Revised: January 7, 2012
Accepted: April 10, 2012
Published online: June 9, 2012
The cannabinoid (CB) receptors, endocannabinoids (eCB) and their synthesizing and catabolizing enzymes and the proteins involved in their transport, constitute what is now recognized as the eCB system. The eCBs are a class of lipids that have been identified as retrograde messengers and produce their effects via presynaptic CB receptors. The major function of the eCBs has been suggested to be that of modulating the release of several neurotransmitters implicated in a number of biological functions that include reward and reinforcement. There is now significant evidence to suggest that the eCB system plays an important role in the development of alcohol tolerance, dependence and relapse. Recent studies suggest that the pharmacological manipulation of the eCB system has the potential not only to block the direct reinforcing properties of alcohol but also alleviate behavioral abnormalities associated with relapse. There is also accumulating evidence that points to the possible utility of the eCB system targeted drugs in the treatment of alcoholism-related behavioral disorders. The agents that block CB1 receptor function or inhibit the synthesis of eCBs are attractive candidate drugs that need to be explored. Further understanding of the role of the eCB system in molecular mechanism/s that underlies alcoholism-related behaviors should lead to a better treatment of this devastating disorder.
- Citation: Hungund BL, Vinod KY. Endocannabinoid system: A newer molecular target for the treatment of alcohol-related behaviors. World J Pharmacol 2012; 1(3): 44-49
- URL: https://www.wjgnet.com/2220-3192/full/v1/i3/44.htm
- DOI: https://dx.doi.org/10.5497/wjp.v1.i3.44
The endocannabinoid (eCB) system consists of cannabinoid (CB) receptors, eCBs, anandamide (AEA) and 2-arachidonyl glycerol (2-AG) and the enzymes involved in their synthesis, transport and degradation[1-4]. The eCBs mimic many of the pharmacological and behavioral effects of tetrahydrocannabinol (THC), a psychoactive component of marijuana and are considered as a new class of neuromodulators[5,6]. Unlike classical neurotransmitters, eCBs are not stored in the vesicles but are released upon demand from membrane phospholipids of postsynaptic neurons[7]. The eCBs have been shown to act as retrograde messengers and regulate neurotransmitter release via binding to presynaptic CB1 receptors[8].
Alcoholism is a complex psychiatric disorder characterized by impaired control over drinking, leading to tolerance, physical dependence and relapse. The mechanisms underlying this disorder are poorly understood at the present time. Alcohol effects appear to be mediated through several intracellular signal transduction pathways involving many classical neurotransmitters and ion channels in different brain regions[9]. There is a growing body of evidence now suggesting a significant role for the eCB system in a number of alcohol-related behaviors that include voluntary alcohol consumption, alcohol tolerance and dependence and addiction to other drugs of abuse. Recent studies have also shown that the drugs targeted against the components of the eCB system may have therapeutic potential in the treatment of a variety of illnesses including drug and alcohol addiction.
The studies investigating the mechanisms underlying the addictive behavior mediated through eCB signaling have been the subject of intensive research. Our laboratory was the first to implicate the eCB system in the development of tolerance to alcohol. Since our first publication in 1998, there have been significant new developments in understanding the role of the eCB system in many of the alcohol-related behaviors. As discussed in the following section, several laboratories including ours have investigated CB1 receptor-mediated mechanisms in explaining the behavioral effects of alcohol that include tolerance and dependence, voluntary alcohol consumption, and fetal alcohol spectrum disorders.
We have previously demonstrated: (1) a downregulation of CB1 receptors and CB1 receptor function in chronic alcohol exposed (alcohol tolerant) mouse synaptic plasma membranes[10,11]; and (2) an increase in the levels of the eCBs, AEA and 2-AG in chronic alcohol exposed neuronal cells in culture in vitro[12,13], and in the levels of AEA in chronic alcohol exposed mouse brain in vivo[14,15]. In addition, the CB1 receptor density and function were found to be significantly downregulated in the cortex, hippocampus, striatum and cerebellum of Swiss-Webster mice that were made alcohol tolerant following 72 h chronic continuous alcohol vapor exposure[15], which returned to normal level 24 h after withdrawal from alcohol[15]. Consistent with rodent studies, the levels of CB1 receptors, CB1 receptor-mediated G-protein signaling and fatty acid amide hydrolase (FAAH) activity were also found to be significantly lower in the postmortem ventral striatum of alcohol-dependent subjects compared to the control group[16].
It has also been demonstrated that co-administration of CB1 receptor antagonist SR141716A during chronic alcohol exposure significantly reduces severity of alcohol withdrawal-induced handling-induced convulsions (HIC) in both alcohol-preferring (AA) C57BL/6J (B6) and alcohol-avoiding DBA/2J (D2) mice[17]. We also have shown that the mice lacking CB1 receptor gene (CB1 KO) chronically exposed to alcohol had reduced HIC[17] consistent with the results reported by Racz et al[18]. Acute administration of SR141716A has also been shown to completely abolish the alcohol deprivation effect (ADE) and alcohol’s motivational properties in AA sP rats[19-21]. The mice lacking FAAH (FAAH-KO), an enzyme that regulates brain AEA levels, has also been shown to display reduced severity of HIC following withdrawal from chronic continuous 72 h alcohol vapor exposure compared to WT littermates[22], while no differences were found in acute alcohol-induced HIC between FAAH KO and WT mice[23]. These studies strongly suggest that the eCB system plays a critical role in the development of tolerance to and dependence on alcohol.
Significant differences in both the density and function of brain CB1 receptors between AA B6 and alcohol-avoiding D2 mice have been reported[17,24,25]. There is also evidence suggesting that the genetic deletion or pharmacologic manipulation of CB1 receptors result in reduced alcohol consumption in rodent models[17,26-36]. For example, the mice lacking the CB1 receptor gene consume significantly less alcohol compared to their wild-type counterparts[17,26-31] similar to the effect produced by pharmacological antagonism of CB1 receptor[32-36]. On the other hand, administration of the agonists CP-55940 and WIN55212 has been shown to enhance alcohol intake in rodents[17,37,38], which was reversed by co-administration of the antagonist SR141716A[17,39]. The FAAH KO mice are severely impaired in their ability to degrade the eCB, AEA, and have been shown to have approximately 15-fold higher brain levels of AEA compared to WT mice[40]. These mice consume significantly more alcohol compared to WT mice[22,41] and these findings have been replicated by another group[23]. In a recent study, a comparison of the expression of the eCB-related genes in AA and alcohol non-preferring (ANA) rats revealed a decrease in the expression of FAAH activity in prefrontal cortex (PFC) of AA rats[42]. The association of impaired FAAH function with alcohol self-administration was further confirmed by the following observations: (1) SR141716A administration dose dependently suppressed self-administration in AA rats when given systemically or locally into PFC; and (2) intra-PFC injection of the competitive FAAH inhibitor URB597 increased alcohol self-administration in non-selected Wistar rats[42]. An increased vulnerability to drug and alcohol abuse in humans has also been suggested to be due to polymorphism in the FAAH gene and reduced FAAH expression and activity[43,44]. These studies suggest that the eCB system at least in part may play a role in many of the alcohol-related behaviors.
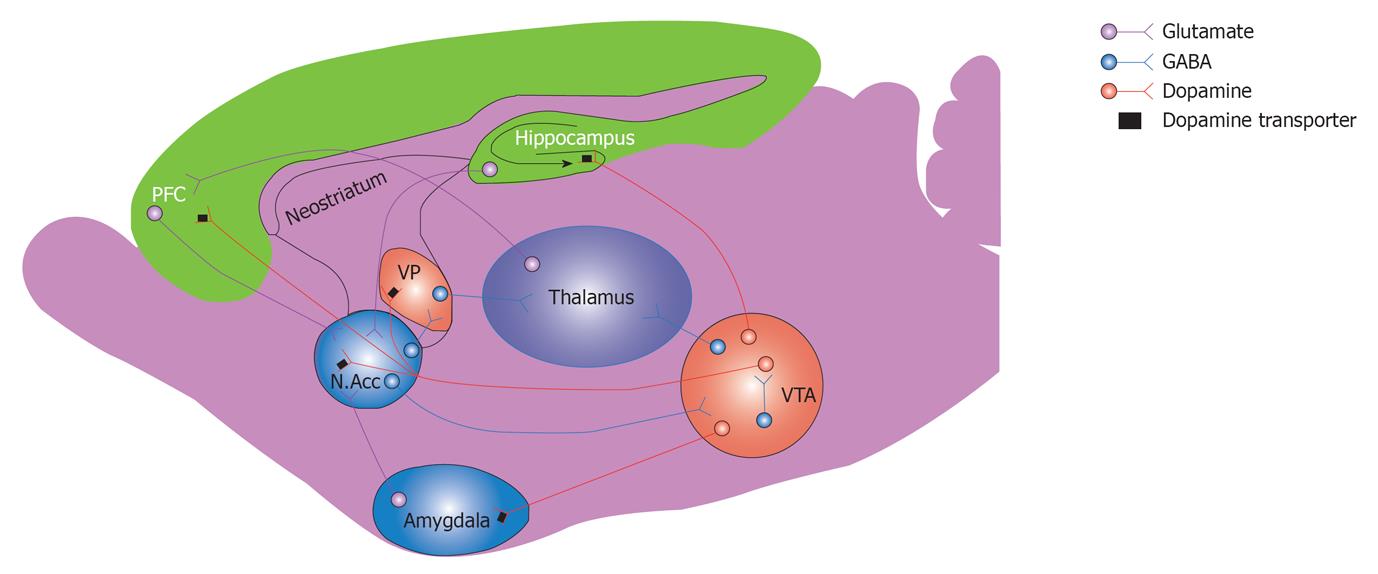
The development of tolerance to and dependence on alcohol could be explained by possible interaction of the eCB system with the mesocorticolimbic system. The mesocorticolimbic dopamine (DA) system represents a common neuronal substrate for reinforcing properties of drugs of abuse including alcohol[45]. Dopaminergic projections originating from the ventral tegmental area (VTA) to the PFC and limbic structures that include nucleus accumbens (NAc) and amygdala have been suggested to play a critical role in reward and reinforcement. The CB1 receptors are present in most brain regions of the reward circuitry, including VTA, NAc, and in several other areas such as PFC, amygdala and hippocampus[46]. The DA neurons of the mesocorticolimbic system are controlled by excitatory and inhibitory inputs that are modulated by CB1 receptors[47,48]. The final effect on the modulation of VTA dopaminergic activity by eCBs depends on the functional balance between the inhibitory GABAergic and excitatory glutamatergic inputs, both of which are inhibited by eCBs under different physiological conditions[47-49]. Alcohol consumption has been shown to increase AEA content in limbic forebrain[42], which appears to activate mesolimbic dopaminergic transmission by increasing the DA release in the shell of NAc[26]. As indicated earlier, a significant reduction in acute alcohol-induced DA release in the NAc shell has been reported in mice that lacked CB1 receptor gene similar to wild type mice treated with CB1 receptor antagonist[26]. It is of interest also to note that subchronic treatment with CB1 receptor agonist WIN-55,212-2 inhibited the release of DA in NAc of rats that were exposed chronically to alcohol[49]. An intravenous administration of both AEA and methandamide (a stable derivative of AEA) and pharmacologic inhibition of FAAH with URB597 led to increase in accumbal DA levels[50] (Figure 1).
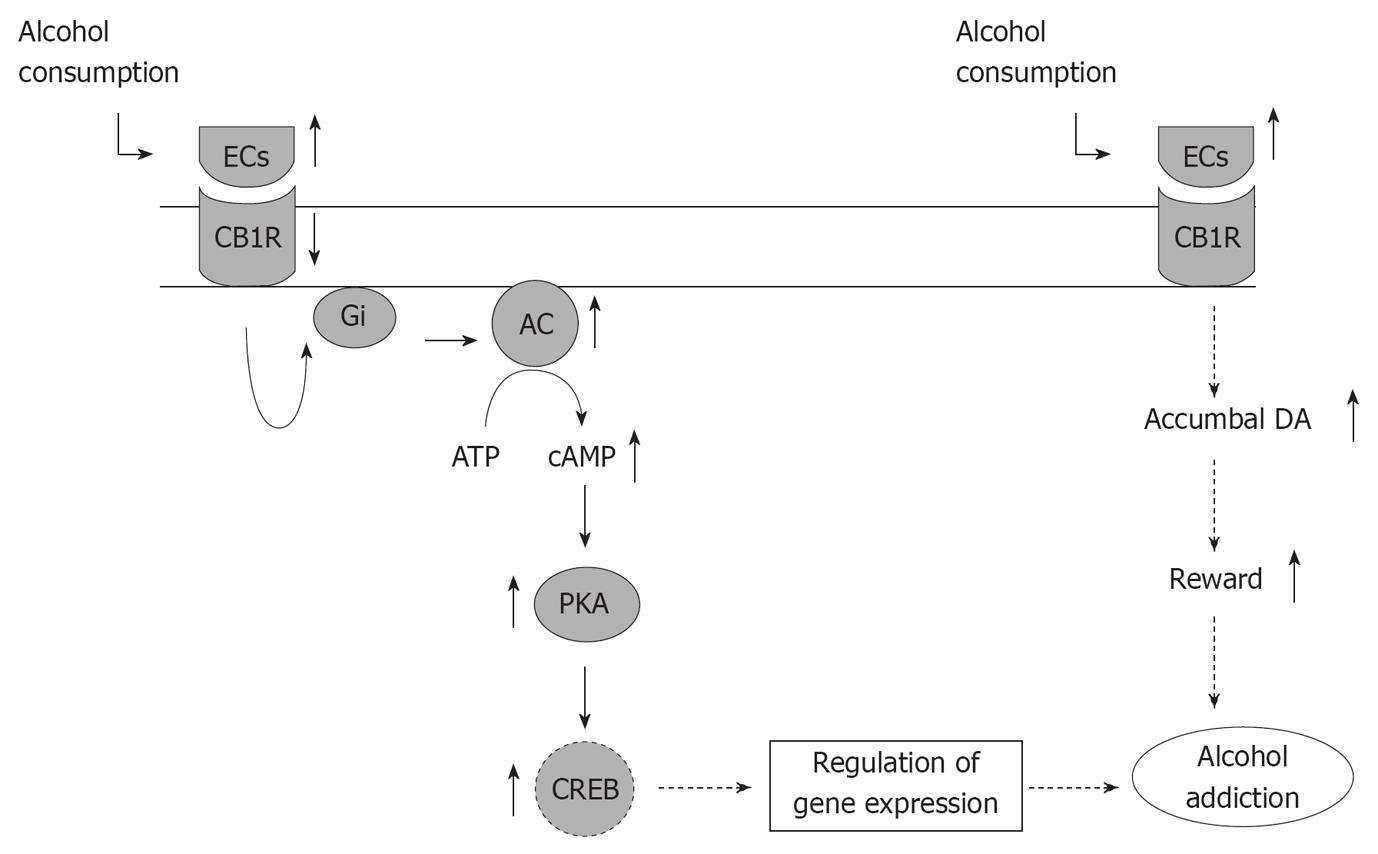
The downregulation of CB1 receptors due to chronic alcohol exposure that results in tolerance to alcohol may be a compensatory neuroadaptation in response to elevation in the eCBs. This in turn might modulate the DA function in reward-related brain regions leading to alcohol addiction (Figure 2).
The dependence and relapse to alcohol may be due to an imbalance between excitatory glutamatergic and inhibitory GABAergic systems caused by altered CB1 receptor function. This could possibly be the result of altered eCB tone induced by chronic alcohol exposure. It is well documented that in the progression to alcohol tolerance and dependence, neuroadaptation occurs resulting in hypoactive GABA receptors and hyperactive NMDA receptors causing a hyperexcitable state that mediates both acute and protracted withdrawal states[45-48]. On the cessation of alcohol use, withdrawal symptoms occur most likely mediated by increased glutamate release. Relapse to alcohol use might reduce these effects and works via negative reinforcement to promote the addicted state[45-48]. These findings strongly implicate a role for the eCB system in reward and reinforcement properties of alcohol and the CB1 receptor targeted drugs should be exploited for future therapeutic drug development.
Despite significant developments in understanding of the neurobiological basis of alcohol tolerance and dependence, thus far no effective treatments are available to treat this devastating disorder. As evidenced in the foregoing discussion the eCB system appears to be a promising target with therapeutic potential. For example, CB1 receptor antagonist/inverse agonist SR141716A has been shown to reduce the development of tolerance to and dependence on alcohol in preclinical rodent models[15,18-20]. It has also been shown to reduce alcohol intake in AA rodents[17,21]. It is worth noting that two Phase II clinical feasibility studies for the effectiveness of SR141716A (rimonabant), a CB1 receptor antagonist, on the prevention of alcohol relapse have been conducted in human alcoholics and found no significant improvement over the placebo patients[51]. In another clinical trial the rimonabant was found to have no effect on alcohol consumption in nontreatment-seeking heavy alcohol drinkers[52]. However, the rimonabant, due to incidence of severe adverse psychiatric side effects, FDA disapproved its commercialization. Nevertheless, further understanding of the neurobiological basis of alcohol abuse-related behaviors involving the components of the eCB system would reveal possible newer targets and future studies should focus on this aspect. Taken together, the available literature strongly support a role for the eCB system in alcohol-related behaviors and the CB1 receptor antagonists and drugs targeted to the eCB metabolizing enzymes are attractive candidates for the treatment of alcoholism.
Peer reviewer: Moses Elisaf, Professor of Internal Medicine, Department of Internal Medicine, University of Ioannina, Medical School, 451 10 Ioannina, Greece
S- Editor Wang JL L- Editor A E- Editor Zheng XM
| 1. | Hungund BL, Vinod KY. Role of the endocannabinoid signaling system in alcohol-related behaviors. Open Neuropsychopharmacol J. 2009;2:31-39. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Basavarajappa BS, Yalamanchili R, Cooper TB, Hungund BL. The Endocannabinoid System. Handbook of Neurochemistry and Molecular Neurobiology. New York: Springer 2008; 343-384. [Cited in This Article: ] |
| 3. | Vinod KY, Hungund BL. Endocannabinoid lipids and mediated system: implications for alcoholism and neuropsychiatric disorders. Life Sci. 2005;77:1569-1583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Vinod KY, Hungund BL. Role of the endocannabinoid system in depression and suicide. Trends Pharmacol Sci. 2006;27:539-545. [PubMed] [Cited in This Article: ] |
| 5. | Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946-1949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3980] [Cited by in F6Publishing: 3864] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 6. | Hungund BL, Basavarajappa BS. Role of brain's own marijuana, anandamide and its cannabinoid receptors (CB1) in alcoholism. Recent Research Developments in Neurochemistry. Trivandrum: Research Signpost 2000; 9-26 Available from: http://cat.inist.fr/aModele=afficheN&cpsidt=15204099. [Cited in This Article: ] |
| 7. | Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol. 2005;53-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 297] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 8. | Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1070] [Cited by in F6Publishing: 1153] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 9. | Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1666] [Cited by in F6Publishing: 1586] [Article Influence: 58.7] [Reference Citation Analysis (1)] |
| 10. | Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Res. 1998;793:212-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 113] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Basavarajappa BS, Hungund BL. Down-regulation of cannabinoid receptor agonist-stimulated [35S]GTP gamma S binding in synaptic plasma membrane from chronic ethanol exposed mouse. Brain Res. 1999;815:89-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Stimulation of cannabinoid receptor agonist 2-arachidonylglycerol by chronic ethanol and its modulation by specific neuromodulators in cerebellar granule neurons. Biochim Biophys Acta. 2000;1535:78-86. [PubMed] [Cited in This Article: ] |
| 13. | Basavarajappa BS, Hungund BL. Chronic ethanol increases the cannabinoid receptor agonist anandamide and its precursor N-arachidonoylphosphatidylethanolamine in SK-N-SH cells. J Neurochem. 1999;72:522-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Hungund BL, Basavarajappa BS, Vadasz C, Kunos G, Rodriguez de Fonseca F, Colombo G, Serra S, Parsons L, Koob GF. Ethanol, endocannabinoids, and the cannabinoidergic signaling system. Alcohol Clin Exp Res. 2002;26:565-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Vinod KY, Yalamanchili R, Xie S, Cooper TB, Hungund BL. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochem Int. 2006;49:619-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Vinod KY, Kassir SA, Hungund BL, Cooper TB, Mann JJ, Arango V. Selective alterations of the CB1 receptors and the fatty acid amide hydrolase in the ventral striatum of alcoholics and suicides. J Psychiatr Res. 2010;44:591-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Vinod KY, Yalamanchili R, Thanos PK, Vadasz C, Cooper TB, Volkow ND, Hungund BL. Genetic and pharmacological manipulations of the CB(1) receptor alter ethanol preference and dependence in ethanol preferring and nonpreferring mice. Synapse. 2008;62:574-581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Racz I, Bilkei-Gorzo A, Toth ZE, Michel K, Palkovits M, Zimmer A. A critical role for the cannabinoid CB1 receptors in alcohol dependence and stress-stimulated ethanol drinking. J Neurosci. 2003;23:2453-2458. [PubMed] [Cited in This Article: ] |
| 19. | Serra S, Brunetti G, Pani M, Vacca G, Carai MA, Gessa GL, Colombo G. Blockade by the cannabinoid CB(1) receptor antagonist, SR 141716, of alcohol deprivation effect in alcohol-preferring rats. Eur J Pharmacol. 2002;443:95-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Colombo G, Vacca G, Serra S, Carai MA, Gessa GL. Suppressing effect of the cannabinoid CB1 receptor antagonist, SR 141716, on alcohol's motivational properties in alcohol-preferring rats. Eur J Pharmacol. 2004;498:119-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Vinod KY, Maccioni P, Garcia-Gutierrez MS, Femenia T, Xie S, Carai MA, Manzanares J, Cooper TB, Hungund BL, Colombo G. Innate difference in the endocannabinoid signaling and its modulation by alcohol consumption in alcohol-preferring sP rats. Addict Biol. 2012;17:62-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Vinod KY, Sanguino E, Yalamanchili R, Manzanares J, Hungund BL. Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. J Neurochem. 2008;104:233-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Blednov YA, Cravatt BF, Boehm SL, Walker D, Harris RA. Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacology. 2007;32:1570-1582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Hungund BL, Basavarajappa BS. Distinct differences in the cannabinoid receptor binding in the brain of C57BL/6 and DBA/2 mice, selected for their differences in voluntary ethanol consumption. J Neurosci Res. 2000;60:122-128. [PubMed] [Cited in This Article: ] |
| 25. | Basavarajappa BS, Hungund BL. Cannabinoid receptor agonist-stimulated [35S]guanosine triphosphate gammaS binding in the brain of C57BL/6 and DBA/2 mice. J Neurosci Res. 2001;64:429-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 266] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 27. | Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci USA. 2003;100:1393-1398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 272] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Poncelet M, Maruani J, Calassi R, Soubrié P. Overeating, alcohol and sucrose consumption decrease in CB1 receptor deleted mice. Neurosci Lett. 2003;343:216-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Naassila M, Pierrefiche O, Ledent C, Daoust M. Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacology. 2004;46:243-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Houchi H, Babovic D, Pierrefiche O, Ledent C, Daoust M, Naassila M. CB1 receptor knockout mice display reduced ethanol-induced conditioned place preference and increased striatal dopamine D2 receptors. Neuropsychopharmacology. 2005;30:339-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Thanos PK, Dimitrakakis ES, Rice O, Gifford A, Volkow ND. Ethanol self-administration and ethanol conditioned place preference are reduced in mice lacking cannabinoid CB1 receptors. Behav Brain Res. 2005;164:206-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Arnone M, Maruani J, Chaperon F, Thiébot MH, Poncelet M, Soubrié P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl). 1997;132:104-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 475] [Cited by in F6Publishing: 480] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 33. | Colombo G, Agabio R, Fà M, Guano L, Lobina C, Loche A, Reali R, Gessa GL. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol Alcohol. 1998;33:126-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 182] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Gallate JE, McGregor IS. The motivation for beer in rats: effects of ritanserin, naloxone and SR 141716. Psychopharmacology (Berl). 1999;142:302-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 154] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 35. | Rodríguez de Fonseca F, Roberts AJ, Bilbao A, Koob GF, Navarro M. Cannabinoid receptor antagonist SR141716A decreases operant ethanol self administration in rats exposed to ethanol-vapor chambers. Zhongguo Yao Li Xue Bao. 1999;20:1109-1114. [PubMed] [Cited in This Article: ] |
| 36. | Freedland CS, Sharpe AL, Samson HH, Porrino LJ. Effects of SR141716A on ethanol and sucrose self-administration. Alcohol Clin Exp Res. 2001;25:277-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 144] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Gallate JE, Saharov T, Mallet PE, McGregor IS. Increased motivation for beer in rats following administration of a cannabinoid CB1 receptor agonist. Eur J Pharmacol. 1999;370:233-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 185] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 38. | Colombo G, Serra S, Brunetti G, Gomez R, Melis S, Vacca G, Carai MM, Gessa L. Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sP rats. Psychopharmacology (Berl). 2002;159:181-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Kelaï S, Hanoun N, Aufrère G, Beaugé F, Hamon M, Lanfumey L. Cannabinoid-serotonin interactions in alcohol-preferring vs. alcohol-avoiding mice. J Neurochem. 2006;99:308-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Lichtman AH, Hawkins EG, Griffin G, Cravatt BF. Pharmacological activity of fatty acid amides is regulated, but not mediated, by fatty acid amide hydrolase in vivo. J Pharmacol Exp Ther. 2002;302:73-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 186] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 41. | Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB, Hungund BL. Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacology. 2006;50:834-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 42. | Hansson AC, Bermúdez-Silva FJ, Malinen H, Hyytiä P, Sanchez-Vera I, Rimondini R, Rodriguez de Fonseca F, Kunos G, Sommer WH, Heilig M. Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacology. 2007;32:117-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 43. | Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci USA. 2002;99:8394-8399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 252] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 44. | Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet. 2004;13:2113-2119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 213] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 45. | Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3538] [Cited by in F6Publishing: 3369] [Article Influence: 240.6] [Reference Citation Analysis (0)] |
| 46. | Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 302] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 47. | Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci. 2004;24:11070-11078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 48. | Lupica CR, Riegel AC. Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology. 2005;48:1105-1116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 49. | Melis M, Pistis M, Perra S, Muntoni AL, Pillolla G, Gessa GL. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci. 2004;24:53-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 374] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 50. | Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98:408-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 154] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 51. | Soyka M, Koller G, Schmidt P, Lesch OM, Leweke M, Fehr C, Gann H, Mann KF. Cannabinoid receptor 1 blocker rimonabant (SR 141716) for treatment of alcohol dependence: results from a placebo-controlled, double-blind trial. J Clin Psychopharmacol. 2008;28:317-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |