesis to fibrosis or adenocarcinoma in advanced stages of ARPKD-CHF will have tremendous clinical potential and decrease the number of hepato-renal transplants in patients with ARPKD-CHF.
The main objectives of our studies were to evaluate a novel PDGFR and VEGFR dual kinase inhibitor, ANG3070, in a PKD-CHF model. These studies could lead to a novel therapeutic approach for fibropolycystic kidney disease.
Renal pathology was confirmed in PCK rats at 6 wk compared to the age and gender-matched wild type SD rats. At 6 wk of age, PCK rats were then randomized to vehicle or ANG3070 for 4 wk. At 10 wk, 24 h urine and left kidneys were collected and rats were continued on treatments for 4 wk. At 14 wk, 24 h urine was collected, rats were sacrificed, and liver and right kidneys were collected for histological evaluation. For Western blot studies, PCK rats were treated with vehicle or ANG3070 for 7 d and sacrificed approximately 30 min after the last treatments.
A well-characterized PCK rat model was used to study fibropolycystic kidney disease. Compared to the wild-type cohort, the PCK kidney (Vehicle cohort) exhibited a marked increase in kidney and liver mass, hepato-renal cystic volume, hepato-renal fibrosis and hepato-renal injury biomarkers. Intervention with ANG3070 in PCK rats decreased kidney weight, reduced renal cystic volume and reduced total kidney hydroxyproline, thus indicating significantly reduced rental interstitial fibrosis compared to the PCK-Vehicle cohort. ANG3070 treatment also mitigated several markers of kidney injury, including urinary neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, Cystatin C and interleukin-18 levels. This treatment also significantly attenuated key indices of renal dysfunction, including proteinuria, albuminuria and serum blood urea nitrogen and creatinine, and improved renal function compared to the PCK-Vehicle cohort. ANG3070 treatment also significantly decreased liver enlargement, hepatic lesions, decreased liver fibrosis and mitigated liver dysfunction compared to the PCK-Vehicle cohort. A dose-response study of ANG3070 needs to be evaluated to establish a minimum dose for maximal therapeutic efficacy in this PCK rat model.
The development of new therapies that prevent the transition from cystogenesis to fibrosis or adenocarcinoma in advanced stages of ARPKD-CHF will have tremendous clinical potential. Studies indicate that PDGF and VEGF are the intermediaries between the cystic and fibrotic components of progressive fibropolycystic disease. We have identified and synthesized a novel small molecule PDGFR + VEGFR/KDR dual kinase inhibitor, ANG3070, using molecular modeling coupled with rational drug design, medicinal chemistry and structure activity relationship. We have evaluated ANG3070 therapeutic effects in a rat model of ARPKD-CHF and have proven it to be efficacious in mitigating kidney and liver injury biomarkers and decreasing hepatic and renal dysfunction. These studies could lead to the identification of a novel therapeutic approach in slowing fibropolycystic disease and decreasing the number of hepato-renal transplants in patients with ARPKD-CHF. These results suggest that ANG3070 has the potential in slowing disease, and may serve as a bridge toward hepato-renal transplantation in patients with fibropolycystic disease.
The results of our studies suggest that ANG3070 has the potential therapeutic effect of slowing disease, and may serve as a bridge toward hepato-renal transplantation in patients with the fibropolycystic disease ARPKD-CHF.
Manuscript source: Unsolicited manuscript
Specialty type: Urology and nephrology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: El-Shabrawi MHF, Gheita TAA, Niu ZS, Tarantino G S- Editor: Ji FF L- Editor: Filipodia E- Editor: Song H
| 1. | Hartung EA, Guay-Woodford LM. Autosomal recessive polycystic kidney disease: a hepatorenal fibrocystic disorder with pleiotropic effects. Pediatrics. 2014;134:e833-e845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Gunay-Aygun M, Avner ED, Bacallao RL, Choyke PL, Flynn JT, Germino GG, Guay-Woodford L, Harris P, Heller T, Ingelfinger J. Autosomal recessive polycystic kidney disease and congenital hepatic fibrosis: summary statement of a first National Institutes of Health/Office of Rare Diseases conference. J Pediatr. 2006;149:159-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Sung JM, Huang JJ, Lin XZ, Ruaan MK, Lin CY, Chang TT, Shu HF, Chow NH. Caroli’s disease and congenital hepatic fibrosis associated with polycystic kidney disease. A case presenting with acute focal bacterial nephritis. Clin Nephrol. 1992;38:324-328. [PubMed] [Cited in This Article: ] |
| 4. | ARPKD/CHF Alliance. ARPKD/CHF Alliance Homepage. Available from: http://www.arpkdchf.org. [Cited in This Article: ] |
| 5. | Nakamura T, Ebihara I, Nagaoka I, Tomino Y, Nagao S, Takahashi H, Koide H. Growth factor gene expression in kidney of murine polycystic kidney disease. J Am Soc Nephrol. 1993;3:1378-1386. [PubMed] [Cited in This Article: ] |
| 6. | Park JH, Woo YM, Ko JY, Kim DY, Li X. Autosomal Dominant Polycystic Kidney Disease Induced by Ciliary Defects. 2015;. [PubMed] [Cited in This Article: ] |
| 7. | Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287-1301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 957] [Cited by in F6Publishing: 910] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 8. | Cowley BD Jr, Smardo FL Jr, Grantham JJ, Calvet JP. Elevated c-myc protooncogene expression in autosomal recessive polycystic kidney disease. Proc Natl Acad Sci USA. 1987;84:8394-8398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 108] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Frick KK, Womer RB, Scher CD. Platelet-derived growth factor-induced c-myc RNA expression. Analysis of an inducible pathway independent of protein kinase C. J Biol Chem. 1988;263:2948-2952. [PubMed] [Cited in This Article: ] |
| 10. | Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65:2003-2017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 349] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 11. | Bello-Reuss E, Holubec K, Rajaraman S. Angiogenesis in autosomal-dominant polycystic kidney disease. Kidney Int. 2001;60:37-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Spirli C, Okolicsanyi S, Fiorotto R, Fabris L, Cadamuro M, Lecchi S, Tian X, Somlo S, Strazzabosco M. ERK1/2-dependent vascular endothelial growth factor signaling sustains cyst growth in polycystin-2 defective mice. Gastroenterology. 2010;138:360-371.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Tao Y, Kim J, Yin Y, Zafar I, Falk S, He Z, Faubel S, Schrier RW, Edelstein CL. VEGF receptor inhibition slows the progression of polycystic kidney disease. Kidney Int. 2007;72:1358-1366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Jiang L, Fang P, Weemhoff JL, Apte U, Pritchard MT. Evidence for a “Pathogenic Triumvirate” in Congenital Hepatic Fibrosis in Autosomal Recessive Polycystic Kidney Disease. Biomed Res Int. 2016;2016:4918798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Rajekar H, Vasishta RK, Chawla YK, Dhiman RK. Noncirrhotic portal hypertension. J Clin Exp Hepatol. 2011;1:94-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Lager DJ, Qian Q, Bengal RJ, Ishibashi M, Torres VE. The pck rat: a new model that resembles human autosomal dominant polycystic kidney and liver disease. Kidney Int. 2001;59:126-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Panicker B, Mishra RK, Lim DS, Oehlen LJWM, Jung D. Antifibrotic Compounds and Uses Thereof. United States patent US9040555. 2012;Jan 26. [Cited in This Article: ] |
| 18. | Narayan P, Huang B, Prani PAKA, Paka L, Goldberg ID. Methods and uses of compounds for treating disease. United States patent US20150105380. 2013;Sep 23. [Cited in This Article: ] |
| 19. | Samuel CS. Determination of collagen content, concentration, and sub-types in kidney tissue. Methods Mol Biol. 2009;466:223-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Takikita-Suzuki M, Haneda M, Sasahara M, Owada MK, Nakagawa T, Isono M, Takikita S, Koya D, Ogasawara K, Kikkawa R. Activation of Src kinase in platelet-derived growth factor-B-dependent tubular regeneration after acute ischemic renal injury. Am J Pathol. 2003;163:277-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Al-Lawati TT. Fibropolycystic disease of the liver and kidney in Oman. Arab J Gastroenterol. 2013;14:173-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Brancatelli G, Federle MP, Vilgrain V, Vullierme MP, Marin D, Lagalla R. Fibropolycystic liver disease: CT and MR imaging findings. Radiographics. 2005;25:659-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Summerfield JA, Nagafuchi Y, Sherlock S, Cadafalch J, Scheuer PJ. Hepatobiliary fibropolycystic diseases. A clinical and histological review of 51 patients. J Hepatol. 1986;2:141-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 159] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Ko JS, Yi NJ, Suh KS, Seo JK. Pediatric liver transplantation for fibropolycystic liver disease. Pediatr Transplant. 2012;16:195-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Wilson PD, Du J, Norman JT. Autocrine, endocrine and paracrine regulation of growth abnormalities in autosomal dominant polycystic kidney disease. Eur J Cell Biol. 1993;61:131-138. [PubMed] [Cited in This Article: ] |
| 26. | Schneider L, Cammer M, Lehman J, Nielsen SK, Guerra CF, Veland IR, Stock C, Hoffmann EK, Yoder BK, Schwab A. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem. 2010;25:279-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 27. | Jain D, Nayak NC, Saigal S. Hepatocellular carcinoma arising in association with von-Meyenburg’s complexes: an incidental finding or precursor lesions? A clinicopatholigic study of 4 cases. Ann Diagn Pathol. 2010;14:317-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Knecht A, Fine LG, Kleinman KS, Rodemann HP, Müller GA, Woo DD, Norman JT. Fibroblasts of rabbit kidney in culture. II. Paracrine stimulation of papillary fibroblasts by PDGF. Am J Physiol. 1991;261:F292-F299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Sweeney WE Jr, Avner ED. Molecular and cellular pathophysiology of autosomal recessive polycystic kidney disease (ARPKD). Cell Tissue Res. 2006;326:671-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Qin S, Taglienti M, Nauli SM, Contrino L, Takakura A, Zhou J, Kreidberg JA. Failure to ubiquitinate c-Met leads to hyperactivation of mTOR signaling in a mouse model of autosomal dominant polycystic kidney disease. J Clin Invest. 2010;120:3617-3628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Norman JT, Orphanides C, Garcia P, Fine LG. Hypoxia-induced changes in extracellular matrix metabolism in renal cells. Exp Nephrol. 1999;7:463-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Raina S, Honer M, Krämer SD, Liu Y, Wang X, Segerer S, Wüthrich RP, Serra AL. Anti-VEGF antibody treatment accelerates polycystic kidney disease. Am J Physiol Renal Physiol. 2011;301:F773-F783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Huang JL, Woolf AS, Kolatsi-Joannou M, Baluk P, Sandford RN, Peters DJ, McDonald DM, Price KL, Winyard PJ, Long DA. Vascular Endothelial Growth Factor C for Polycystic Kidney Diseases. J Am Soc Nephrol. 2016;27:69-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Nieto JA, Yamin MA, Goldberg ID, Narayan P. An Empirical Biomarker-Based Calculator for Cystic Index in a Model of Autosomal Recessive Polycystic Kidney Disease-The Nieto-Narayan Formula. PLoS One. 2016;11:e0163063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |