Published online Dec 24, 2015. doi: 10.5500/wjt.v5.i4.267
Peer-review started: July 27, 2015
First decision: August 25, 2015
Revised: October 9, 2015
Accepted: November 3, 2015
Article in press: November 4, 2015
Published online: December 24, 2015
The deterioration of endothelial structure plays a very important role in the development of vascular diseases. It is believed that endothelial dysfunction starts in the early stage of kidney disease and is a risk factor of an unfavorable cardiovascular prognosis. Because a direct assessment of biological states in endothelial cells is not applicable, the measurement of endothelial microparticles (EMPs) detached from endothelium during activation or apoptosis is thought to be a marker of early vascular disease and endothelial dysfunction in children with chronic kidney disease (CKD). Few studies have shown increased circulating EMPs and its relationship with cardiovascular risk factors in patients with CKD. MPs contain membrane proteins and cytosolic material derived from the cell from which they originate. EMPs having CD144, CD 146, CD31+/CD41-, CD51 and CD105 may be used to evaluate the vascular endothelial cell damage and determine asymptomatic patients who might be at higher risk of developing cardiovascular disease in CKD and renal transplant.
Core tip: In chronic kidney disease (CKD), cardiovascular disease is a leading cause of mortality and morbidity even after renal transplantation. Classical cardiovascular risk factors are insufficient to explain the entire story in the development of atherosclerosis. The existence of endothelial dysfunction may serve as a marker of a poor cardiovascular outcome. The need for a reliable and clinically significant marker of early vascular disease and endothelial dysfunction in atherosclerosis and early detection of graft rejection in renal transplant recipients is emerging. Although the precise molecular mechanism of microparticle formation is not clear, it has recently emerged as a marker of vascular disease. The dynamics of circulating endothelial microparticles in CKD and transplantation will be reviewed in this manuscript.
- Citation: Dursun I, Yel S, Unsur E. Dynamics of circulating microparticles in chronic kidney disease and transplantation: Is it really reliable marker? World J Transplant 2015; 5(4): 267-275
- URL: https://www.wjgnet.com/2220-3230/full/v5/i4/267.htm
- DOI: https://dx.doi.org/10.5500/wjt.v5.i4.267
Cardiovascular disease is one of the most common causes of mortality and morbidity in adults and children with chronic kidney disease (CKD) even after renal transplantation, which is the ideal renal replacement therapy for children with end-stage kidney disease (ESKD). Atherosclerosis in patients with CKD is the most powerful independent predictor of all-cause and primarily cardiovascular mortality[1-5]. Hypertension, a second common post transplant complication, and cardiovascular events are risk factors for unfavorable outcome in children with renal transplant[4]. The classical cardiovascular risk factors are insufficient to explain the entire story in the development of atherosclerosis in uremia and how specific pathogenic uremic factors could be involved[6,7]. The deterioration of endothelial integrity plays a major role in the development of vascular diseases, including atherosclerosis and vascular calcification, and it is believed that endothelial dysfunction begins in the early stage of CKD[2]. The existence of endothelial dysfunction may serve as a reliable marker of poor cardiovascular outcome in patients with CKD[3].
The investigation for a reliable and clinically significant indicator of early vascular disease and endothelial dysfunction in atherosclerosis and the early detection of graft rejection in renal transplant recipients are hot topics[8]. Because a direct assessment of biological states in endothelial cells is often invasive or costly, biomarkers might be an alternative and reliable option in identifying the pathology and evaluating the risk of diseases[9]. Biomarkers are objectively measurable indicators of normal biological situations, pathogenic processes or pharmacological responses to treatments[10].
Recently, released vesicles into the extracellular space in both normal and stress conditions have been thought to be an indicator of early vascular disease and impaired endothelial function in children with CKD, vasculitis and obesity[11-13].
The term microparticle may be used to define a number of similarly sized particles that comprise the membrane, lipoprotein, protein aggregates and other debris. Membrane microparticles are microparticles (MPs) that consist of a cell-derived vesicle, which is resulted from the outer blebbing of the plasma membrane and sequential dropping into the extracellular space. Therefore, MPs contain membrane proteins and cytosolic material extracted from the cell from which they originate[14-16]. Endothelial microparticles (EMPs) are small (< 1.5 μm) vesicular particles of the endothelial cell membrane detached from endothelium during the process of activation or apoptosis. They are considered to be markers of endothelial dysfunction[9]. They act like diffusible vectors of biologic activities in our body and are involved in the exchange of information between the circulating cells and the endothelium[15-17]. The characteristics of EMPs are presented in Table 1.
| Characteristic | Microparticles |
| Size | 100-1000 nm |
| Mechanism of formation | Outward blebbing of plasma membrane |
| How detected | Flow cytometry, capture-based assays and electron microscopy |
| Characteristic features | Annexin V-positivity and presence of cell-specific surface markers |
| Composition | Protein, RNA and miRNA |
| Membrane properties | Externalized phosphatidylserine, rich in lipid rafts and impermeable |
| Name of antigens | CD31 (PECAM-1) |
| CD51 (vitronectin receptor, αv β3) | |
| CD105 (endoglin) | |
| CD144 (VE-cadherin) | |
| CD146 (S-Endo 1-associated antigen) |
Some interventions such as fish-oil supplementation, statins, anti-TNF agents, acetylsalicylate and vitamin C supplementation may affect microparticle formation and reduce number of circulating microparticles[18-22]. For this reason, analysis of circulating microparticles could give useful information about the efficacy of treatment[23].
The vascular endothelium plays a key role as a barrier between the circulating blood and the vessel wall. The protracted or excessive endothelial activation by pathophysiological stimuli or agonists, like proinflammatory cytokines, growth factors, infectious agents, lipoproteins and oxidative stress and uremic toxins, results in impairment in endothelial function and circulating EMPs separated from a blood vessel[6,24-26].
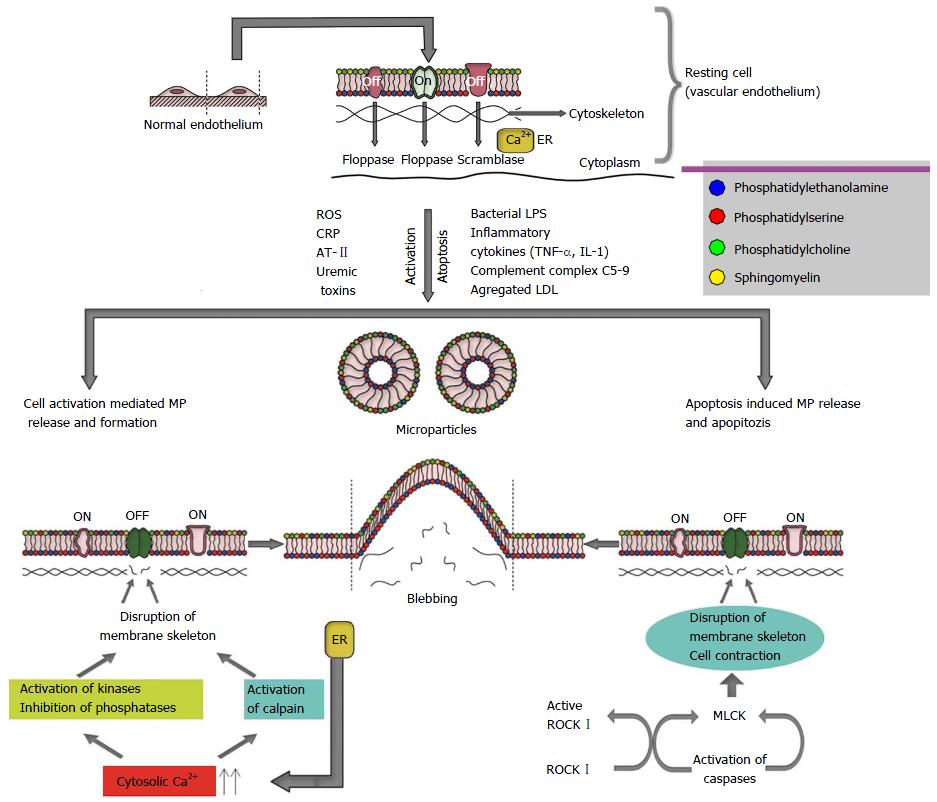
Although the precise molecular mechanism of MP formation is not clear, the breakdown of the membrane skeleton and the loss of phospholipid asymmetry are thought to be essential[9]. Figure 1 shows the proposed mechanisms leading to MP formation. The outer blebbing of the plasma membrane is the first step that begins the MP formation[9]. A second event involved in the MP formation is the externalization of phosphatidylserine (PS)[9]. The composition and the distribution of cell membrane phospholipids are highly special: Phosphatidyl-ethanolamine (PE) and PS are found in the inner side of the cell membrane, whereas phosphatidylcholine and sphingomyelin are located in the external membrane layer. The maintenance of this asymmetry is crucial and is maintained by three distinct enzymes: Flippases, floppases and scramblases[9,14]. Flippases contribute to the translocation of PS and PE against their electrochemical gradient towards the inner membrane. Floppases catalyze the transport of PS to the outer membrane. Finally, scramblases are ATP-independent and facilitate the movement of PS between both membrane leaflets[27,28]. The loss of phospholipid asymmetry results from activation; apoptosis and necrosis uncover PS on the outer cell surface, which is a key event of the formation of MPs[9,14].
Cell activation and apoptosis are two well-known processes causing the formation of MPs[29]. Vascular endothelium can release MPs in the case of cell activation caused by bacterial lipopolysaccharides, the inflammatory cytokines, including tumor necrosis factor or interleukin-1, the complement complex C5b-9, accumulated low density lipoproteins, uremic toxins, high glucose and reactive oxygen species[9,15,30]. Cell activation causes a prompt release of intracellular calcium from the endoplasmic reticulum (Figure 1). The rise of cytosolic calcium triggers a change in the transmembrane usual state, which activates cytosolic enzymes, including calpain that leads to the disruption of cytoskeleton filaments. Ultimately, such cell membrane changes generate blebbing and dropping of the membrane-derived MP into the extracellular fluid[8] (Figure 1).
Apoptosis is the process of programmed cell death characterized by blebbing, cell contraction, nuclear disruption, increased chromatin concentration and chromosomal DNA fragmentation. When the cells enter the apoptotic process, they cause rapid cellular membrane blebbing. Creation of bleb results from the actin cytoskeleton and actin-myosin contraction tightly controlled by caspase 3-produced Rho kinase I activation[31-33] (Figure 1).
The surface of the released MPs has special biochemical features leading to important consequences in the blood and tissue. First, PS binds to annexin V, which is usually used to define and count total MP amounts. However, the binding of annexin V is unspecific. Second, PS abundance supplies multiple binding sites for the coagulation factors providing MP pro-coagulant activity. Finally, lipid and protein content of MP membrane may help characterize the MP and identify their potential biological effects[29].
Although there is consensus on the importance of EMPs, obtained results may show variation even within the same disease likely due to diversity in methodology used for microparticle measurement[34]. For example, freezing may decrease EMPs level regardless of storage duration[34]. In another study[35], It was demonstrated that there was no significant difference in terms of the levels of EMPs between fresh and frozen samples, however, long term storage of samples at -80°, all types of MPs were significantly reduced.
Solid phase capture assay, flow cytometry and ELISA have been used to identify and measure EMPs level in blood. The solid-phase capture assay is able to perform the capture of most of MPs and functional assessment of the circulating MPs having procoagulant potential, irrespective of the capture ligand. The most important weakness of this method is underestimation of MP levels by antigenic capture due to possible interaction of soluble antigens[36]. Flow cytomety is the most widely used technique to quantify EMPs. It can capable of the analysis of thousands of MPs and differentiate the MPs based on their cellular origins[35,36]. Major disadvantages of flow cytometry are its labor-intensiveness, costs and ineffective to detect MPs smaller than 300 nm in diameter[34-36].
Endothelial dysfunction has a major role in the evolution of atherosclerosis. Deterioration of endothelial function evolves in the early stage of kidney disease when the glomerular filtration rate starts to decline and blood pressure increases[2]. The presence of endothelial dysfunction may serve as a marker of an unfavorable cardiovascular prognosis[3,37]. Because EMPs are able to directly impair endothelium-dependent vasodilator mechanisms, the levels of EMPs in patients with CKD are thought to be inversely correlated with endothelial function measured by flow-mediated vasodilatation[25]. In patients with CKD, EMPs may provide not only useful information regarding endothelial dysfunction but may also accelerate preexisting vascular dysfunction by impairing the nitric oxide release from the vascular endothelial (VE) cells[38].
The carotid intima-media thickness (cIMT), carotid artery and primary femoral artery pulse wave velocity (PWV) are used as indicators of early atherosclerosis[11,39]. Recently, we demonstrated that EMPs in the circulation were strongly related to atherosclerosis and arterial stiffness. We showed that PWV and cIMT were increased in uremic children and that both were positively correlated with CD144+ EMP and CD146+ EMP. CD144+ EMP and mean blood pressure values were independent predictors of arterial stiffness, which was measured by PWV[11].
Although the reason of the increased circulating EMPs in hypertensive patients is not completely clear[40], it has been shown that EMPs may induce the progression of impaired endothelial function that already exists via expression of different adhesion molecules, endothelial cyclooxygenase type 2, the release of cytokines, and the impairment of nitric oxide released from VE cells[23,25]. This may cause atherosclerosis, hypertension and target organ damage such as hypertensive nephropathy, which is one of the common complications of high blood pressure. Hypertension is one of the leading causes of CKD in adult and EMPs are involved in impaired renal function in patients with hypertension[41]. Hsu et al[41] studied the relationship between circulating MPs and decline in glomerular filtration rate (GFR) in hypertensive subjects and demonstrated that the ratio of circulating EMP to endothelial progenitor cell (EPC) was associated with deterioration of kidney function. This is likely explained by the impaired vascular repair capacity and increased endothelial damage indicated by higher EMP to EPC ratios may accelerate the decline in GFR in patients with hypertension[41].
Increased MP levels have been reported in a variety of diseases that are especially associated with vascular injury[8]. Soriano et al[42] evaluated the possible relation between VC and the number of EMPs in CKD and investigated whether MPs from CKD patients may directly take part in the pathogenesis of VC. They showed that VC patients had an increased number of EMPs compared to non-VC patients and that MPs from CKD patients having VC raised 3-fold increase of osteocalcin expression, known as an active player in vascular calcification, in vascular smooth muscle cells[42,43]. Chen et al[44] the number of circulating MPs in patients with cardio-renal syndrome with and without coronary artery disease (CAD). They found that CAD was an independent predictor of increased EMPs in patients with CKD and that an increased creatinine level was related to the number of circulating of MPs. On the contrary, Faure et al[6] investigated EMP levels of patients with and without a clinical history of cardiovascular diseases and detected that the ones without a cardiovascular history did not have lower EMP levels compared to the ones with a cardiovascular history. They concluded that CKD patients without vascular diseases suffered from vascular injury associated with high EMP levels.
To date, few studies have examined the circulating EMPs on CKD[6,11,25,38,45]. Boulanger et al[45] indicated that EMPs were increased in end-stage renal disease through low shear stress, which is a major determinant of endothelial apoptosis[45]. Faure et al[6] enumerated the levels of circulating EMPs in pre-dialysis patients with CKD and in HD patients, and they examined the capability of uremic toxins to generate the release of EMP in HUVEC. They demonstrated that the levels of CD144 and CD146+ EMP in the pre-dialysis and hemodialysis groups were significantly higher than those in the healthy controls. They also found out that uremic toxins significantly induced the high level of EMP release by cultured HUVEC. In addition, they demonstrated that there was no difference in CD146+ or CD144 + EMP levels in terms of dialysis membrane (cellulosic vs synthetic) and that HD session did not affect CD146+ or CD144 + EMP levels. Amabile et al[25] demonstrated similar findings. In addition, they examined the relationship between circulating EMPs and arterial dysfunction. They showed that the levels of EMPs correlate the loss of flow-mediated dilatation, increased PWV and an increased carotid artery augmentation index[25]. The increased levels of EMPs in patients with ESRD could be directly related to the presence of uremic toxins, such as p-cresol[6], p-cresyl sulfate[46], indoxyl sulfate[6] and homocysteine[47]. The elevation of EMP may exaggerate endothelial injury caused by the uremic state[6]. The p-cresol limits endothelial cell activation caused by inflammatory cytokines[48]. Both p-cresol and indoxyl sulfate inhibit endothelial proliferation. They are produced by amino acid catabolism as end-products and protein-bound uremic solutes. Thus, they are badly removed by conventional hemodialysis[49]. Altogether, this finding could explain the reason that HD sessions do not change CD146+ or CD144 + EMP levels. The pathogenic role of p-cresol and indoxyl sulfate in the formation of EMPs has been established[50,51]. It is shown that CKD patients had increased serum level of p-cresol and indoxyl sulfate are increased[52]. The p-cresol and indoxyl sulfate can stimulate the vesiculation of cultured endothelial cells in two ways. First, p-cresol affects the endothelial cell cytoskeleton in a Rho kinase-dependent way required for endothelial cell vesiculation[53,54]. Second, p-cresol modifies the actin cytoskeleton organization in endothelial cells, and its inhibitory effect on endothelial proliferation could, in part, be related to its effects on the endothelial actin cytoskeleton[55].
Similar to the case reported in a previous adult study, in our pediatric study, children with CKD (both dialysis and pre-dialysis group) had significantly increased circulating EMPs and cardiovascular risk factors (e.g., blood pressures, PTH, CRP, low albumin, anemia and low GFR) were associated with an increase in EMPs. Additionally, we demonstrated that HD patients had significantly increased EMPs showing endothelial dysfunction compared to PD patients. From this perspective, the data suggested that the deterioration of endothelial function in PD patients is slightly milder than in HD patients[11].
VE-cadherin (CD144) is an endothelial-specific adhesion molecule positioned at junctions between the endothelial cells. It controls special cellular processes, like cell proliferation and apoptosis, and regulates VE growth factor receptor functions[56]. CD 146 known as S-Endo 1-associated antigen is an integral membrane glycoprotein and located at the cell-cell junction in all of the endothelial cells[57]. CD31 known as platelet endothelial cell adhesion molecule-1 is expressed on the both early and mature endothelial cells, platelets, and the majority of leukocyte subpopulations. Its expression on endothelial cells is intensified at cell-cell junctions. CD31 works such a sensor of endothelial cell response to fluid shear stress and participated in the regulation of leukocyte migration along the venular walls[58]. CD51 (Vitronectin receptor α) is a member of type I transmembrane protein and exist on endothelial cells, monocytes, macrophages, and platelets. It is involved in leukocyte homing and rolling. CD105 known as endoglin is a type I membrane glycoprotein presented on the cell surfaces and is a component of the TGF beta receptor complex. It is involved in the cytoskeletal organization affecting cell morphology and migration and has very important function in the development of the cardiovascular system and in vascular remodeling[59]. Hence, EMPs having CD144, CD 146, CD31+/CD41-, CD51 and CD105 may be used to measure the existence and severity of VE cell damage[15]. Unfortunately, we do not have data giving the normal reference of MP in adult and pediatric population and its level based on CKD stage. Recently, we have demonstrated the patients with CKD stage 3-5 had increased EMPs compare to control subjects[11].
CV disease is a major cause of mortality and substantially reduces the life expectancy in patients with CKD[60]. Because arterial damage is thought to be a major contributor to cardiovascular mortality[61], Amabile et al[61] performed a prospective study in 81 stable, hemodialyzed, ESRD patients. They examined the influence of EMPs on all-cause mortality and fatal major cardiovascular events. The preliminary data showed that high levels of EMPs were associated with poor outcome. They were also independent predictors of all-cause and cardiovascular mortality. The most interesting findings in their study was that they determined a cut-off value (1190 events/μL) for global death prediction with 63% sensitivity and 82% specificity (The areas under the curve 0.73 ± 0.065) and a cut-off value (1040 events/μL) for CV death prediction with 83% sensitivity and 75% specificity (the areas under the curve 0.876 ± 0.06)[38]. Hence, the monitoring of EMP levels in patients with CKD might be a useful approach for determining the ones without any symptom for high risk of developing CV diseases. This strategy would provide better risk stratification and introduce inexpensive prophylactic treatments[38].
Although the survival of patients who undergo renal transplantation has improved and more than doubled the expected lifetime of a person with ESRD[62], renal transplant recipients still have high risk of vascular complications, in part due to the effect of immunosuppressive medications[63]. To our knowledge, three studies have examined the role of EMPs in kidney transplantation and the impact of immunosuppressive agents on the kinetics of EMPs in renal transplant recipients (RTRs) during the post-transplant phase[64-66]. Al-Massarani et al[64] analyzed the levels of EMPs at 4 h to 6 h before the graft and at 3, 6, 9 and 12 mo after the transplantation. Similar to previous studies, before the graft, the RTRs had significantly high level of EMPs compared to healthy donors. Following one year post transplant, EMPs levels were significantly decreased regardless of the immunosuppressive treatment. They did not find any difference in the EMP levels between two therapeutic arms (CsA/AZA vs Tac/MMF). They also evaluated the ones with and without a clinical history of cardiovascular disease (HCVD) in terms of EMP levels, and they demonstrated that patients with HCVD had significantly increased EMP levels compared to the patients without HCVD. There was a significant decline in EMP levels in patients without HCVD one year after transplant. The most interesting findings of the study were that patients with CMV infection had high level of EMP and that the presence of CMV was an independent predictor of enhanced EMP[64]. Increased EMP levels in CMV infection are attributed to virus tropism for endothelial cells[67,68].
Endothelial dysfunction observed in dialysis patients improves after kidney transplant, which is likely secondary to the decline in cardiovascular risk factors, like anemia, volume overload, uremic toxins and oxidative stress[53]. The amelioration of cardiovascular risk factors and the recovery of renal function in RTRs could decrease cellular activation and the EMP levels[65].
Although the population of renal transplant recipients with functioning allograft has significantly increased, graft rejection that occurred by cellular, humoral or mix mediated is still one of the major causes for allograft failure[66]. It is well known that the endothelium is the primary target of immunological attack in allograft rejection that could be detected early for effective patient care and management[66]. Unfortunately, serum creatinine (SCr) is a non-specific marker for the diagnosis of allograft dysfunction and kidney biopsy, which is the gold standard diagnostic procedure for the assessment of allograft rejection and is an invasive and expensive procedure. Qamri et al[66] measured EMP and SCr levels in blood plasma before (baseline) and periodically on days 7, 14 and 21, and 2 mo after transplantation and investigated whether the changes in circulating EMP levels were different based on underlying causes of CKD. They showed that the circulating EMP levels from baseline to two months post-transplant in patients with diabetes mellitus who received only kidney allograft, patients with obstructive/inherited isolated kidney disease and patients with immune-complex mediated glomerulonephritis were decreased. An increased circulating EMP level was associated with rejection. When they classified patients based on peritubular capillary (PTC) C4d staining, the circulating EMPs in patients with negative PTC C4d staining were rapidly decreased after treatment for rejection; however, the circulating EMP level decreased more slowly in patients with positive PTC C4d staining that likely showed endothelial activation[66]. Based on the results of the study, it is perceived that antibody mediated endothelial cell injury is involved in allograft rejection. Increased circulating EMPs may give useful information about vascular endothelium in the setting of graft rejection and may provide novel tools for defining or adapting post-transplant therapeutic management[64]. In conclusion, EMPs are small vesicular particles of the endothelial cell membrane detached from endothelium during the process of activation or apoptosis and are considered as a marker of injury in the microvascular endothelial cells, which is a prominent characteristic of acute vascular rejection and chronic allograft nephropathy[9,66]. The circulating EMPs could be used as a marker of VE cell damage and to determine asymptomatic patients who might be at higher risk of developing cardiovascular disease in CKD and renal transplant. However, normal values should be obtained by conducting measurements in healthy subjects, including children from birth to 16 years of age, to use EMP as a reliable marker of vascular dysfunction in clinical practice. We also need a general agreement on methodological aspects of MP assessments to provide an opportunity of inter-laboratory comparisons of the results and determination of normal levels of MPs
We would like to thank Mahmut Albayrak for his help to draw nice Figure.
P- Reviewer: Artunc F, Tain YL
S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 2. | Morris ST, McMurray JJ, Rodger RS, Jardine AG. Impaired endothelium-dependent vasodilatation in uraemia. Nephrol Dial Transplant. 2000;15:1194-1200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 4. | Yamada A, Tashiro A, Hiraiwa T, Komatsu T, Kinukawa T, Ueda N. Long-term outcome of pediatric renal transplantation: a single center study in Japan. Pediatr Transplant. 2014;18:453-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK. Long-term survival in renal transplant recipients with graft function. Kidney Int. 2000;57:307-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 6. | Faure V, Dou L, Sabatier F, Cerini C, Sampol J, Berland Y, Brunet P, Dignat-George F. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J Thromb Haemost. 2006;4:566-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 7. | Vanholder R, Glorieux G, De Smet R, Lameire N. New insights in uremic toxins. Kidney Int Suppl. 2003;53:S6-S10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 8. | Chironi GN, Boulanger CM, Simon A, Dignat-George F, Freyssinet JM, Tedgui A. Endothelial microparticles in diseases. Cell Tissue Res. 2009;335:143-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 325] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 9. | Burger D, Schock S, Thompson CS, Montezano AC, Hakim AM, Touyz RM. Microparticles: biomarkers and beyond. Clin Sci (Lond). 2013;124:423-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 261] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 10. | Biomarkers Definition Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4510] [Cited by in F6Publishing: 3883] [Article Influence: 168.8] [Reference Citation Analysis (0)] |
| 11. | Dursun I, Poyrazoglu HM, Gunduz Z, Ulger H, Yykylmaz A, Dusunsel R, Patyroglu T, Gurgoze M. The relationship between circulating endothelial microparticles and arterial stiffness and atherosclerosis in children with chronic kidney disease. Nephrol Dial Transplant. 2009;24:2511-2518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Dursun I, Düsünsel R, Poyrazoglu HM, Gunduz Z, Patıroglu T, Ulger H, Gurgoze MK. Circulating endothelial microparticles in children with Henoch-Schönlein purpura; preliminary results. Rheumatol Int. 2011;31:1595-1600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Gündüz Z, Dursun İ, Tülpar S, Baştuğ F, Baykan A, Yıkılmaz A, Patıroğlu T, Poyrazoglu HM, Akın L, Yel S. Increased endothelial microparticles in obese and overweight children. J Pediatr Endocrinol Metab. 2012;25:1111-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21:157-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 535] [Cited by in F6Publishing: 557] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 15. | Combes V, Simon AC, Grau GE, Arnoux D, Camoin L, Sabatier F, Mutin M, Sanmarco M, Sampol J, Dignat-George F. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999;104:93-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 549] [Cited by in F6Publishing: 552] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 16. | Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121-1132. [PubMed] [Cited in This Article: ] |
| 17. | Sabatier F, Roux V, Anfosso F, Camoin L, Sampol J, Dignat-George F. Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood. 2002;99:3962-3970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 211] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 18. | Wu SY, Mayneris-Perxachs J, Lovegrove JA, Todd S, Yaqoob P. Fish-oil supplementation alters numbers of circulating endothelial progenitor cells and microparticles independently of eNOS genotype. Am J Clin Nutr. 2014;100:1232-1243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Suades R, Padró T, Alonso R, Mata P, Badimon L. Lipid-lowering therapy with statins reduces microparticle shedding from endothelium, platelets and inflammatory cells. Thromb Haemost. 2013;110:366-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Pelletier F, Garnache-Ottou F, Biichlé S, Vivot A, Humbert P, Saas P, Seillès E, Aubin F. Effects of anti-TNF-α agents on circulating endothelial-derived and platelet-derived microparticles in psoriasis. Exp Dermatol. 2014;23:924-925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Bulut D, Becker V, Mügge A. Acetylsalicylate reduces endothelial and platelet-derived microparticles in patients with coronary artery disease. Can J Physiol Pharmacol. 2011;89:239-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Morel O, Jesel L, Hugel B, Douchet MP, Zupan M, Chauvin M, Freyssinet JM, Toti F. Protective effects of vitamin C on endothelium damage and platelet activation during myocardial infarction in patients with sustained generation of circulating microparticles. J Thromb Haemost. 2003;1:171-177. [PubMed] [Cited in This Article: ] |
| 23. | Mateos-Cáceres PJ, Zamorano-León JJ, Rodríguez-Sierra P, Macaya C, López-Farré AJ. New and old mechanisms associated with hypertension in the elderly. Int J Hypertens. 2012;2012:150107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Brodsky SV, Zhang F, Nasjletti A, Goligorsky MS. Endothelium-derived microparticles impair endothelial function in vitro. Am J Physiol Heart Circ Physiol. 2004;286:H1910-H1915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 279] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 25. | Amabile N, Guérin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London GM, Tedgui A, Boulanger CM. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16:3381-3388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 394] [Cited by in F6Publishing: 419] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 26. | Dignat-George F, Sampol J. Circulating endothelial cells in vascular disorders: new insights into an old concept. Eur J Haematol. 2000;65:215-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 27. | Bevers EM, Comfurius P, Dekkers DW, Zwaal RF. Lipid translocation across the plasma membrane of mammalian cells. Biochim Biophys Acta. 1999;1439:317-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 286] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 28. | Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 731] [Cited by in F6Publishing: 664] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 29. | VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res. 2003;59:277-287. [PubMed] [Cited in This Article: ] |
| 30. | Llorente-Cortés V, Otero-Viñas M, Camino-López S, Llampayas O, Badimon L. Aggregated low-density lipoprotein uptake induces membrane tissue factor procoagulant activity and microparticle release in human vascular smooth muscle cells. Circulation. 2004;110:452-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Boulanger CM, Amabile N, Tedgui A. Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension. 2006;48:180-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 32. | Sebbagh M, Renvoizé C, Hamelin J, Riché N, Bertoglio J, Bréard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 669] [Cited by in F6Publishing: 693] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 33. | Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3:339-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 927] [Cited by in F6Publishing: 925] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 34. | Shah MD, Bergeron AL, Dong JF, López JA. Flow cytometric measurement of microparticles: pitfalls and protocol modifications. Platelets. 2008;19:365-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 35. | Ayers L, Kohler M, Harrison P, Sargent I, Dragovic R, Schaap M, Nieuwland R, Brooks SA, Ferry B. Measurement of circulating cell-derived microparticles by flow cytometry: sources of variability within the assay. Thromb Res. 2011;127:370-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 36. | Jy W, Horstman LL, Jimenez JJ, Ahn YS, Biró E, Nieuwland R, Sturk A, Dignat-George F, Sabatier F, Camoin-Jau L. Measuring circulating cell-derived microparticles. J Thromb Haemost. 2004;2:1842-1851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 280] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 37. | Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol. 2010;6:21-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 38. | Amabile N, Guérin AP, Tedgui A, Boulanger CM, London GM. Predictive value of circulating endothelial microparticles for cardiovascular mortality in end-stage renal failure: a pilot study. Nephrol Dial Transplant. 2012;27:1873-1880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 39. | Covic A, Gusbeth-Tatomir P, Goldsmith DJ. Arterial stiffness in renal patients: an update. Am J Kidney Dis. 2005;45:965-977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Wang JM, Su C, Wang Y, Huang YJ, Yang Z, Chen L, Wu F, Xu SY, Tao J. Elevated circulating endothelial microparticles and brachial-ankle pulse wave velocity in well-controlled hypertensive patients. J Hum Hypertens. 2009;23:307-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Hsu CY, Huang PH, Chiang CH, Leu HB, Huang CC, Chen JW, Lin SJ. Increased circulating endothelial apoptotic microparticle to endothelial progenitor cell ratio is associated with subsequent decline in glomerular filtration rate in hypertensive patients. PLoS One. 2013;8:e68644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Soriano S, Carmona A, Triviño F, Rodriguez M, Alvarez-Benito M, Martín-Malo A, Alvarez-Lara MA, Ramírez R, Aljama P, Carracedo J. Endothelial damage and vascular calcification in patients with chronic kidney disease. Am J Physiol Renal Physiol. 2014;307:F1302-F1311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Idelevich A, Rais Y, Monsonego-Ornan E. Bone Gla protein increases HIF-1alpha-dependent glucose metabolism and induces cartilage and vascular calcification. Arterioscler Thromb Vasc Biol. 2011;31:e55-e71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Chen YL, Chen CH, Wallace CG, Wang HT, Yang CC, Yip HK. Levels of circulating microparticles in patients with chronic cardiorenal disease. J Atheroscler Thromb. 2015;22:247-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Boulanger CM, Amabile N, Guérin AP, Pannier B, Leroyer AS, Mallat CN, Tedgui A, London GM. In vivo shear stress determines circulating levels of endothelial microparticles in end-stage renal disease. Hypertension. 2007;49:902-908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 46. | Meijers BK, Van Kerckhoven S, Verbeke K, Dehaen W, Vanrenterghem Y, Hoylaerts MF, Evenepoel P. The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am J Kidney Dis. 2009;54:891-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 198] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 47. | Sekuła M, Janawa G, Stankiewicz E, Stępień E. Endothelial microparticle formation in moderate concentrations of homocysteine and methionine in vitro. Cell Mol Biol Lett. 2011;16:69-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Dou L, Cerini C, Brunet P, Guilianelli C, Moal V, Grau G, De Smet R, Vanholder R, Sampol J, Berland Y. P-cresol, a uremic toxin, decreases endothelial cell response to inflammatory cytokines. Kidney Int. 2002;62:1999-2009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 49. | Lesaffer G, De Smet R, Lameire N, Dhondt A, Duym P, Vanholder R. Intradialytic removal of protein-bound uraemic toxins: role of solute characteristics and of dialyser membrane. Nephrol Dial Transplant. 2000;15:50-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 189] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 50. | Niwa T, Ise M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J Lab Clin Med. 1994;124:96-104. [PubMed] [Cited in This Article: ] |
| 51. | Enomoto A, Takeda M, Tojo A, Sekine T, Cha SH, Khamdang S, Takayama F, Aoyama I, Nakamura S, Endou H. Role of organic anion transporters in the tubular transport of indoxyl sulfate and the induction of its nephrotoxicity. J Am Soc Nephrol. 2002;13:1711-1720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 241] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 52. | Niwa T, Takeda N, Tatematsu A, Maeda K. Accumulation of indoxyl sulfate, an inhibitor of drug-binding, in uremic serum as demonstrated by internal-surface reversed-phase liquid chromatography. Clin Chem. 1988;34:2264-2267. [PubMed] [Cited in This Article: ] |
| 53. | Cerini C, Dou L, Anfosso F, Sabatier F, Moal V, Glorieux G, De Smet R, Vanholder R, Dignat-George F, Sampol J. P-cresol, a uremic retention solute, alters the endothelial barrier function in vitro. Thromb Haemost. 2004;92:140-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Kanthou C, Tozer GM. The tumor vascular targeting agent combretastatin A-4-phosphate induces reorganization of the actin cytoskeleton and early membrane blebbing in human endothelial cells. Blood. 2002;99:2060-2069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 239] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 55. | Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, Berland Y, Brunet P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004;65:442-451. [PubMed] [Cited in This Article: ] |
| 56. | Petzelbauer P, Halama T, Gröger M. Endothelial adherens junctions. J Investig Dermatol Symp Proc. 2000;5:10-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 57. | Bardin N, George F, Mutin M, Brisson C, Horschowski N, Francés V, Lesaule G, Sampol J. S-Endo 1, a pan-endothelial monoclonal antibody recognizing a novel human endothelial antigen. Tissue Antigens. 1996;48:531-539. [PubMed] [Cited in This Article: ] |
| 58. | Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27:2514-2523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 380] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 59. | Sanz-Rodriguez F, Guerrero-Esteo M, Botella LM, Banville D, Vary CP, Bernabéu C. Endoglin regulates cytoskeletal organization through binding to ZRP-1, a member of the Lim family of proteins. J Biol Chem. 2004;279:32858-32868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 60. | Zoccali C. Cardiovascular risk in uraemic patients-is it fully explained by classical risk factors? Nephrol Dial Transplant. 2000;15:454-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 61. | Amabile N, Boulanger CM, Guerin AP, Tedgui A, London GM. Circulating endothelial microparticles: a novel biomarker for prediction of subsequent death and cardiovascular events in end-stage renal disease. Circulation. 2009;120:s1010. [Cited in This Article: ] |
| 62. | 2007 Annual Report of the U. S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1997–2006. Rockville, MD, Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation 2007; . [Cited in This Article: ] |
| 63. | Silkensen JR. Long-term complications in renal transplantation. J Am Soc Nephrol. 2000;11:582-588. [PubMed] [Cited in This Article: ] |
| 64. | Al-Massarani G, Vacher-Coponat H, Paul P, Widemann A, Arnaud L, Loundou A, Robert S, Berland Y, Dignat-George F, Camoin-Jau L. Impact of immunosuppressive treatment on endothelial biomarkers after kidney transplantation. Am J Transplant. 2008;8:2360-2367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Al-Massarani G, Vacher-Coponat H, Paul P, Arnaud L, Loundou A, Robert S, Moal V, Berland Y, Dignat-George F, Camoin-Jau L. Kidney transplantation decreases the level and procoagulant activity of circulating microparticles. Am J Transplant. 2009;9:550-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 66. | Qamri Z, Pelletier R, Foster J, Kumar S, Momani H, Ware K, Von Visger J, Satoskar A, Nadasdy T, Brodsky SV. Early posttransplant changes in circulating endothelial microparticles in patients with kidney transplantation. Transpl Immunol. 2014;31:60-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 67. | Grefte A, van der Giessen M, van Son W, The TH. Circulating cytomegalovirus (CMV)-infected endothelial cells in patients with an active CMV infection. J Infect Dis. 1993;167:270-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 178] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 68. | Kas-Deelen AM, de Maar EF, Harmsen MC, Driessen C, van Son WJ, The TH. Uninfected and cytomegalic endothelial cells in blood during cytomegalovirus infection: effect of acute rejection. J Infect Dis. 2000;181:721-724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |