Published online Jul 6, 2017. doi: 10.5527/wjn.v6.i4.209
Peer-review started: March 7, 2017
First decision: April 18, 2017
Revised: May 15, 2017
Accepted: May 30, 2017
Article in press: May 31, 2017
Published online: July 6, 2017
To investigate the relationship between circadian variations in blood pressure (BP) and albuminuria at rest, and during exercise in non-hypertensive type 2 diabetes (T2D) patients.
We conducted a cross-sectional study in well controlled T2D patients, non-hypertensive, without clinical proteinuria and normal creatinine clearance. In each participant, we recorded the BP using ambulatory blood pressure monitoring (ABPM) for 24-h, and albuminuria at rest and after a standardized treadmill exercise.
We enrolled 27 type 2 patients with a median age of 52; and a mean duration of diabetes and HbA1c of 3.6 ± 0.8 years and 6.3% ± 0.5% respectively. Using a 24-h ABPM, we recorded a mean diurnal systolic blood pressure (SBP) of 128 ± 17 mmHg vs nocturnal of 123 ± 19 mmHg (P = 0.004), and mean diurnal diastolic blood pressure (DBP) of 83 ± 11 mmHg vs nocturnal 78 ± 14 mmHg (P = 0.002). There was a significant difference between albuminuria at rest [median = 23 mg, interquartile range (IQR) = 10-51] and after exercise (median = 35 mg, IQR = 23-80, P < 0.001). Patients with exercise induced albuminuria had an increase in nocturnal BP values on all three components (128 mmHg vs 110 mmHg, P = 0.03 for SBP; 83 mmHg vs 66 mmHg, P = 0.04; 106 vs 83, P = 0.02 for mean arterial pressure), as well as albuminuric patients at rest. Moreover, exercise induced albuminuria detect a less increase in nocturnal DBP (83 vs 86, P = 0.03) than resting albuminuria.
Exercise induced albuminuria is associated with an increase in nocturnal BP values in T2D patients.
Core tip: Many studies have reported association between abnormalities in ambulatory blood pressure monitoring (ABPM) and albuminuria at rest, or the link between exercise induced albuminuria and future development of microalbuminuria. However, the relationship between exercise-induced albuminuria and nocturnal abnormalities of BP on ABPM have not been investigate. The current study aimed to investigate the potential relationship between exercise-induced albuminuria and circadian variations in BP in a sub-Saharan type 2 diabetes population. We found that exercise-induced albuminuria is associated with less important nocturnal abnormalities of BP than resting albuminuria suggesting that exercise-induced albuminuria be used to detect early abnormalities of nighttime BP.
- Citation: Tankeu AT, Kaze FF, Noubiap JJ, Chelo D, Dehayem MY, Sobngwi E. Exercise-induced albuminuria and circadian blood pressure abnormalities in type 2 diabetes. World J Nephrol 2017; 6(4): 209-216
- URL: https://www.wjgnet.com/2220-6124/full/v6/i4/209.htm
- DOI: https://dx.doi.org/10.5527/wjn.v6.i4.209
Type 2 diabetes mellitus (T2DM) is the leading cause of chronic kidney disease (CKD) worldwide[1,2]. It represents with hypertension - the most common cause of end-stage renal disease (ESRD); affecting at least one-third of patients starting chronic dialysis worldwide, and represents the main cause of mortality in diabetes patients[3]. Early detection and intervention in diabetic nephropathy can help to slow loss in renal function, prevent complications, and decrease cardiovascular events; thereby improving survival and quality of life in T2DM patients[3]. As the global incidence of T2D continues to rise, controlling diabetic nephropathy is becoming a health priority. Fifty years ago, the development of assays for detection of microalbuminuria (MA) revolutionized diabetes management. Microalbuminuria, also called moderate albuminuria (urinary albumin excretion ≥ 30 mg/24 h but less than 300 mg/24 h), measured at rest became the earliest marker of diabetic nephropathy, and was thought to predict overt diabetic nephropathy in 80% of patients[4]. However, recent evidence have shown that only 30% of patients with microalbuminuria will develop overt diabetic nephropathy[5]. Moreover, screening with albumin excretion rate alone would miss 20% of progressive disease[6]. These evidences suggest that a need for methods for early detection of diabetic nephropathy. Physiologically, it is well known that patients with subclinical nocturnal blood pressure (BP) abnormalities are prone to renal insult and hence at risk of developing microalbuminuria[7]. So, non-dipping nocturnal BP pattern defined as a less than 10% decrease in nocturnal BP, increase the risk of organ damage such as microalbuminuria[7]. In the other hand, exercise-induced albuminuria has been proposed as a long term predictive factor of overt diabetic nephropathy, but there is still a dearth of evidence on its usefulness in routine screening[8]. We sought to explore a possible relationship between resting and exercise-induced albuminuria, and circadian variations in BP levels on 24-h ambulatory blood pressure monitoring (ABPM).
This study was carried out at the National Obesity Center of the Yaoundé Central Hospital, a reference diabetes center in this area. Eligible participants were: Type 2 diabetics, according to the WHO definition[9]; without clinical proteinuria (≤ 30 mg/dL), with clinical BP less than 140/90 mmHg, HbA1c less than 7%, an estimated glomerular filtration rate (eGFR) ≥ 60 mL/min per square meter using the MDRD formula which was found to be the most accurate formula for sub-Saharan African diabetic patients[10]. We excluded patients working during the night, those receiving drugs for hypertension or any other drugs able to modify albuminuria, patients with contraindication to exercise or presenting signs of urinary tract infection. Patients having a fever and pregnant women were also excluded. All patient included were on oral anti-diabetic treatment.
Study design and duration: This was a cross sectional study conducted from February to June 2015 a period of five months.
This study was conducted to investigate the relationship between exercise-induced albuminuria and early diabetic nephropathy assessed by nocturnal elevation of ABPM. To this end, albuminuria was measured at rest for microalbuminuria, and after a moderate and standardized exercise for exercise induced albuminuria. Then comparison between patients were planned to shows that exercise induced albuminuria is similar or even higher to microalbuminuria at rest for the detection of abnormal nocturnal BP patterns.
All the patients attending the clinic were assessed, and eligible patients were invited to participate to the study. The procedure was comprised of an inclusion visit and three exploratory visits. Within the two weeks following an information visit, a careful clinical exam including BP measurement, urinary dipstick exam, and resting electrocardiogram were performed on eligible patients. The sample size was estimated using Withley et al[11] formula: n = (2/d2) × Cp, power.
The primary outcome was a significant difference of at least 10 mmHg of SBP and/or DBP in patients having exercise induced albuminuria. The standardized difference for BP in T2DM patients used was set at 10 mmHg (UKPDS). Considering α = 5% and β = 10%, with a statistical power of 90%, the minimal size was 11 patients.
The standardized difference, d = (expected difference)/(standard deviation): D = 10/10 = 1.
N = (2/d²) × Cp, power where Cp, power is a constant defined by the chosen value of the statistical power and the value p. This constant is available in statistical tables[11] C0.05, 90% = 10.5.
Thus the minimum size of the sample is: n = 2/(1²) × 10.5.
n = 10.5 rounded to 11 subjects.
To improve statistical power, we increased the study sample at 30 patients. Patients were screened clinically for eligibility with three clinical BP measurements, a urinary dipstick, resting electrocardiogram and a blood sample for creatinine estimation and creatinine clearance calculation. Of the 30 eligible patients invited, 3 were excluded because they had signs of urinary tract infection on dipstick.
Participants were invited to arrive at the hospital between 8:00 and 10:00 am for all exploratory visits. On arrival, participants were maintained in a sitting position for at least five minutes before BP measurement. Three serial BP measurements taken in the sitting position were obtained from non-dominant arm placed at the level of the heart, using adults cuffs (32-42 cm) adapted to an automated sphygmomanometer Omron HEM-705 CP (Omron Corporation, Tokyo, Japan). The average of the three measurements was used for all analyses. Weight was measured in light clothed subjects to the nearest 0.5 kg with a mechanical scale, while height was measured in an upright position to the nearest 0.5 cm. The body mass index (BMI in kg/m²) was calculated as weight (kg)/[height (m) × height (m)]. Waist circumference was measured horizontally, midway between the lower rib margin and the iliac crest with a measuring tape. Macro or clinical albuminuria was assessed by dipstick of spot urine, and considered positive for at least 1+ (< 30 mg/dL). Electrocardiography was done with the CardiMax FX-7302 electrocardiograph (Fukuda Den Shi, Tokyo, Japan).
Twenty-four hour BP was measured by a portable, light weight monitor device i-mapa CE 004 1.1 TM (High-tech Medical St Louis, Paris). The purpose of the examination and practical modalities including: the measurement procedure and related constraints were explained to the participants. The device was then connected to an adult cuff, tested and calibrated. The practical procedure consisted of two major steps.
Programming the measurement over 24 h with patient information: For this purpose, demographic information and anthropometric measurements were entered into the i-mapa software, and then the medical history was recorded, allowing us to have an electronic medical record of the patient. Then a measurement protocol was selected from those proposed by the software and a 24-h measurement cycle was activated.
Installation of the measurement monitor on the patient: Then, the standard cuff was placed around the patient’s arm 2.5 cm above the antecubital dimple in direct contact with the skin and the case fixed at the level of the waist. The patient performs some movements to ensure comfort. After the patient had put on his clothes, a manual measurement was made, which enabled the selected measurement protocol to start and ensure functionality of the device. Thereafter, the patient returned to his daily activities with instructions concerning the handling of the device, and a paper where he/her was thought to notice all particular activity during the day such as medication taken with the hour, any important emotional or physical changing (diary) and the appointment was made the next day at the same time. The patient brought us back the monitor 24 h later. Once arrived, the device was turned off and unplugged. The measurements made were then transferred to a computer using a Bluetooth key enabling communication between the software and the monitor.
Reading the 24-h measurement: Readings were obtained automatically at 15-mn intervals during the day time define from 07:00 to 22:00 and twice an hour from 22:00 to 07:00. This interval was set using a fix clock time but control with a personal diary for each patient.
The protocol used for physical exertion was set up by our research team. It consisted of a simple step at a constant speed of 1 m/s for 30 min with a slope set at 5% with the aim of achieving a sub-maximal physical effort, resulting in a rise in the heart rate to 50%-70% of the patient’s maximum HR. For each participant, a complete emptying of the bladder was performed 10 min prior to exercise. In the preceding days, he was recommended to continue to carry out his usual activities, avoid moderate to vigorous physical activity 48 h before the start of each phase of investigations, namely: Jogging, rapid walking for 1 h, and also avoid taking medications that may modify the evaluated parameters such as angiotensin converting enzyme inhibitors.
The exercise was carried out under medical supervision. For this phase, the volunteer was invited to the exploration room of our research laboratory to the National Obesity Center of the Yaoundé central hospital between 08:00 and 10:00 in the morning. Upon arrival, blood glucose was performed; the data sheet was revisited and updated. The patient was driven and installed in the exercise room. The treadmill was then switched on with accessories such as the heart rate monitor. A urine and blood sample was collected at rest and preserved before the beginning of the exercise. The patient was then placed on the treadmill after having installed the heart rate monitor on the chest, the electrodes of the heart being in direct contact with the skin and safety belt installation. Continuous heart rate (HR) recording allowed us to ensure that the patient remained within the limits set for our study 50%-70% of maximal HR. At the end of the exercise, the patient was placed in an armchair to recover and received water for rehydration and to promote diuresis.
Urinary albumin excretion was calculate using albumin to creatinine ratio to reduce effect of urinary concentration after exercise on albuminuria and expressed in mg/g. First void urine collection was used for rest albuminuria and a random sample urine was collected within the 30 mn following physical exercise to measure exercise-induced albuminuria. Retinopathy was assessed by fundoscopy and creatinine measured using modified Jaffe kinetic reaction with colorimetric methods. Albuminuria was measured by turbidimetry and others biochemical parameters using an automatic spectrophotometer.
Data acquisition was done by Epi-data3.1 software and statistical analysis was performed using Statistical Package for Social Science (SPSS) version 21.0 for Windows (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, version 21.0. Armonk, NY: IBM Corp.) and Stata 12.0 software. Continuous variables are expressed as median (interquartile range = IQR) or mean ± SD where appropriate, and categorical variables as count (percentage). The Spearman rank coefficient was used to test correlations. The χ2 test, Mann-Whitney rank sum test were used to test associations between qualitative variables and difference between two respectively. A P value < 0.05 was considered statistically significant.
We included 27 patients (18 males) with a median age of 52 (IQR = 36-56) years, a mean duration of diabetes of 3.6 ± 0.8 years and a mean HbA1c of 6.3% ± 0.5%. Twenty four hours ABPM recorded a mean diurnal systolic blood pressure (SBP) of 128 ± 17 mmHg vs nocturnal of 123 ± 19 mmHg (P = 0.004), and mean diurnal diastolic blood pressure (DBP) of 83 ± 11 mmHg vs nocturnal DBP 78 ± 14 mmHg (P = 0.002) for a mean day vs night difference of 05 mmHg for SBP and 05 mmHg for DBP. Four/Twenty-seven of patients had diabetic retinopathy.
Our population study was divided three times in two subgroups, according to albuminuria before and after exercise, these groups were subsequently compared pairs. These comparisons are illustrated in Tables 1, 2 and 3. They included clinical and ABPM findings in order to compare possible differences on BP profile associate with albuminuria.
| Variables | Normoalb0 | Microalb0 | P value |
| Frequencies | 19 | 8 | / |
| Age | 52 (40-56) | 48 (34-56) | 0.56 |
| BMI (kg/m²) | 25.4 (23.1-28.5) | 26.1 (24-31.6) | 0.62 |
| Waist circumference | 92 (87-96) | 93 (84-97) | 0.99 |
| SBP (mmHg) | 122 (112-133) | 137 (122-142) | 0.68 |
| DBP (mmHg) | 77 (73-87) | 89 (79-99) | 0.04 |
| HbA1c (%) | 6.6 (5.9-7.0) | 6.2 (6-7) | 0.99 |
| eGFR (mL/min) | 93 (77-104) | 89 (70-104) | 0.52 |
| ACR at rest | 13.5 (8.6-21.9) | 75.3 (46-94.1) | 0.0001 |
| Exercise induced ACR | 29.2 (20.5-33.3) | 92.3 (56.3-128.7) | 0.0001 |
| Variables | Normoalb30 | Microalb30 | P value |
| Frequencies | 11 | 16 | / |
| Diurnal SBP | 117 (112-133) | 132 (125-136) | 0.71 |
| Nocturnal SBP | 110 (98-125) | 128 (122-138) | 0.03 |
| 24 h SBP | 116 (109-131) | 132 (124-136) | 0.05 |
| Diurnal DBP | 78 (76-85) | 85 (80-92) | 0.19 |
| Nocturnal DBP | 66 (59-82) | 83 (74-89) | 0.044 |
| 24 h DBP | 76 (72-84) | 85 (78-89) | 0.11 |
| Diurnal MAP | 93 (89-107) | 107 (102-112) | 0.05 |
| Nocturnal MAP | 83 (77-102) | 106 (99-110) | 0.02 |
| 24 h MAP | 89 (85-105) | 108 (99-112) | 0.03 |
| Variables | Microalb0 | Microalb30 | P value |
| Frequencies | 8 | 8 | |
| Diurnal SBP | 131 (126-136) | 132 (125-136) | 0.95 |
| Nocturnal SBP | 130 (126-138) | 128 (122-138) | 0.38 |
| 24 h SBP | 133 (126-135) | 132 (124-136) | 0.60 |
| Diurnal DBP | 85 (83-93) | 85 (80-92) | 0.22 |
| Nocturnal DBP | 86 (80-92) | 83 (74-89) | 0.03 |
| 24 h DBP | 86 (83-93) | 85 (78-89) | 0.11 |
| Diurnal MAP | 107 (104-111) | 107 (102-112) | 0.77 |
| Nocturnal MAP | 107 (102-112) | 106 (99-110) | 0.14 |
| 24 h MAP | 107 (104-110) | 108 (99-112) | 0.68 |
The first two groups compared were patients with albuminuria at rest vs patients without resting albuminuria (Table 1). We found that patients with albuminuria at rest had more elevated DBP on clinical measure than non-albuminuric patients at rest (89 mmHg vs 77 mmHg, P = 0.04). Using ABPM, there was a marked increase in all component of nocturnal BP (130 mmHg vs 115 mmHg, P = 0.06 for SBP; 86 mmHg vs 72 mmHg for DBP, P = 0.02; and 107 vs 90, P = 0.03 for mean arterial BP).
The 2nd comparison concerned patients presenting with exercise-induced albuminuria and those without albuminuria after exercise (Table 2). This revealed that patients with exercise-induced albuminuria had an increase in nocturnal BP values on all three components [128 mmHg vs 110 mmHg, P = 0.03 for SBP; 83 mmHg vs 66 mmHg, P = 0.04; 106 vs 83, P = 0.02 for mean arterial pressure (MAP)].
The last comparison was made between patients presenting albuminuria only after exercise and those with albuminuria at rest (Table 3). Since patients with exercise-induced albuminuria had elevated night time BP values compared to those with albuminuria at rest, we sought to determine if exercise induced albuminuria is associated with a particular abnormality in BP profile. We found that patients with exercise induced albuminuria had slightly lower BP values
on clinical measure (130/87 mmHg vs 137/89 mmHg, P = 0.08). However, on ABPM, exercise induced albuminuria was associated with a slight and significant decrease in nocturnal DBP (83 mmHg vs 86 mmHg, P = 0.03) and a rise of percentage of abnormal SBP value (P = 0.01). Apart from the aforementioned abnormalities, both groups were similar in other parameters. Nevertheless, both groups had an increase in BP value compare to the rest of population.
Using ABPM, the mean BP of our population study was 128 ± 17 mmHg vs 124 ± 19 mmHg and 127 ± 17 mmHg (P day vs night = 0.004) for systolic day time, night time and 24 h values respectively; and 84 ± 11 mmHg vs 79 ± 14 mmHg and 83 ± 12 mmHg for diastolic component.
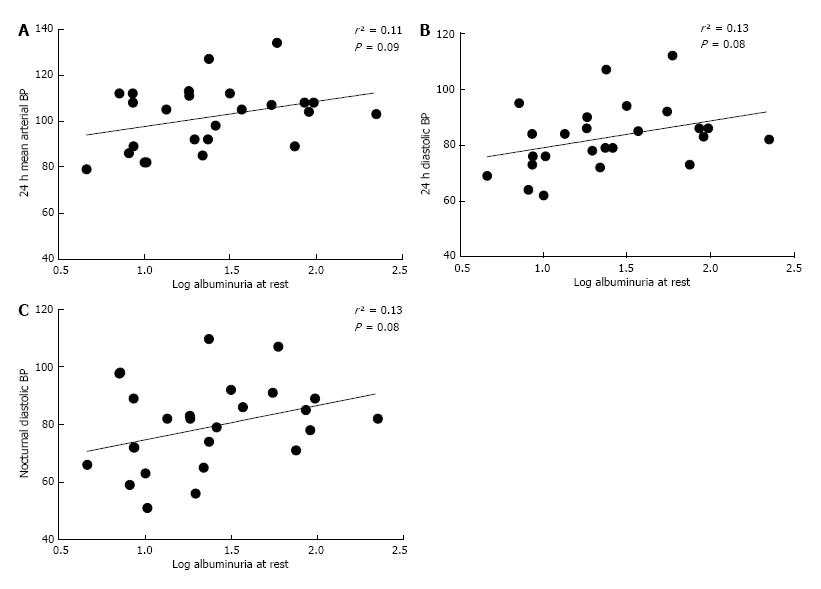
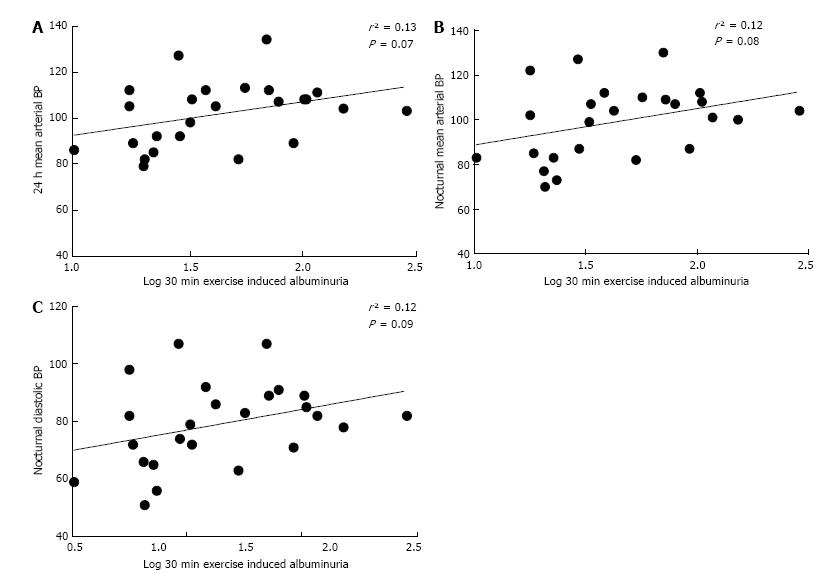
Correlation done between albuminuria and different components of BP on a sample population showed that albuminuria at rest is weakly correlated with 24 h MAP (r² = 0.11, P = 0.09; Figure 1A), 24 h DBP (r² = 0.13, P = 0.08; Figure 1B) and nocturnal DBP (r² = 0.13, P = 0.08; Figure 1C). On the other hand, exercise induced albuminuria correlated better with 24-h mean arterial blood pressure (MAP) (r² = 0.13, P = 0.07; Figure 2A), night time DBP (r² = 0.12, P = 0.09; Figure 2B) and nocturnal MAP (r² = 0.12, P = 0.09; Figure 2C).
This study was carried out to investigate a relationship between urinary albumin excretion during exercise and circadian variations in BP. We found an average drop of 3.1% during the night with a high proportion of non-dippers (20/27 or 74%) in our study population, with a blunted nighttime BP. The proportion of non-dippers is higher than those of others study on type 2 diabetic population[12]. The increase of non-dippers in our study could be due to the tendency of having elevated nocturnal BP in black subject as described by Hebert et al[13] in 1996. So, the non-dipper profile could be more common in black populations. On the other hand, the high frequency of non-dipper status found in our study may reflect an incipient nephropathy in our population of diabetic patients without proteinuria. Since the reduction of nighttime dipping BP is consider as an earlier sign of diabetic nephropathy in diabetic patients with normal BP.
Patients were subsequently separated according to their urinary albumin excretion at rest and during exercise. Firstly, we compared albuminuric and non albuminuric patients at rest, and found that patients with albuminuria at rest exhibited more BP abnormalities compared to those without albuminuria. This include an elevated clinical DBP on clinical measure suggesting that albuminuria at rest is consistent with a slight elevation of clinical BP more marked on the diastolic component. Using ABPM, there was an increase in all diastolic components of BP (diurnal, nocturnal and mean arterial) as well as nocturnal SBP and nocturnal mean MAP in albuminuric patients at rest. These data are consistent with those found in literature where albuminuria at rest is associated with abnormal patterns of nocturnal BP[13-15]. However, our study highlights the fact that this increase in nocturnal BP is more marked on diastolic component of BP. Therefore, clinical and nocturnal DBP must be checked and managed meticulously in T2D patients to detect even a slight elevation or a moderate rise in baseline DBP comparing with precedent measures. This is to ensure early detection of these patients, thereby improve their management to prevent diabetic nephropathy.
Secondly, we compared the BP of patients exhibiting exercise induced albuminuria at rest and during exercise with those without this abnormality on clinical measure and using ABPM. We found that, though there was no difference in SBP and DBP on clinical measure, but ABPM detected an increase in nocturnal BP including night time SBP, DBP as well as nocturnal MAP. This important finding supports and confirms our research hypothesis stipulating that exercise induced albuminuria is associated with abnormal circadian BP profile particularly abnormalities in night time BP pattern.
Finally, we compared patients exhibiting exercise induced albuminuria without resting albuminuria to those having albuminuria at rest in order to check if there is a difference between BP profiles of these two groups of patients. Comparing with non-albuminuric patients, these two groups had elevated BP values. However, patients with moderate resting albuminuria tend to have a higher BP values than those presenting only exercise-induced albuminuria. This association could be of importance since mild variations in nocturnal BP has been associated with renal injuries in T2D patients. Our findings are consistent with the fact that exercise induced albuminuria is associated with the same abnormalities in BP profile as albuminuria at rest, and therefore could be useful in management of T2D patients to detect the same patients with blunted nighttime BP. Nevertheless, there is an urgent need for longitudinal studies to verify this hypothesis. Moreover, exercise induced albuminuria could be associated with very subtle variations in circadian BP.
Albuminuria at rest had a positive but weak correlation with nighttime DBP and 24 h BP, sustaining the fact that an increase in 24 h BP is associated with an elevation in albuminuria at rest which could be a marker of renal deterioration[16]. Therefore, an increase in nighttime DBP and/or 24 h DBP could be associated with greater kidney damage in diabetic patients without hypertension. These results corroborate with those Rossing in 1993[17]. Similarly, exercise induced albuminuria correlated better, and positively, with night DBP and 24 h MAP. Thus, exercise induced albuminuria is correlated with the same characteristics as albuminuria at rest.
There was a strong positive correlation between albuminuria at rest and during exercise albuminuria (r = 0.7 and P < 0.001). This suggests that patients with a greater resting albuminuria therefore tend to have a higher albuminuria after exercise. Considering the fact that albuminuria at rest is a marker of incipient diabetic nephropathy, this suggests that exercise induced albuminuria would be greater in case of pre-existing renal impairment[18]. This hypothesis is supported by physiology, since it is known that subclinical lesions can be unmasked even by moderate physical exercise[8]. The results obtained in this study shows a significant proportion of abnormal circadian BP profile in patients with T2D, considered normotensive on clinic-based measurements.
This study presents some limitations related to the study design. Since this is a cross-sectional study, the predictive value of these abnormalities needs to be evaluated with prospective studies. This could bring more evidence and basic arguments to recommend management of T2D patients presenting exercise-induced albuminuria. This study depicts the importance of ABPM in the management of diabetic patients, due to its efficacy in detecting abnormalities in BP values, which is a better predictor of renal damage compared with clinic-based BP measurements. We also found that exercise induced albuminuria discriminate the same patients as resting albuminuria stressing the need to revisit importance of exercise-induced albuminuria in the earlier diagnosis and management of T2D patients. This will require prospective studies in order to evaluate the predictive capacity of exercise induced albuminuria on nighttime BP abnormalities what have not been done yet to date.
Exercise-induced albuminuria is associated with nocturnal blood pressure abnormalities in T2D patients and could discriminate patients with more precocious increase of nighttime blood pressure than albuminuria at rest.
We gratefully acknowledge the biostatistician (Olivier T Donfack) of the Tropical school who accepted to review the statistical methods used in this study and all the patients who have accepted to take part in this study. We also thank Dr. Ndip Valirie Agbor for proofreading services.
Exercise induced albuminuria has long been proposed as early marker of diabetes nephropathy but was not approved due to lack of evidence and clinical importance. Nocturnal blood pressure abnormalities and represent an early marker diabetes kidney disease.
A relation between nocturnal blood pressure abnormalities detected by ambulatory blood pressure monitoring (ABPM) and exercise-induced albuminuria would be of great importance since this will suggest that the latter can serve as earlier marker of diabetes nephropathy.
Most of studies conducted to determine the clinical importance of exercise-induced albuminuria have been done in type 1 diabetes individuals and has compared it only to albuminuria at rest. This study shows an association between nocturnal blood pressure abnormalities and exercise-induced albuminuria suggesting that it could be useful to discriminate type 2 diabetes (T2D) individuals with early renal diabetes kidney disease. As the best of our knowledge, this study is the first reporting this association in T2D patients who represent more than 90% of diabetes patients worldwide.
Economically, the separate or combined use of ABPM, and exercise induced albuminuria in assessing diabetic patients clinically normotensive, for the detection of subclinical diabetic nephropathy lesions could represent a significant gain in health spending by its low cost compared to new markers currently in test and would significantly reduce health care spending. Indeed, by allowing very early detection of subclinical renal damage, they would allow a more prompt and early treatment, increasing the chances of controlling the disease and slow its progression to end-stage renal disease (ESRD) and chronic kidney disease. This would be a significant reduction in costs for health systems today face a real inflation of the prevalence of ESRD and dialysis diabetes-related admissions.
Albuminuria: Presence of albuminuria in urine which is a marker of renal damage; Microalbuminuria or moderate albuminuria: Urinary albumin excretion ≥ 30 mg/24 h or 30 mg/g with albumin to creatinin ratio; Exercise-induced albuminuria: Increase or presence of urinary albumin excretion after an exercise.
Well done important information supporting the view that patients regarded as not afflicted with diabetic nephropathy may indeed be progressing towards renal damage that is preventable if treated before diabetic nephropathy progresses to a 24 h disorder.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: Cameroon
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Friedman EA, Fujigaki Y, Gheith O S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Sheen YJ, Sheu WH. Risks of rapid decline renal function in patients with type 2 diabetes. World J Diabetes. 2014;5:835-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 41] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Sobngwi E, Enoru S, Ashuntantang G, Azabji-Kenfack M, Dehayem M, Onana A, Biwole D, Kaze F, Gautier JF, Mbanya JC. Day-to-day variation of insulin requirements of patients with type 2 diabetes and end-stage renal disease undergoing maintenance hemodialysis. Diabetes Care. 2010;33:1409-1412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Kim SS, Kim JH, Kim IJ. Current Challenges in Diabetic Nephropathy: Early Diagnosis and Ways to Improve Outcomes. Endocrinol Metab (Seoul). 2016;31:245-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Currie G, McKay G, Delles C. Biomarkers in diabetic nephropathy: Present and future. World J Diabetes. 2014;5:763-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 76] [Cited by in F6Publishing: 73] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 5. | Rossing P, Hougaard P, Parving HH. Progression of microalbuminuria in type 1 diabetes: ten-year prospective observational study. Kidney Int. 2005;68:1446-1450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S14-S80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2830] [Cited by in F6Publishing: 2933] [Article Influence: 293.3] [Reference Citation Analysis (0)] |
| 7. | Shalaby NM, Shalaby NM. Study of ambulatory blood pressure in diabetic children: prediction of early renal insult. Ther Clin Risk Manag. 2015;11:1531-1537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | O'Brien SF, Watts GF, Powrie JK, Shaw KM. Exercise testing as a long-term predictor of the development of microalbuminuria in normoalbuminuric IDDM patients. Diabetes Care. 1995;18:1602-1605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | WHO. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia [Internet]. WHO. [cited 2017 Jan 14]. Available from: http://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/. [Cited in This Article: ] |
| 10. | Agoons DD, Balti EV, Kaze FF, Azabji-Kenfack M, Ashuntantang G, Kengne AP, Sobngwi E, Mbanya JC. Performance of three glomerular filtration rate estimation equations in a population of sub-Saharan Africans with Type 2 diabetes. Diabet Med. 2016;33:1291-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Whitley E, Ball J. Statistics review 4: sample size calculations. Crit Care. 2002;6:335-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 398] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 12. | Cuspidi C, Sala C, Valerio C, Negri F, Mancia G. Nocturnal hypertension and organ damage in dippers and nondippers. Am J Hypertens. 2012;25:869-875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Hebert LA, Agarwal G, Ladson-Wofford SE, Reif M, Hiremath L, Carlton SG, Nahman NS, Falkenhain ME, Agarwal A. Nocturnal blood pressure in treated hypertensive African Americans Compared to treated hypertensive European Americans. J Am Soc Nephrol. 1996;7:2130-2134. [PubMed] [Cited in This Article: ] |
| 14. | Xu H, Huang X, Risérus U, Cederholm T, Sjögren P, Lindholm B, Ärnlöv J, Carrero JJ. Albuminuria, renal dysfunction and circadian blood pressure rhythm in older men: a population-based longitudinal cohort study. Clin Kidney J. 2015;8:560-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Poulsen PL, Hansen KW, Mogensen CE. Ambulatory blood pressure in the transition from normo- to microalbuminuria. A longitudinal study in IDDM patients. Diabetes. 1994;43:1248-1253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 78] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Rosenstock J, Raskin P. Early diabetic nephropathy: assessment and potential therapeutic interventions. Diabetes Care. 1986;9:529-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Rossing P, Hommel E, Smidt UM, Parving HH. Impact of arterial blood pressure and albuminuria on the progression of diabetic nephropathy in IDDM patients. Diabetes. 1993;42:715-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 83] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Mogensen CE, Vittinghus E. Urinary albumin excretion during exercise in juvenile diabetes. A provocation test for early abnormalities. Scand J Clin Lab Invest. 1975;35:295-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |