Published online Dec 24, 2013. doi: 10.5500/wjt.v3.i4.78
Revised: July 15, 2013
Accepted: July 23, 2013
Published online: December 24, 2013
While life expectancy is greatly improved after a heart transplant, survival is still limited, and compared to the general population, the exercise capacity and health-related quality of life of heart transplant recipients are reduced. Increased exercise capacity is associated with a better prognosis. However, although several studies have documented positive effects of exercise after heart transplantation (HTx), little is known about the type, frequency and intensity of exercise that provides the greatest health benefits. Moreover, the long-term effects of exercise on co-morbidities and survival are also unclear. Exercise restrictions apply to patients with a denervated heart, and for decades, it was believed that the transplanted heart remained denervated. This has since been largely disproved, but despite the new knowledge, the exercise restrictions have largely remained, and up-to-date guidelines on exercise prescription after HTx do not exist. High-intensity, interval based aerobic exercise has repeatedly been documented to have superior positive effects and health benefits compared to moderate exercise. This applies to both healthy subjects as well as in several patient groups, such as patients with metabolic syndrome, coronary artery disease or heart failure. However, whether the effects of this type of exercise are also applicable to heart transplant populations has not yet been fully established. The purpose of this article is to give an overview of the current knowledge about the exercise capacity and effect of exercise among heart transplant recipients and to discuss future exercise strategies.
Core tip: It is time to rethink exercise strategies among heart transplant populations. Chronotropic incompetence is not necessarily a factor that limits exercise capacity in heart transplant recipients, and the exercise restrictions that have traditionally been applied to patients with a denervated heart can be disregarded. High-intensity, interval-based aerobic exercise is superior to moderate exercise in patients with coronary artery disease and heart failure, and the positive effects of this type of exercise seem to also be largely reproducible among heart transplant recipients.
- Citation: Nytrøen K, Gullestad L. Exercise after heart transplantation: An overview. World J Transplant 2013; 3(4): 78-90
- URL: https://www.wjgnet.com/2220-3230/full/v3/i4/78.htm
- DOI: https://dx.doi.org/10.5500/wjt.v3.i4.78
Heart transplantation (HTx) gives numerous patients with end-stage heart disease a second chance at life. However, although life expectancy is greatly improved, survival is reduced, mainly due to the increased frequency of late complications. Additionally, the patient’s exercise capacity and health-related quality of life (HRQoL) are reduced compared with the general population[1]. Exercise capacity improves after a HTx when compared with end-stage heart failure[2-8], but continues to be subnormal when compared with age-matched values in healthy individuals. In most studies, the peak oxygen uptake (VO2peak) levels range from 50% to 70% of the general population (Table 1), and a reduced VO2peak level is generally associated with a poorer prognosis[9]. Only a few studies have reported individuals reaching close to normal VO2peak levels[10,11]. Both central hemodynamics and peripheral physiological abnormalities may explain the reduced exercise capacity of these patients (Table 2). These factors include reduced cardiac output due to chronotropic incompetence or reduced stroke volume, peripheral abnormalities, including reduced muscle strength and oxidative capacity, and abnormal blood supply due to impaired vasodilatory capacity or capillary density[12].
| Study | n | Mean age (yr) | Mean time after HTx | VO2peak: mL/kg per minute or L/min | VO2peak (% of age-predicted value) | Percent of age-predicted HRmax or actual HRmax (bpm) |
| Renlund et al[123] | 110 | 51 ± 10 | 26 mo | 17.7 ± 0.3 mL | 64% ± 1% | 85% |
| Mandak et al[51] | 60 | 52 ± 10 | 1 yr | 16.2 ± 3.8 mL | NA | 137 ± 24 |
| Osada et al[124] | 56 | 50 ± 12 | 3 yr | 20.0 ± 5.0 mL | 70% ± 17% | 88% ± 11% |
| Notarius et al[12] | 12 | 51 ± 4 | 8 mo | 17.3 ± 1.7 mL | 57% | 147 ± 7 |
| Douard et al[30] | 85 | 52 ± 12 | 0-100 mo | 21.1 ± 6 mL | NA | 85% ± 13% |
| Squires et al[24] | 95 | 48 ± 14 | 1 yr | 19.9 ± 4.8 mL | 61% ± 15% | 138 ± 22 |
| Gullestad et al[48] | 174 | 51 ± 1 | 3.5 yr | 19.4 ± 0.4 mL | 70% ± 1% | 146 ± 2 |
| Myers et al[125] | 47 | 47 ± 12 | 4.8 yr | 9.4 ± 2.6 mL | 59% ± 14% | 129 ± 18 |
| Schmid et al[126] | 17 | 58 ± 13 | 65 ± 27 | 20.9 ± 5.2 mL | NA | 136 ± 12 |
| Richard et al[127] | 7 | 40 ± 13 | 2 yr | NA | 101% ± 12% | 93% ± 9% |
| Carter et al[2] | 47 | 48 | 5 yr | 16.1 ± 0.5 mL | 51% ± 1.5% | 74% ± 1% |
| Ulubay et al[118] | 7 | 43 ± 14 | 19 mo | 1.45 ± 0.33 L | NA | 114 ± 41 |
| Central factors |
| Reduced cardiac output |
| Chronotropic incompetence |
| Reduced stroke volume |
| Systolic dysfunction |
| Diastolic dysfunction |
| Pulmonary dysfunction |
| Pulmonary hypertension |
| Lung disease |
| Pulmonary congestion |
| Peripheral factors |
| Decreased skeletal muscle function |
| Reduced muscle mass |
| Reduced muscle strength |
| Reduced capillary density |
| Reduced oxidative capacity |
| Reduced mitochondrial function |
| Corticosteroid induced myopathy |
| Impaired vasodilatory capacity |
| Endothelial dysfunction |
| Deconditioning |
| Potential factors contributing to reduced exercise capacity |
| Increasing age |
| Donor age |
| Donor match |
| Ischemic time |
| Pre transplant de-conditioning |
| Primary diagnosis |
| Co-morbidities |
| Smoking |
| Cultural differences |
| Gender differences |
| Anxiety and depression |
| Socio-economic status |
| Reduced health-related quality of life |
While several studies have demonstrated an effect of aerobic exercise after HTx, the majority of studies used a protocol consisting of moderate training (Table 3). Traditionally, mainly due to chronotropic incompetence because of denervation, HTx recipients have not been exposed to interval-based exercise with higher intensity because it has been considered “unphysiological”. High-intensity interval training (HIT) has repeatedly proven to be a highly efficient form of exercise for improving the physical capacity of both normal subjects and patients with coronary artery disease (CAD) and heart failure[13-15]. While the exact results in various patient groups are varied, HIT has demonstrated improvements in both central and peripheral factors, such as stroke volume, left ventricle (LV) remodeling, blood volume and flow, blood pressure, endothelial function, biochemical markers, skeletal muscle function and HRQoL[14-16]. However, except for two small studies published in 2004 and 2011[11,16], HIT has not been used as an intervention among HTx recipients. A systematic review and meta-analysis regarding the effects of exercise in solid organ transplant recipients were recently published[17], concluding that “exercise training is a promising but unproven intervention for improving cardiovascular outcomes of solid organ transplant recipients”. Because the existing randomized controlled trials (RCTs) are small and of relatively short duration, more large-scale RCTs are urgently needed to develop clear and evidence-based guidelines regarding exercise prescriptions after HTx.
| Study | n, mean age (yr) | Mean time after HTx | Intervention | Mean change in VO2peak (mL/kg per minute) within groups | Mean change in VO2peak (mL/kg per minute) between groups |
| Kobashigawa et al[3] | 27, 52 | 1 mo | 6 mo partly supervised rehabilitation program vs controls CG | Ex: 9.2 → 13.6 | 2.5 |
| Walking: cycling and upper and lower limb exercises for 30 min × 1-3/wk | Con: 10.4 → 12.3 | ||||
| Tegtbur et al[26] | 32, 55 | 5 yr | 1 yr home-based exercise program vs controls | Ex: 18.6 → 20.0 | 1.3 |
| Cycling every other day for 1 yr at 80%-90% of maximum HR | Con: 18.9 → 19.0 | ||||
| Bernardi et al[6] | 24, 52 | 6 mo | 6 mo home training vs controls | Ex: 14.9 → 19.6 | 3.4 |
| Cycling at 60%-70% of VO2peak 30 min × 5/wk × 6 mo | Con: 14.3 → 15.6 | ||||
| Karapolat et al[5] | 28, 42 | 1.5 yr | 8 wk supervised hospital training vs home-based training | ||
| 1.5 h of multiple exercises: including aerobic exercise for 30 min at 60%-70% of VO2peak× 3/wk | Ex: 16.7 → 19.5 | 3.4 | |||
| The controls received written guidelines on exercises and a walking program | Con: 20.1 → 19.5 | ||||
| Wu et al[27] | 37, 56 | 2 yr | 8 wk home training vs controls | ||
| Strength training and aerobic exercise at 60%-70% of VO2peak: 35-40 min × 3/wk | Ex: 12.1 → 13.2 | 1.6 | |||
| Con: 13.7 → 13.2 | |||||
| Haykowsky et al[25] | 43, 59 | 5 yr | 12 wk aerobic/strength training vs controls | ||
| First 8 wk: continuous aerobic exercise at 60%-80% of VO2peak: 30-45 min × 2/wk | |||||
| Continuous aerobic training at 80% of VO2peak, 45 min × 2/wk and bicycle interval training for 30 s at 90%-100% of VO2peak: followed by 60 s rest for 10-25 reps × 2/wk in the final 4 wk | Ex: 21.2 → 24.7 | 3.5 | |||
| Resistance training at 50% of 1RM: 10-15 reps × 1-2 sets × 4 exercises × 2/wk for 12 wk | Con: 18.2 → 18.2 | ||||
| Hermann et al[16] | 27, 50 | 7 yr | 8 wk high-intensity interval training vs controls | ||
| Interval blocks of 4 min/2 min/30 s: corresponding to 80%: 85% and 90% of VO2peak: respectively: and recovery periods of ½ min: and finally staircase running at 80% of VO2peak: followed by recovery walking 60 min × 3/wk | Ex: 23.9 → 28.3 | 5.6 | |||
| Con: 24.6 → 23.4 | |||||
| Nytrøen et al[18] | 48, 51 | 4 yr | 1 yr high-intensity interval training vs controls | ||
| 4 interval blocks of 4 min each performed at 91% of peak HR: with 3 min active recovery periods between each block | Ex: 27.7 → 30.9 | 3.6 | |||
| 3 periods of 8 wk distributed throughout 1 yr with exercise 3/wk for a total of 72 exercise sessions | Con: 28.5 → 28.0 |
The most recent RCT assessing effect of exercise after HTx[18], which was also the largest to date, was published too late to be included in that systematic review regarding the effects of exercise in solid organ transplant recipients[17], but the study is presented in Table 3 and will be referred to throughout this article.
In contrast to the chronotropic response of a normal heart to exercise, a newly transplanted heart is denervated, which causes higher resting heart rate (HR) and reduced HR response (chronotropic incompetence)[8]. The HR response during exercise is mainly controlled by catecholamines from the adrenal glands, resulting in a significantly slower increase of the HR at onset of exercise, a reduced peak HR, and a delayed return towards resting values after cessation of exercise[4,8,19-21]. It is a common belief that this slow HR is of great importance when designing rehabilitation programs early after HTx, especially during the very first year. An improved HR response to exercise has been demonstrated during the first year after surgery[21], but it is unclear if this is due to reinnervation, and if so, what the functional importance of possible reinnervation is[22-24].
Knowledge about the denervated heart is important in order to adjust the exercise protocol to achieve the optimal effect of physical exercise. There are several small studies which have shown that aerobic exercise gives a higher exercise capacity in HTx recipients[3,5,6,25-28]. The exercise protocols used, which have mainly consisted of steady-state training with moderate intensity, have shown positive effects[3,5,6,26-28]. However, the increase in exercise capacity and the VO2peak levels reached are moderate[3,5,6,26-28].
Several reports have been published on the effects of rehabilitation and exercise in non-transplant patients. The main conclusion is that high-intensity, aerobic training, especially interval-based training, is a favorable type of exercise that yields improvements in both peripheral and central factors[13,14,29]. Wisløff et al[14] showed that interval training improved VO2peak with 46% in patients with heart failure, but it has been unclear whether this type of exercise is suitable for HTx patients.
It is assumed that the delayed HR response after HTx is a limitation in regard to adapting to interval training. Presently, it is commonly believed that because of the slow HR of these patients, the session should begin with a thorough warm-up period, which should be followed by steady-state (Steady-state training refers to no rapid changes in intensity or exercising with an even HR) aerobic exercise. Although the HR response to exercise improves with time after HTx, the prevailing opinion is that these patients should not participate in interval training. This considerably limits their possibilities in joining existing rehabilitation programs in their home environment. Additionally, it has not yet been thoroughly investigated if this form of exercise is really unsuitable for this group of patients.
Both central hemodynamic and peripheral physiological factors most likely contribute to the reduced exercise capacity in HTx recipients (Table 2). The central factors include chronotropic incompetence, impaired LV function or greater arteriovenous oxygen difference, while peripheral limitations include reduced muscle mass, anabolic resistance due to reduced muscle strength and oxidative capacity, or abnormal blood supply due to impaired vasodilatory capacity and capillary density. Several factors specific for HTx patients, such as immunosuppressive regimens, donor age, and ischemic time, as well as general factors, such as smoking status, prolonged de-conditioning, co-morbidities, socio-economic status, and cultural differences, may contribute to their reduced performance[7,12,20,30-33] (Table 2).
The American College of Sports Medicine[34] and American Heart Association[35] recommend exercising with an intensity between 50% and 90% of maximum VO2, which refers to approximately 60%-95% of the maximum HR. In comparison to the detailed prescription of different medications, this is a very imprecise recommendation. As such, it causes difficulties for both the health personnel who are obligated to give advice based on these recommendations, and the patients who are trying to carry out these vague exercise prescriptions. One of the reasons for the imprecise recommendations is that there has been uncertainty and disagreement regarding how VO2peak improves most efficiently. The majority of researchers in the field now agree that the major factor limiting an individual’s VO2peak is the stroke volume. Given that maximum HR cannot be increased, the stroke volume is the limiting (and only) factor that, through exercise, may improve cardiac output[36]. It is reasonable to think that exercising at a near to maximum stroke volume would give the best results. The previous belief was that maximum stroke volume was reached at approximately 50%-70% of maximum HR[37,38], and this is still stated in most textbooks[36], even though it was shown as early as in the 1960s that stroke volume does not necessarily plateau in this range[39]. Additional and more recent research has documented, both in untrained, moderate and well trained subjects, that the stroke volume often does not reach a plateau until the HR is close to its maximum[40-44]. This has not been documented in patients with CAD, but several studies of high-intensity exercise interventions have documented a superior effect of such exercise compared to exercise at a moderate intensity, both in patients with heart failure and CAD[13,14,45-47].
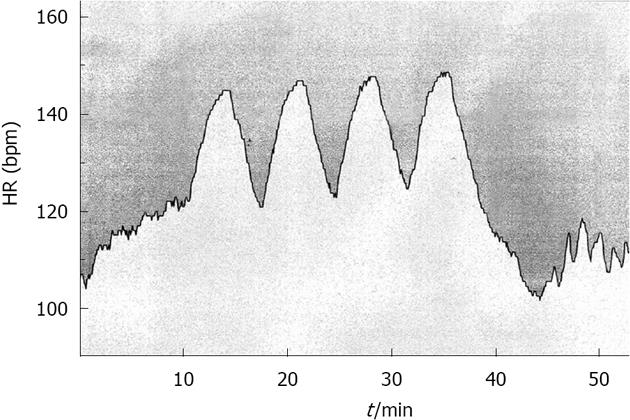
Most individuals should be able to reach an intensity of approximately 90%-95% of their maximum HR within 1-2 min. Based on this, the leading research community of this field in Norway (Norwegian University of Science and Technology, Trondheim) have proposed and documented that 4 exercise bouts of 4 min each (4 × 4) (Figure 1), with an active break in between, is an exercise prescription that is highly effective and works very well[38].
As shown in Table 3, only a limited number of RCTs have investigated the effect of exercise in HTx recipients. Furthermore, most of these studies compared some form of rehabilitation/exercise program with a control group that was not receiving any specific type of exercise strategy, and most of the exercises performed in the intervention groups were performed in-hospital and at a moderate intensity. It is challenging to conduct large-scale, high-quality, in-hospital exercise studies because the HTx recipients often live very far away from the transplantation centers, and it is difficult and expensive to recruit enough patients to participate in a long-term exercise program very far away from their home. Thus, a training program that is close to the patients’ home might be more desirable. We tested such an approach in a study investigating the effect of HIT in HTx recipients[18], and even though Norway is a small country with a very small HTx population, this was manageable because we designed the intervention to be carried out individually, in each of the patients’ home communities.
Each participant was assigned to a local physical therapist who cooperated closely with our hospital and the people in charge of the trial. The exercise intervention, which lasted for 1 year, was divided into three main, intensive exercise periods lasting 8 wk each. After each 8-wk period, the project group and the local physical therapist discussed the results and the improvements in each individual patient thus far and made plans for the next intensive exercise period. The evaluation of the training period included HR analyses from the HR monitors and exercise logs, re-testing of the patient’s maximum HR and adjustment of the desired training zones, if necessary.
When the study first started, we were anxious to see whether this fairly ambitious exercise intervention would and could be sustained by the participants for a full year. Of the 52 patients who were initially recruited, 48 completed the follow-up, and the results exceeded our expectations, showing that a mean of 96% of the planned exercise sessions were completed at the target intensity throughout the year, without any adverse events. Four patients did not complete the study due to other reasons. Thus, we feel that it is safe to conclude that this form of exercise is both highly applicable and safe in stable, long-term HTx recipients[18]. Our results are in accordance with the only other two existing studies that introduced HIT to HTx recipients[11,16], but future large-scale studies are needed to confirm the observed effects on peak exercise capacity and determine the mechanisms by which this occurs. Additionally, the studies should examine whether HIT has beneficial effects besides improving exercise capacity, such as in reducing transplant related complications. Furthermore, HIT also needs to be compared to other exercise strategies and not only control groups receiving no particular training regimen.
The first RCT investigating the effect of exercise in HTx recipients was published by Kobashigawa et al[3]. The trial included patients who were 1 mo post HTx and compared a 6-mo rehabilitation program to a control group receiving no specific exercise strategy. The mean change between the groups at follow-up was 3.4 mL/kg per minute (P < 0.001), but the VO2peak level reached at the end of the intervention was low compared to the values predicted for healthy subjects of similar age and sex. This was also reflected in most of the subsequent trials. Table 3 describes the details of the published RCTs during the period from 1999 to 2012. The mean VO2peak values reached after the exercise interventions ranged from 13.2 to 28.3 mL/kg per minute in the various studies, and the mean change between the control and exercise groups ranges from 1.3 to 5.6 mL/kg per minute. Except from the two previously mentioned randomized HIT-studies[16,18], the mean intensity of the aerobic training was reported to be 60%-80% of VO2peak in the majority of the studies, the exercise duration ranged from 30 min to 1.5 h, 2-5 times per week for 8 wk up to 1 year, the mean time after HTx ranged from 1 mo up to 7 years, and the number of participants ranged from 24 to 43 (Table 3).
Based on the great differences between these trials, the somewhat inconsistent results and the lack of a control group undergoing a different exercise strategy in most of the RCTs, it can only be concluded that exercise improves VO2peak; it is not yet possible to state which type of exercise, intensity and duration gives optimal results, although a HIT-intervention seems to be favorable.
Most of the studies describing VO2peak levels in HTx recipients reported that the levels to be between 50% and 70% of predicted values[2,7,20,48]. The highest level reported was from a study carried out at our center[18]. The exercise group improved their VO2peak level from 27.7 to 30.9 mL/kg per minute, corresponding to 80% to 89% of predicted values in healthy subjects (Table 1). The high baseline VO2peak values may have been a result of selection bias, based on the design of the study, the exercise intervention and the inclusion criteria. However, considering the wide range of VO2peak values (from 13.9 to 44.0 mL/kg per minute, corresponding to 46% to 130% of predicted) and the normally distributed data, this suggests a heterogeneous group rather than a selected group of well fit HTx recipients, and the data might mirror the stable and healthy Norwegian HTx population quite well. Our high levels are supported by another recent Nordic RCT investigating the effect of high-intensity exercise in HTx recipients[16]. In that study, the authors demonstrated higher than average VO2peak values, with a baseline value of 23.9 improving to 28.3 mL/kg per minute at follow-up, even with a considerably higher mean time after HTx of 7 years vs 4 years in our study. It is unclear whether this could be a reflection that Scandinavian HTx recipients have levels above average, or if it is due to type of test protocol used in other studies and/or uncertainty about whether maximal intensity really was reached during the exercise test. However, we believe that HIT interventions[16,18] likely induced a greater effect than moderate training. This is in accordance with previous studies among patients with CAD[13] and left ventricular dysfunction[14], which have used comparable HIT protocols.
At follow-up, the mean change in VO2peak between the groups in our study[18] was 3.6 mL/kg per minute. This is similar to three of the other RCTs involving moderate training that are presented in Table 3[6,25,28]. However, it is important to note that two of these studies[6,28] had considerably lower baseline VO2peak values and that it is well known that subjects with low initial VO2peak levels easily gain greater improvements than those with fairly high baseline values[49]. Haykowsky et al[25] demonstrated a similar improved mean VO2peak follow-up value in the exercise group (24.7 mL/kg per minute vs 30.9 mL/kg per minute) and reported a similar mean change between the groups of 3.5 mL/kg per minute. This exercise intervention[25] also included elements of HIT, which makes a more suitable comparison for our study. Haykowsky’s study[25], which was published in April 2009, the same year we started including patients in our RCT, was then the RCT with the highest demonstrated VO2peak improvement among HTx recipients. In 2011, during the course of our study, Hermann et al[16] published the results from their study, demonstrating an overwhelming difference of 5.6 mL/kg per minute between the exercise group and the control group after 8 wk of exercising 3 times per week. Although it is questionable why and how the control group reduced their VO2peak level from 24.6 to 23.4 mL/kg per minute in only 8 wk, which contributed to the large mean difference between the groups, the exercise group still had a remarkable mean improvement of 4.4 mL/kg per minute, substantially supporting HIT as a highly effective form of exercise in long-term HTx recipients. Similarly, our study supports HIT as a safe, applicable and effective form of exercise, and the field is now ready and in need of future studies investigating the effects of HIT compared to other exercise interventions. The timing is also important, as the health benefits may be even greater if the intervention is started earlier, that is, shortly after HTx.
In the 1990s, it was widely believed that “total denervation persists in the human heart following cardiac transplantation”[50] and “the lack of alteration in the HR response over time, suggests that no significant functional reinnervation occurs”[51]. This was the common belief in most research communities in early studies among HTx recipients. However, in last decade, a body of evidence has repudiated this statement. Nevertheless, several studies evaluating sympathetic and parasympathetic reinnervation have yielded somewhat contradicting results, especially with respect to possible parasympathetic reinnervation[52-56]. The evidence of sympathetic reinnervation seems to be more frequent and certain, but is inconsistent in nature[57-63]. Multiple different direct and indirect methods of evaluating reinnervation, such as HR variability analysis[64], cardial release of noradrenalin[22], positron-emission tomography[65] and the evaluation of the chronotropic response as a sign of functional reinnervation, have also yielded varying results[66].
The normalization of the chronotropic responses is associated with functional reinnervation and better exercise capacity[6,30,57-60,65]. Along the same lines, the reduced exercise capacity in HTx recipients is generally associated with chronotropic incompetence due to denervation. Multiple studies showing partial normalization of the HR response have reported discrepant results regarding the degree of normalization and percent of subjects developing normalization, in addition to great differences according the time after HTx when the improved chronotropic response is confirmed. Bernardi et al[6] showed that autonomic nervous control can be improved by physical training, while others have proposed that reinnervation occurs independently over time[4,61,67]. Richard et al[10] and Pokan et al[11] have shown peak HR values close to or above age-predicted in HTx recipients. These findings are supported by a study from our center[21], in which we documented a high degree of normalization of chronotropic responses within 6 mo after HTx. This occurred earlier and with a higher degree of normalization than demonstrated by others[2,24,62,67-69]. The high degree of normalization during the first year after HTx[21] was confirmed in a different long-term HTx population in the previously discussed HIT-intervention study[18]. The exercise group in this study significantly improved their peak HR from 154 to 163 bpm, whereas the control group remained unchanged (154 bpm vs 153 bpm). This finding supports Bernardi et al[6], but it is still unclear whether time alone may result in the normalization of chronotropic responses or whether it occurs in combination with exercise or others factors.
Because it has been assumed that chronotropic incompetence in HTx recipients is a limitation towards adapting to interval training, and because the patients have atypical central and peripheral responses to exercise, previously described training regimens have mostly consisted of moderate, steady-state intensity exercise[4,17,32] (Table 3). Only a few previous studies have described close to normal HR responses in HTx recipients[10,11]. These, together with our recent studies[18,21], have provided increasing evidence suggesting that HR response is not a limiting factor for exercise capacity in the majority of HTx patients. Hopefully, this finding will contribute to minimizing the persistent exercise restrictions that apply to patients with denervated hearts. A future challenge is to identify which factors influence the reinnervation process and why it is inconsistent and does not occur in all HTx subjects.
The absence of parasympathetic activity is clearly evident in the denervated heart, which has an elevated resting HR, often more than 100 bpm[70]. Resting HR reflects vagal tone and HR recovery is known to be a marker of parasympathetic activity[71-74] while HR increase at onset of exercise and peak HR reflect sympathetic activity[19,70,75]. The improved HR increase and close to normal peak HR and HR reserve observed in several studies[10,11,18,21] support the notion of functional, sympathetic reinnervation. In one of our studies[21], we found improved HR recovery, a marker of parasympathetic activity[70,72,73,76], thus suggesting parasympathetic reinnervation. In contrast, persistent elevated resting HR[21] was not consistent with vagal reinnervation. Although we also found a significant lower resting HR in another study[18], suggesting improved vagal reinnervation, our results only confirm the inconsistencies in the literature regarding reinnervation in general.
Pulmonary diffusion, cardiac output and blood volume are regarded as the main central limitations to oxygen delivery, while the role of peripheral factors limiting VO2peak has been an object of greater discussion[77]. While it is agreed upon that VO2peak is dependent on the interaction between O2-transport and muscle (mitochondrial) consumption of O2, there is disagreement as to which of these is the main determinant[77]. The results vary largely in trained vs sedentary subjects or in different patient groups vs normal subjects. In athletes, as in patients with chronic lung disease, pulmonary diffusion seems to be the greatest limitation. In healthy, untrained subjects and in patients with heart failure, the principal limiting factor is cardiac output, often combined with skeletal muscle limitations[78]. In HTx recipients, it is assumed that reduced exercise capacity is due to a combination of central and peripheral physiological abnormalities[12,79], but the mechanisms behind this subnormal capacity is not completely understood. Thus, we initially hypothesized in our recent trial[18,80] that a possible increase in VO2peak after a HIT intervention would be positively affected by both central and peripheral factors. To study myocardial performance, we performed thorough examinations of the systolic and diastolic function of all participants using newer echocardiographic techniques, both at rest and during sub-maximal exercise (bicycle ergometer). In contrast to the documented improved cardiac function of HIT in patients with cardiovascular diseases in general, we found, rather surprisingly, no alterations of clinical importance, either in cardiac systolic or diastolic function as assessed by echocardiographic measurements[80]. This suggests that HTx recipients respond differently to HIT than other groups of patients. Muscle diffusion capacity, mitochondrial enzyme levels and capillary density are other potential peripheral sites for VO2peak limitation[33,77]. Although most research supports cardiovascular delivery as the central component in VO2peak, the importance of skeletal muscle function should not be underestimated[33]. Borrelli et al[82] found that the main gain in VO2peak was at the peripheral level. In accordance with this, we found that muscular exercise capacity and amount of body fat were strong factors predicting VO2peak in a group of HTx recipients[83]. These findings were confirmed in a follow-up study[18], where the same peripheral factors made the most significant contributions to the improvement in VO2peak. These findings suggests that peripheral muscular and metabolic alterations have a substantial impact on the aerobic exercise capacity in HTx recipients and that they may have a greater impact than cardiac limitations[33].
However, despite the absence of detectable echocardiographic improvements in the current study[80], the HIT group demonstrated a higher O2-pulse and lower HR at sub-maximal exercise levels, which indicate increased stroke volume. In addition, the chronotropic response index (CRI), which reflects both the maximum HR and the resting HR, significantly increased from 0.89 to 0.95. These somewhat contradictory findings regarding cardiac function may be explained by the small number of observations, possibly causing a type 2 statistical error. Echocardiographic measurements during peak exercise and a higher number of observations could reveal an undetected improvement in cardiac function in future studies.
Because of the initially high and close to normal CRI, and a mean time of 4 years after HTx, we did not expect any notable improvement in the chronotropic responses. Nevertheless, at follow-up, the HIT group showed a significant increase in their CRI as a result of both a lower resting HR and a higher peak HR[18]. However, it is still unclear whether improved autonomic nervous control is a result of exercise[6] or if it occurs independently, as a result of time[4,63]. As previously mentioned, a large number of studies have documented partial sympathetic reinnervation. This appears to be greatest during the first few years after a HTx and then gradually decreases[4,59]. The findings in our study[18] indicate that the peak HR, despite a close to normal level at baseline (4 years post HTx), can still be influenced by intensive exercise and fully reach, or even exceed, the expected maximum HR. A total of 30% of the patients in the current study had an expected maximum HR > 100% (range 100%-111%).
In summary, it seems quite clear that HTx recipients respond differently to exercise than other patient groups and that the beneficial training effects in a number of studies[18,25,82] predominantly rely on peripheral mechanisms, especially muscular exercise capacity. However, some of the findings in our study[18] also suggest some improvements in cardiac function and that autonomic regulation is improved and close to normal. Further investigation is needed to establish why and how the transplanted hearts respond differently to high-intensity exercise than normal subjects and other patients groups.
It is well known that many HTx recipients develop a number of complications, such as cardiac allograft vasculopathy (CAV), graft failure, renal failure, cancer, gout, metabolic disturbances, including hyperlipidemia and diabetes mellitus, and reduced HRQoL. These may all contribute to increased morbidity and reduced survival. In general, exercise might have beneficial effects on several of these parameters in the general population and in different patients groups. However, little is known about the role of exercise on transplant related complications. The few RCTs that have been published[17,32] (Table 3) have mainly documented the effects of exercise on exercise capacity, muscle strength, body composition, endothelial function, some inflammatory biomarkers and HRQoL. However, detailed knowledge regarding other effects of exercise, such as on metabolic disturbances, renal function and co-morbidities, does not exist. Studies describing factors associated with exercise capacity also provide limited and uncertain information because the specific factors considered varies greatly[30,31,48,83].
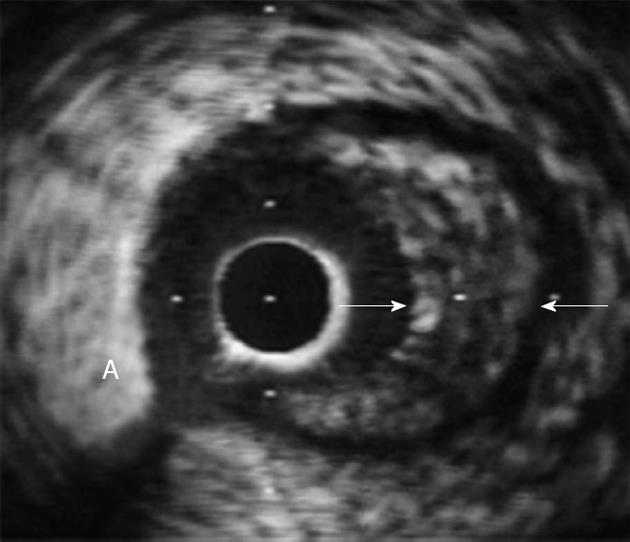
We recently examined the effect of HIT on CAV[84]. CAV (Figure 2) is a rapidly progressive form of atherosclerosis that occurs in HTx recipients and involves diffuse thickening and occlusion of the coronary arteries[85]. The classic early sign of CAV, intimal thickening, is present in approximately 58% of the arteries during the first year after HTx[86,87]. Later, luminal stenosis of the epicardial branches and occlusion of the smaller arteries develop, resulting secondarily in myocardial ischemia and infarction[88]. Treating CAV is a great therapeutic challenge, and it is the leading cause of late graft-loss and death among HTx patients. The only real cure for severe CAV is retransplantation[85,88]. Because of denervation, myocardial ischemia may be asymptomatic in many cases, and CAV can only be diagnosed by coronary angiography or intravascular ultra sound (IVUS). IVUS increases the sensitivity for early diagnosis compared to traditional angiography[85] (Figure 2).
Existing therapy options, both for the prevention and treatment of CAV, including immunosuppressive drugs and statin therapy early after HTx, have so far not given the desired results[88-90]. In clinical practice, prophylaxis of CAV involves modification of general risk factors, such as smoking, obesity, diabetes and hypertension, as well as the implementation of physical activity[91]. However, although the atheroprotective effect of exercise and the effect of physical activity on established CAD is well documented[92-94], with HIT being reported to have a more pronounced effect[14,38], knowledge about the specific effect of exercise on CAV is very limited.
CAV continues to limit the long term success of HTx; CAV is, in addition to malignancy, the most important causes of death in patients who survive the first year after HTx. In our recent study[84], we demonstrated, using serial IVUS measurements, that the progression of CAV was reduced by more than 50% in the HIT group compared to the control group[84]. Although no previous reports on the effect of exercise on CAV progression in HTx patients exist, our finding is in accordance with the effect of exercise on progression of coronary atherosclerosis among patients with CAD[95,96], specifically, an increased threshold for chest pain[97]. In a recent study by Yoshikawa et al[95], it was shown that a high VO2peak level was associated with healthier tissue composition and less coronary plaque in patients with CAD. Additionally, a recent review article summarizing the impact of exercise training on arterial wall thickness concluded that exercise can decrease arterial wall thickness in subjects with CAD or with cardiovascular risk factors, as well as in healthy subjects[96]. However, what type of exercise, frequency, duration and intensity that gives the best results remain to be determined.
In contrast to most studies investigating the effect of exercise on cardiovascular health, our study[84] had a long-term, high-intensity exercise intervention. If the effect on CAV is due to this mode of exercise is uncertain and needs to be confirmed in future studies. However, we believe that a high-intensity program is needed, given that the control group in our study must be considered a moderate training group as they performed a considerable amount of exercise during the study period. Specifically, only 33% exercised little or not at all, and 67% exercised 2 times or more per week.
Several mechanisms are involved in the initiation and progression of CAV, including innate and adaptive immune responses, as well as risk factors, such as smoking, hypertension, hyperglycemia, hypercholesterolemia, body mass index (BMI) and metabolic disturbances[98]. In our study, we found that the progression of CAV, as assessed by an increase in percent atheroma volume (PAV) > 1.5%, was associated with a significantly higher mean change in weight, BMI and visceral fat. Because a high BMI and visceral fat are associated with increased inflammation, which is a well-known factor contributing to the development of endothelial dysfunction, atherosclerosis and CAV[99], a possible mechanism by which HIT affects CAV progression could be mediated by a reduction in the inflammatory burden. This is consistent with studies among patients with CAD that have suggested that the effect of exercise on atherosclerosis may be explained, to some extent, by its influence on metabolism and its anti-inflammatory effects[100,101]. However, in the present study[84], other than significantly lower IL-8 level at follow-up (within-group), a numerically lower level of C-reactive protein (CRP) and a numerically higher level of IL-6 in the HIT group, there was no clear effect of HIT on the differential expression inflammatory mediators between the groups. This contrasts the findings of Hermann et al[16], who carried out a comparable HIT intervention and found a significant reduction of CRP in the HTx exercise group. The reason for the discrepant finding is unclear but could be related to patient population, duration of exercise or timing of blood sampling. We cannot rule out that exercise could have a beneficial effect on vascular inflammation that is not associated with a systemic inflammatory response. Lower increases in PAV were associated with a reduction in visceral fat, as well as BMI and weight[84], which could be due to an effect of HIT on adipocyte-derived mediators. This needs to be clarified in future studies.
Studies investigating HRQoL after HTx have clearly demonstrated that HTx recipients have significantly improved HRQoL compared to the pre transplant stage[102-107]. These studies have mainly used generic questionnaires or a combination of generic and disease specific questionnaires[102-107]. Several studies have reported that the improved HRQoL also remains high in the long-term after HTx[102,103,108-111]. In contrast, we previously found reduced HRQoL among HTx patients in the long term after surgery compared with newly transplanted patients[112]. Compared with HRQoL scores in general populations, HTx populations demonstrate varied results. Some studies report no HRQoL differences between HTx recipients and the general population[106,110,111], whereas others have reported that HTx populations have significantly lower HRQoL scores compared with a reference population[103,108,109,112-116]. Furthermore, reduced HRQoL is associated with anxiety and depression after HTx[105,107,114,117].
In contrast to the previous study from our center demonstrating a higher frequency of depression and anxiety[112], both groups in the most recent study from our center had high scores on HRQoL and no symptoms of anxiety or depression at baseline[18]. This might be due to our inclusion criteria, allowing only stable and healthy HTx recipients to participate. The high baseline HRQoL scores among both groups limited the possibility of revealing an actual effect of exercise in this area, but despite the ceiling-effect, there was a clear trend towards a better overall HRQoL in the HIT group compared to the control group in all domains of SF36[18]. This was also confirmed by a significantly higher rating in the HIT group on the VAS scale, which assessed their subjective opinion on whether participation in the HIT intervention generated positive influences on their general health[18]. This supports previously documented evidence on the association between increased exercise capacity and better HRQoL[105,118-122].
In the past few years, there has been increasing focus on using physical exercise as a tool in both the primary and secondary prophylaxis of cardiovascular diseases, which is the main cause of sickness and death in the western world. Despite the increased focus and great benefits of regular exercise, it is still underutilized as a therapeutic intervention.
Traditionally, several exercise restrictions have applied to the transplanted, denervated heart, which seems to be based more on caution than scientific evidence. The time seems to be right for rethinking the use of exercise among HTx recipients and to offer an “up to date” physical training principle to this group of patients who are presently not recommended to participate in HIT programs.
The high degree of normalization of chronotropic responses among HTx recipients should be a major factor in support of reducing the exercise restrictions that have applied to the denervated heart. Accumulating evidence suggest that chronotropic incompetence is not a factor limiting exercise capacity in the majority of HTx recipients and that HIT is a feasible, safe and effective way to improve exercise capacity and general health in stable, long term HTx recipients. This type of exercise should be introduced and used more frequently among a broader audience. However, the transplanted heart seems to respond differently to this type of exercise, resulting mainly in peripheral improvements rather than improved cardiac function. Larger studies and more basic research are needed to investigate these mechanisms. Future research is also needed to determine if the positive effects on CAV are reproducible, to examine which mechanisms cause these effects and to determine whether such an intervention has an effect on long term survival. The important question regarding optimal timing for introducing HIT after HTx also needs to be assessed. At present there is not (yet) sufficient evidence to conclude that HIT is superior to moderate exercise in HTx-recipients.
P- Reviewers: Cantarovich F, Cooper DKC, Fourtounas C, Holan V, Pauliks L S- Editor: Gou SX L- Editor: A E- Editor: Zheng XM
| 1. | Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, Kirk R, Rahmel AO, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report--2011. J Heart Lung Transplant. 2011;30:1078-1094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 397] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 2. | Carter R, Al-Rawas OA, Stevenson A, Mcdonagh T, Stevenson RD. Exercise responses following heart transplantation: 5 year follow-up. Scott Med J. 2006;51:6-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Kobashigawa JA, Leaf DA, Lee N, Gleeson MP, Liu H, Hamilton MA, Moriguchi JD, Kawata N, Einhorn K, Herlihy E. A controlled trial of exercise rehabilitation after heart transplantation. N Engl J Med. 1999;340:272-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 222] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Kavanagh T. Exercise rehabilitation in cardiac transplantation patients: a comprehensive review. Eura Medicophys. 2005;41:67-74. [PubMed] [Cited in This Article: ] |
| 5. | Karapolat H, Eyigor S, Zoghi M, Yagdi T, Nalbantgil S, Durmaz B, Ozbaran M. Effects of cardiac rehabilitation program on exercise capacity and chronotropic variables in patients with orthotopic heart transplant. Clin Res Cardiol. 2008;97:449-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Bernardi L, Radaelli A, Passino C, Falcone C, Auguadro C, Martinelli L, Rinaldi M, Viganò M, Finardi G. Effects of physical training on cardiovascular control after heart transplantation. Int J Cardiol. 2007;118:356-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Squires RW. Exercise therapy for cardiac transplant recipients. Prog Cardiovasc Dis. 2011;53:429-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Braith RW, Edwards DG. Exercise following heart transplantation. Sports Med. 2000;30:171-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Kavanagh T, Mertens DJ, Hamm LF, Beyene J, Kennedy J, Corey P, Shephard RJ. Prediction of long-term prognosis in 12169 men referred for cardiac rehabilitation. Circulation. 2002;106:666-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 410] [Cited by in F6Publishing: 440] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 10. | Richard R, Verdier JC, Duvallet A, Rosier SP, Leger P, Nignan A, Rieu M. Chronotropic competence in endurance trained heart transplant recipients: heart rate is not a limiting factor for exercise capacity. J Am Coll Cardiol. 1999;33:192-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Pokan R, Von Duvillard SP, Ludwig J, Rohrer A, Hofmann P, Wonisch M, Smekal G, Schmid P, Pacher R, Bachl N. Effect of high-volume and -intensity endurance training in heart transplant recipients. Med Sci Sports Exerc. 2004;36:2011-2016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Notarius CF, Levy RD, Tully A, Fitchett D, Magder S. Cardiac versus noncardiac limits to exercise after heart transplantation. Am Heart J. 1998;135:339-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Rognmo Ø, Hetland E, Helgerud J, Hoff J, Slørdahl SA. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2004;11:216-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 443] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 14. | Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086-3094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1299] [Cited by in F6Publishing: 1330] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 15. | Arena R, Myers J, Forman DE, Lavie CJ, Guazzi M. Should high-intensity-aerobic interval training become the clinical standard in heart failure? Heart Fail Rev. 2013;18:95-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Hermann TS, Dall CH, Christensen SB, Goetze JP, Prescott E, Gustafsson F. Effect of high intensity exercise on peak oxygen uptake and endothelial function in long-term heart transplant recipients. Am J Transplant. 2011;11:536-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Didsbury M, McGee RG, Tong A, Craig JC, Chapman JR, Chadban S, Wong G. Exercise training in solid organ transplant recipients: a systematic review and meta-analysis. Transplantation. 2013;95:679-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Nytrøen K, Rustad LA, Aukrust P, Ueland T, Hallén J, Holm I, Rolid K, Lekva T, Fiane AE, Amlie JP. High-intensity interval training improves peak oxygen uptake and muscular exercise capacity in heart transplant recipients. Am J Transplant. 2012;12:3134-3142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Orizio C, Perini R, Comandè A, Castellano M, Beschi M, Veicsteinas A. Plasma catecholamines and heart rate at the beginning of muscular exercise in man. Eur J Appl Physiol Occup Physiol. 1988;57:644-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Marconi C, Marzorati M. Exercise after heart transplantation. Eur J Appl Physiol. 2003;90:250-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Nytrøen K, Myers J, Chan KN, Geiran OR, Gullestad L. Chronotropic responses to exercise in heart transplant recipients: 1-yr follow-up. Am J Phys Med Rehabil. 2011;90:579-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Wilson RF, Christensen BV, Olivari MT, Simon A, White CW, Laxson DD. Evidence for structural sympathetic reinnervation after orthotopic cardiac transplantation in humans. Circulation. 1991;83:1210-1220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 196] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Kaye DM, Esler M, Kingwell B, McPherson G, Esmore D, Jennings G. Functional and neurochemical evidence for partial cardiac sympathetic reinnervation after cardiac transplantation in humans. Circulation. 1993;88:1110-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 109] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Squires RW, Leung TC, Cyr NS, Allison TG, Johnson BD, Ballman KV, Wagner JA, Olson LJ, Frantz RP, Edwards BS. Partial normalization of the heart rate response to exercise after cardiac transplantation: frequency and relationship to exercise capacity. Mayo Clin Proc. 2002;77:1295-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Haykowsky M, Taylor D, Kim D, Tymchak W. Exercise training improves aerobic capacity and skeletal muscle function in heart transplant recipients. Am J Transplant. 2009;9:734-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Tegtbur U, Busse MW, Jung K, Pethig K, Haverich A. Time course of physical reconditioning during exercise rehabilitation late after heart transplantation. J Heart Lung Transplant. 2005;24:270-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Wu YT, Chien CL, Chou NK, Wang SS, Lai JS, Wu YW. Efficacy of a home-based exercise program for orthotopic heart transplant recipients. Cardiology. 2008;111:87-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Karapolat H, Eyigör S, Zoghi M, Yagdi T, Nalbangil S, Durmaz B. Comparison of hospital-supervised exercise versus home-based exercise in patients after orthotopic heart transplantation: effects on functional capacity, quality of life, and psychological symptoms. Transplant Proc. 2007;39:1586-1588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Tjønna AE, Lee SJ, Rognmo Ø, Stølen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slørdahl SA. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118:346-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 735] [Cited by in F6Publishing: 761] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 30. | Douard H, Parrens E, Billes MA, Labbe L, Baudet E, Broustet JP. Predictive factors of maximal aerobic capacity after cardiac transplantation. Eur Heart J. 1997;18:1823-1828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Leung TC, Ballman KV, Allison TG, Wagner JA, Olson LJ, Frantz RP, Edwards BS, Dearani JA, Daly RC, McGregor CG. Clinical predictors of exercise capacity 1 year after cardiac transplantation. J Heart Lung Transplant. 2003;22:16-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Hsieh PL, Wu YT, Chao WJ. Effects of exercise training in heart transplant recipients: a meta-analysis. Cardiology. 2011;120:27-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Williams TJ, McKenna MJ. Exercise limitation following transplantation. Compr Physiol. 2012;2:1937-1979. [PubMed] [Cited in This Article: ] |
| 34. | American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 8th ed. Baltimore: Wolters Kluwer, Lippincott Williams and Wilkins 2009; 60-104. [Cited in This Article: ] |
| 35. | Fletcher GF, Balady GJ, Amsterdam EA, Chaitman B, Eckel R, Fleg J, Froelicher VF, Leon AS, Piña IL, Rodney R. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:1694-1740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1172] [Cited by in F6Publishing: 1105] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 36. | Åstrand PO, Rodahl K, Dahl HA, Strømme SB. Textbook of work physiology: physiological bases of exercise. 4th ed. Champaign: Human Kinetics Publishers 2003; . [Cited in This Article: ] |
| 37. | Vella CA, Robergs RA. A review of the stroke volume response to upright exercise in healthy subjects. Br J Sports Med. 2005;39:190-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Wisløff U, Ellingsen Ø, Kemi OJ. High-intensity interval training to maximize cardiac benefits of exercise training? Exerc Sport Sci Rev. 2009;37:139-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 39. | Chapman CB, Fisher JN, Sproule BJ. Behavior of stroke volume at rest and during exercise in human beings. J Clin Invest. 1960;39:1208-1213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 115] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Zhou B, Conlee RK, Jensen R, Fellingham GW, George JD, Fisher AG. Stroke volume does not plateau during graded exercise in elite male distance runners. Med Sci Sports Exerc. 2001;33:1849-1854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Gledhill N, Cox D, Jamnik R. Endurance athletes’ stroke volume does not plateau: major advantage is diastolic function. Med Sci Sports Exerc. 1994;26:1116-1121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 207] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 42. | Wang E, Solli GS, Nyberg SK, Hoff J, Helgerud J. Stroke volume does not plateau in female endurance athletes. Int J Sports Med. 2012;33:734-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Krip B, Gledhill N, Jamnik V, Warburton D. Effect of alterations in blood volume on cardiac function during maximal exercise. Med Sci Sports Exerc. 1997;29:1469-1476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 99] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 44. | Martino M, Gledhill N, Jamnik V. High VO2max with no history of training is primarily due to high blood volume. Med Sci Sports Exerc. 2002;34:966-971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Kemi OJ, Wisloff U. High-intensity aerobic exercise training improves the heart in health and disease. J Cardiopulm Rehabil Prev. 2010;30:2-11. [PubMed] [Cited in This Article: ] |
| 46. | Karlsen T, Hoff J, Støylen A, Skovholdt MC, Gulbrandsen Aarhus K, Helgerud J. Aerobic interval training improves VO2 peak in coronary artery disease patients; no additional effect from hyperoxia. Scand Cardiovasc J. 2008;42:303-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Helgerud J, Høydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, Simonsen T, Helgesen C, Hjorth N, Bach R. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. 2007;39:665-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 701] [Cited by in F6Publishing: 710] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 48. | Gullestad L, Myers J, Edvardsen T, Kjekshus J, Geiran O, Simonsen S. Predictors of exercise capacity and the impact of angiographic coronary artery disease in heart transplant recipients. Am Heart J. 2004;147:49-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Anderssen SA, Strømme SB. [Physical activity and health--recommendations]. Tidsskr Nor Laegeforen. 2001;121:2037-2041. [PubMed] [Cited in This Article: ] |
| 50. | Shephard RJ. Responses of the cardiac transplant patient to exercise and training. Exerc Sport Sci Rev. 1992;20:297-320. [PubMed] [Cited in This Article: ] |
| 51. | Mandak JS, Aaronson KD, Mancini DM. Serial assessment of exercise capacity after heart transplantation. J Heart Lung Transplant. 1995;14:468-478. [PubMed] [Cited in This Article: ] |
| 52. | Uberfuhr P, Frey AW, Fuchs A, Paniara C, Roskamm H, Schwaiger M, Reichart B. Signs of vagal reinnervation 4 years after heart transplantation in spectra of heart rate variability. Eur J Cardiothorac Surg. 1997;12:907-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Beckers F, Ramaekers D, Speijer G, Ector H, Vanhaecke J, Verheyden B, Van Cleemput J, Droogné W, Van de Werf F, Aubert AE. Different evolutions in heart rate variability after heart transplantation: 10-year follow-up. Transplantation. 2004;78:1523-1531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 54. | Halpert I, Goldberg AD, Levine AB, Levine TB, Kornberg R, Kelly C, Lesch M. Reinnervation of the transplanted human heart as evidenced from heart rate variability studies. Am J Cardiol. 1996;77:180-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Arrowood JA, Minisi AJ, Goudreau E, Davis AB, King AL. Absence of parasympathetic control of heart rate after human orthotopic cardiac transplantation. Circulation. 1997;96:3492-3498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Uberfuhr P, Frey AW, Reichart B. Vagal reinnervation in the long term after orthotopic heart transplantation. J Heart Lung Transplant. 2000;19:946-950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Lovric SS, Avbelj V, Trobec R, Zorman D, Rakovec P, Hojker S, Gersak B, Milcinski M. Sympathetic reinnervation after heart transplantation, assessed by iodine-123 metaiodobenzylguanidine imaging, and heart rate variability. Eur J Cardiothorac Surg. 2004;26:736-741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Wilson RF, Johnson TH, Haidet GC, Kubo SH, Mianuelli M. Sympathetic reinnervation of the sinus node and exercise hemodynamics after cardiac transplantation. Circulation. 2000;101:2727-2733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 59. | Uberfuhr P, Ziegler S, Schwaiblmair M, Reichart B, Schwaiger M. Incomplete sympathic reinnervation of the orthotopically transplanted human heart: observation up to 13 years after heart transplantation. Eur J Cardiothorac Surg. 2000;17:161-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 60. | Schwaiblmair M, von Scheidt W, Uberfuhr P, Ziegler S, Schwaiger M, Reichart B, Vogelmeier C. Functional significance of cardiac reinnervation in heart transplant recipients. J Heart Lung Transplant. 1999;18:838-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Bengel FM, Ueberfuhr P, Ziegler SI, Nekolla S, Reichart B, Schwaiger M. Serial assessment of sympathetic reinnervation after orthotopic heart transplantation. A longitudinal study using PET and C-11 hydroxyephedrine. Circulation. 1999;99:1866-1871. [PubMed] [Cited in This Article: ] |
| 62. | Ferretti G, Marconi C, Achilli G, Caspani E, Fiocchi R, Mamprin F, Gamba A, Ferrazzi P, Cerretelli P. The heart rate response to exercise and circulating catecholamines in heart transplant recipients. Pflugers Arch. 2002;443:370-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Bengel FM, Ueberfuhr P, Karja J, Schreiber K, Nekolla SG, Reichart B, Schwaiger M. Sympathetic reinnervation, exercise performance and effects of beta-adrenergic blockade in cardiac transplant recipients. Eur Heart J. 2004;25:1726-1733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Sanatani S, Chiu C, Nykanen D, Coles J, West L, Hamilton R. Evolution of heart rate control after transplantation: conduction versus autonomic innervation. Pediatr Cardiol. 2004;25:113-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 65. | Bengel FM, Ueberfuhr P, Schiepel N, Nekolla SG, Reichart B, Schwaiger M. Effect of sympathetic reinnervation on cardiac performance after heart transplantation. N Engl J Med. 2001;345:731-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 228] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 66. | Gallego-Page JC, Segovia J, Alonso-Pulpón L, Alonso-Rodríguez M, Salas C, Ortíz-Berrocal J. Re-innervation after heart transplantation: a multidisciplinary study. J Heart Lung Transplant. 2004;23:674-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Mercier J, Ville N, Wintrebert P, Caillaud C, Varray A, Albat B, Thévenet A, Préfaut C. Influence of post-surgery time after cardiac transplantation on exercise responses. Med Sci Sports Exerc. 1996;28:171-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 68. | Gullestad L, Haywood G, Ross H, Bjornerheim R, Geiran O, Kjekshus J, Simonsen S, Fowler M. Exercise capacity of heart transplant recipients: the importance of chronotropic incompetence. J Heart Lung Transplant. 1996;15:1075-1083. [PubMed] [Cited in This Article: ] |
| 69. | Carvalho VO, Pascoalino LN, Bocchi EA, Ferreira SA, Guimarães GV. Heart rate dynamics in heart transplantation patients during a treadmill cardiopulmonary exercise test: a pilot study. Cardiol J. 2009;16:254-258. [PubMed] [Cited in This Article: ] |
| 70. | Freeman JV, Dewey FE, Hadley DM, Myers J, Froelicher VF. Autonomic nervous system interaction with the cardiovascular system during exercise. Prog Cardiovasc Dis. 2006;48:342-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 71. | Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351-1357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1254] [Cited by in F6Publishing: 1187] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 72. | Dogru MT, Gunaydin S, Simsek V, Tulmac M, Guneri M. Correlation of Chronotropic Index and Heart Rate Recovery to Heart Rate Variability and Diastolic Function Values in Men. Inter J Cardiol. 2007;4 Available from: http://ispub.com/IJC/4/2/11549. [Cited in This Article: ] |
| 73. | Lauer M, Froelicher ES, Williams M, Kligfield P. Exercise testing in asymptomatic adults: a statement for professionals from the American Heart Association Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention. Circulation. 2005;112:771-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 74. | Desai MY, De la Peña-Almaguer E, Mannting F. Abnormal heart rate recovery after exercise as a reflection of an abnormal chronotropic response. Am J Cardiol. 2001;87:1164-1169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 75. | Londeree BR, Moeschberger ML. Influence of age and other factors on maximal heart rate. J Card Rehabil. 1984;4:44-49. [Cited in This Article: ] |
| 76. | Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehabil. 2007;14:215-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 77. | Bassett DR, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32:70-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1095] [Cited by in F6Publishing: 1118] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 78. | Middlekauff HR. Making the case for skeletal myopathy as the major limitation of exercise capacity in heart failure. Circ Heart Fail. 2010;3:537-546. [PubMed] [Cited in This Article: ] |
| 79. | Mettauer B, Levy F, Richard R, Roth O, Zoll J, Lampert E, Lonsdorfer J, Geny B. Exercising with a denervated heart after cardiac transplantation. Ann Transplant. 2005;10:35-42. [PubMed] [Cited in This Article: ] |
| 80. | Rustad LA, Nytrøen K, Amundsen BH, Gullestad L, Aakhus S. One year of high-intensity interval training improves exercise capacity, but not left ventricular function in stable heart transplant recipients: A randomisedcontrolled trial. Eur J Prev Cardiol. 2012;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 81. | Molmen-Hansen HE, Stolen T, Tjonna AE, Aamot IL, Ekeberg IS, Tyldum GA, Wisloff U, Ingul CB, Stoylen A. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Prev Cardiol. 2012;19:151-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 82. | Borrelli E, Pogliaghi S, Molinello A, Diciolla F, Maccherini M, Grassi B. Serial assessment of peak VO2 and VO2 kinetics early after heart transplantation. Med Sci Sports Exerc. 2003;35:1798-1804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 83. | Nytrøen K, Rustad LA, Gude E, Hallén J, Fiane AE, Rolid K, Holm I, Aakhus S, Gullestad L. Muscular exercise capacity and body fat predict VO2peak in heart transplant recipients. Eur J Prev Cardiol. 2012;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | Nytrøen K, Annette Rustad L, Erikstad I, Aukrust P, Ueland T, Lekva T, Gude E, Wilhelmsen N, Hervold A, Aakhus S. Effect of high-intensity interval training on progression of cardiac allograft vasculopathy. J Heart Lung Transplant. 2013;. [PubMed] [Cited in This Article: ] |
| 85. | Avery RK. Cardiac-allograft vasculopathy. N Engl J Med. 2003;349:829-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 86. | Billingham ME. The pathologic changes in long-term heart and lung transplant survivors. J Heart Lung Transplant. 1992;11:S252-S257. [PubMed] [Cited in This Article: ] |
| 87. | Stoica SC, Goddard M, Large SR. The endothelium in clinical cardiac transplantation. Ann Thorac Surg. 2002;73:1002-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 88. | Weis M. Cardiac allograft vasculopathy: prevention and treatment options. Transplant Proc. 2002;34:1847-1849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 89. | Segovia J, Gómez-Bueno M, Alonso-Pulpón L. Treatment of allograft vasculopathy in heart transplantation. Expert Opin Pharmacother. 2006;7:2369-2383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 90. | Wang SS. Treatment and prophylaxis of cardiac allograft vasculopathy. Transplant Proc. 2008;40:2609-2610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 91. | Chou NK, Jan CF, Chi NH, Lee CM, Wu IH, Huang SC, Chen YS, Yu HY, Tsao CI, Ko WJ. Cardiac allograft vasculopathy compared by intravascular ultrasound sonography: everolimus to mycophenolate mofetil--one single-center experience. Transplant Proc. 2012;44:897-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 92. | Szostak J, Laurant P. The forgotten face of regular physical exercise: a ‘natural’ anti-atherogenic activity. Clin Sci (Lond). 2011;121:91-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 93. | Ahmed HM, Blaha MJ, Nasir K, Rivera JJ, Blumenthal RS. Effects of physical activity on cardiovascular disease. Am J Cardiol. 2012;109:288-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 94. | Swift DL, Lavie CJ, Johannsen NM, Arena R, Earnest CP, O’Keefe JH, Milani RV, Blair SN, Church TS. Physical activity, cardiorespiratory fitness, and exercise training in primary and secondary coronary prevention. Circ J. 2013;77:281-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 232] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 95. | Yoshikawa D, Ishii H, Kurebayashi N, Sato B, Hayakawa S, Ando H, Hayashi M, Isobe S, Okumura T, Hirashiki A. Association of cardiorespiratory fitness with characteristics of coronary plaque: assessment using integrated backscatter intravascular ultrasound and optical coherence tomography. Int J Cardiol. 2013;162:123-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 96. | Thijssen DH, Cable NT, Green DJ. Impact of exercise training on arterial wall thickness in humans. Clin Sci (Lond). 2012;122:311-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 97. | Wienbergen H, Hambrecht R. Physical exercise and its effects on coronary artery disease. Curr Opin Pharmacol. 2013;13:218-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 98. | Schmauss D, Weis M. Cardiac allograft vasculopathy: recent developments. Circulation. 2008;117:2131-2141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 294] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 99. | Weis M, von Scheidt W. Cardiac allograft vasculopathy: a review. Circulation. 1997;96:2069-2077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 283] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 100. | Pedersen BK. The diseasome of physical inactivity--and the role of myokines in muscle--fat cross talk. J Physiol. 2009;587:5559-5568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 410] [Cited by in F6Publishing: 377] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 101. | Lavie CJ, Church TS, Milani RV, Earnest CP. Impact of physical activity, cardiorespiratory fitness, and exercise training on markers of inflammation. J Cardiopulm Rehabil Prev. 2011;31:137-145. [PubMed] [Cited in This Article: ] |
| 102. | Burra P, De Bona M. Quality of life following organ transplantation. Transpl Int. 2007;20:397-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 103. | Grady KL. Quality of life after heart transplantation: are things really better? Curr Opin Cardiol. 2003;18:129-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Martín-Rodríguez A, Pérez-San-Gregorio MA, Díaz-Domínguez R, Pérez-Bernal J. Health-related quality of life evolution in patients after heart transplantation. Transplant Proc. 2008;40:3037-3038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 105. | Karapolat H, Eyigor S, Durmaz B, Yagdi T, Nalbantgil S, Karakula S. The relationship between depressive symptoms and anxiety and quality of life and functional capacity in heart transplant patients. Clin Res Cardiol. 2007;96:593-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 106. | Kugler C, Tegtbur U, Gottlieb J, Bara C, Malehsa D, Dierich M, Simon A, Haverich A. Health-related quality of life in long-term survivors after heart and lung transplantation: a prospective cohort study. Transplantation. 2010;90:451-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 107. | Evangelista LS, Dracup K, Moser DK, Westlake C, Erickson V, Hamilton MA, Fonarow GC. Two-year follow-up of quality of life in patients referred for heart transplant. Heart Lung. 2005;34:187-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 108. | Saeed I, Rogers C, Murday A. Health-related quality of life after cardiac transplantation: results of a UK National Survey with Norm-based Comparisons. J Heart Lung Transplant. 2008;27:675-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 109. | Dew MA, Switzer GE, Goycoolea JM, Allen AS, DiMartini A, Kormos RL, Griffith BP. Does transplantation produce quality of life benefits? A quantitative analysis of the literature. Transplantation. 1997;64:1261-1273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 263] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 110. | Jokinen JJ, Hämmäinen P, Lemström KB, Lommi J, Sipponen J, Harjula AL. Association between gastrointestinal symptoms and health-related quality of life after heart transplantation. J Heart Lung Transplant. 2010;29:1388-1394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 111. | Petroski RA, Grady KL, Rodgers S, Backer CL, Kulikowska A, Canter C, Pahl E. Quality of life in adult survivors greater than 10 years after pediatric heart transplantation. J Heart Lung Transplant. 2009;28:661-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 112. | Sivertsen B, Relbo A, Gullestad L, Hellesvik M, Grov I, Andreassen A, Simonsen S, Geiran O, Havik OE. [Self-assessed health and psychological symptoms after heart transplantation]. Tidsskr Nor Laegeforen. 2007;127:3198-3201. [PubMed] [Cited in This Article: ] |
| 113. | Politi P, Piccinelli M, Fusar-Poli P, Klersy C, Campana C, Goggi C, Viganò M, Barale F. Ten years of “extended” life: quality of life among heart transplantation survivors. Transplantation. 2004;78:257-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 114. | Fusar-Poli P, Martinelli V, Klersy C, Campana C, Callegari A, Barale F, Viganò M, Politi P. Depression and quality of life in patients living 10 to 18 years beyond heart transplantation. J Heart Lung Transplant. 2005;24:2269-2278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 115. | Kugler C, Malehsa D, Tegtbur U, Guetzlaff E, Meyer AL, Bara C, Haverich A, Strueber M. Health-related quality of life and exercise tolerance in recipients of heart transplants and left ventricular assist devices: a prospective, comparative study. J Heart Lung Transplant. 2011;30:204-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 116. | Loge JH, Kaasa S. Short form 36 (SF-36) health survey: normative data from the general Norwegian population. Scand J Soc Med. 1998;26:250-258. [PubMed] [Cited in This Article: ] |
| 117. | Grady KL, Naftel DC, Kobashigawa J, Chait J, Young JB, Pelegrin D, Czerr J, Heroux A, Higgins R, Rybarczyk B. Patterns and predictors of quality of life at 5 to 10 years after heart transplantation. J Heart Lung Transplant. 2007;26:535-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 118. | Ulubay G, Ulasli SS, Sezgin A, Haberal M. Assessing exercise performance after heart transplantation. Clin Transplant. 2007;21:398-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 119. | Karapolat H, Eyigor S, Durmaz B, Nalbantgil S, Yagdi T, Zoghi M. The effect of functional performance, respiratory function and osteopenia on the quality of life after heart transplantation. Int J Cardiol. 2008;124:381-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 120. | Christensen SB, Dall CH, Prescott E, Pedersen SS, Gustafsson F. A high-intensity exercise program improves exercise capacity, self-perceived health, anxiety and depression in heart transplant recipients: a randomized, controlled trial. J Heart Lung Transplant. 2012;31:106-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 121. | Hsu CJ, Chen SY, Su S, Yang MC, Lan C, Chou NK, Hsu RB, Lai JS, Wang SS. The effect of early cardiac rehabilitation on health-related quality of life among heart transplant recipients and patients with coronary artery bypass graft surgery. Transplant Proc. 2011;43:2714-2717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 122. | Buendía F, Almenar L, Martínez-Dolz L, Sánchez-Lázaro I, Navarro J, Agüero J, Muñoz B, Sánchez-Gómez JM, Cebrian M, Salvador A. Relationship between functional capacity and quality of life in heart transplant patients. Transplant Proc. 2011;43:2251-2252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 123. | Renlund DG, Taylor DO, Ensley RD, O’Connell JB, Gilbert EM, Bristow MR, Ma H, Yanowitz FG. Exercise capacity after heart transplantation: influence of donor and recipient characteristics. J Heart Lung Transplant. 1996;15:16-24. [PubMed] [Cited in This Article: ] |
| 124. | Osada N, Chaitman BR, Donohue TJ, Wolford TL, Stelken AM, Miller LW. Long-term cardiopulmonary exercise performance after heart transplantation. Am J Cardiol. 1997;79:451-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 125. | Myers J, Gullestad L, Bellin D, Ross H, Vagelos R, Fowler M. Physical activity patterns and exercise performance in cardiac transplant recipients. J Cardiopulm Rehabil. 2003;23:100-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 126. | Schmid JP, Gaillet R, Noveanu M, Mohacsi P, Saner H, Hullin R. Influence of the exercise protocol on peak VO2 in patients after heart transplantation. J Heart Lung Transplant. 2005;24:1751-1756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 127. | Richard R, Verdier JC, Doutreleau S, Piquard F, Gény B, Rieu M. Exercise limitation in trained heart and kidney transplant recipients: central and peripheral limitations. J Heart Lung Transplant. 2005;24:1774-1780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |