Published online Nov 25, 2017. doi: 10.5495/wjcid.v7.i4.50
Peer-review started: June 15, 2017
First decision: July 20, 2017
Revised: August 2, 2017
Accepted: September 12, 2017
Article in press: September 13, 2017
Published online: November 25, 2017
The infection due to human immunodeficiency virus (HIV) is characterized by the progressive reduction of CD4+ T lymphocytes and the compromise of other cell lines of the immune system, resulting in immunosuppression. In this context, autoimmune diseases could be considered contradictory, however, cases of autoimmune diseases during this infection have been described, including autoimmune hepatitis (AIH), which is uncommon and has few case reports within medical literature, none of them from Latin America. In this case report where a patient with an HIV infection on combined antiretroviral treatment developed acute elevation of transaminases, hyperbilirubinemia, and deterioration in hepatic synthetic function. Although initially an antiretroviral drug-induced liver injury was suspected, during the study a diagnosis of autoimmune hepatitis was proven, which required treatment with corticosteroid and azathioprine, obtaining a satisfactory response and managing to continue the antiretroviral therapy. Autoimmune diseases in HIV infection must be taken into account. In the case of hepatitis in patients with HIV on antiretroviral treatment, the differentiation between viral hepatitis caused by autoimmune diseases or medications is essential to establish an adequate treatment, and avoid the suspension of the antiretroviral therapy.
Core tip: In the combined antiretroviral therapy era, human immunodeficiency virus (HIV) infection can show diverse manifestations others than infections, been autoimmunity a paradoxical but well described phenomena in this scenario. The objective of this case report is to illustrate a rare condition as autoimmune hepatitis in HIV infected patients on therapy, with an additional literature review, to help clinicians in the approach of this disease and the differentiation with drug induced liver injury related to antiretroviral therapy.
- Citation: Noreña I, Morantes-Caballero JA, Garcés A, Gómez BJ, Rodríguez G, Saavedra C, Otero W. Autoimmune hepatitis in human immunodeficiency virus infection: Case report and literature review. World J Clin Infect Dis 2017; 7(4): 50-57
- URL: https://www.wjgnet.com/2220-3176/full/v7/i4/50.htm
- DOI: https://dx.doi.org/10.5495/wjcid.v7.i4.50
The global incidence of infection due to human immunodeficiency virus (HIV) reached its peak in 1997. Since then it has remained relatively constant. However, the prevalence has increased as the survival rate has improved thanks to the combination antiretroviral therapy (cART)[1]. With these therapies there is a reduction of the mortality as well as the risk of developing severe events related to the acquired immune deficiency syndrome (AIDS) in 57% of cases, regardless of age, gender and CD4+ T lymphocyte count[1,2], and constitutes the most effective strategy for preventing onwards HIV-1 infections[3]. The early onset of therapy results in better outcomes for patients, even with an advanced disease[2]. In 2%-18% of patients, treatment must be discontinued due to adverse effects, especially in the liver, preventing patients to benefit from this treatment[4]. Drug induced liver injury (DILI) manifests itself through hepatitis and an increase of the aminotransferases, making it indistinguishable from any other hepatitis[5].
HIV affects the CD4+ lymphocytes and alters other cell lines of the innate immune system (macrophages, monocytes and dendritic cells)[6]. This can lead to autoimmune diseases[5-7]. So far, few cases have been published of HIV/AIDS patients with concomitant autoimmune diseases, such as vasculitis, systemic lupus erythematosus, psoriasis, Graves’ disease, and less commonly, autoimmune hepatitis (AIH)[5-9]. After reviewing the literature, there were only 22 cases of autoimmune hepatitis described in patients with HIV, in the described cases the CD4+ lymphocyte count was above 100 cells/mm3, and was initially considered as liver toxicity caused as a side effect of the antiretroviral therapy[10-18]. Taking into account the little information on these two entities occurring simultaneously, the case of a patient with HIV, who, in the course of their disease, developed AIH, is presented.
A 26-year-old male, student by occupation, who due to a self-suspicion screening for syphilis was diagnosed with a 2-year HIV infection; he was infected by a former partner in an unprotected man-man relation. In addition, he had a history of intravenous drug use, and no family history was identified. The initial study found an HIV viral load of 179488 copies/mL and a CD4+ T lymphocyte count of 298 cells/mm3 and CD8+ of 2067 cells/mm3. The complete blood count and liver and kidney profiles were normal. The serology for hepatitis A, B and C were negative, as well as the tuberculin test and the serology for syphilis. No opportunistic infections were documented. Treatment was started using Efavirenz/Tenofovir disoproxil fumarate/Emtricitabine, having a good tolerance and achieving a lower HIV viral load and satisfactory CD4+ T lymphocyte recovery. One year after starting the treatment, in a follow-up appointment clinical hypothyroidism of an autoimmune etiology was documented (anti-microsomal antibodies, anti-thyroglobulin and positive anti-thyroid peroxidase), and treatment with levothyroxine was started.
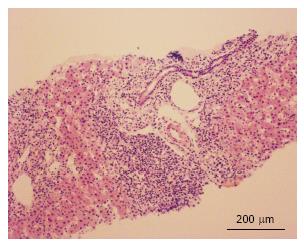
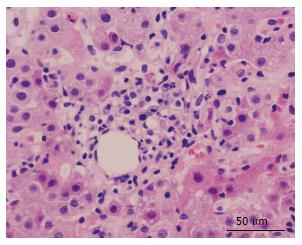
Several months later he was admitted to the emergency room due to jaundice. Hyperbilirubinemia (> 15 mg/dL) was found, with a predominance of direct bilirubin, severe elevation of transaminases (> 2000 IU/L), prolongation of the prothrombin time INR (1.95), and a discrete increase of alkaline phosphatase and gamma glutamyl transferase. Among other differential diagnostics, hepatotoxicity by cART was suspected, and this therapy was immediately discontinued. During hospitalization, the serology for hepatotropic viruses was negative (A, B, C and E), viral loads for virus B, C, Epstein Bar (EBV) and cytomegalovirus (CMV) were undetectable. The hepato-biliary ultrasound and portal doppler were normal. The antinuclear antibodies were positive 1:160 dils, with mottled pattern, and negative anti-mitochondrial and anti-muscle antibodies. High levels of immunoglobulin G were found. A liver biopsy was performed, which reported a lymphoplasmacytic inflammatory infiltration with eosinophils and severe interface activity, hepatocytes with peri-central inflammation and focal necrosis (“compatible with autoimmune hepatitis”) (Figures 1 and 2). Treatment with oral prednisolone 1 mg/kg per day was started, with a significant improvement and a quick normalization of amino transferases and bilirubin, and was discharged without restarting antiretroviral therapy.
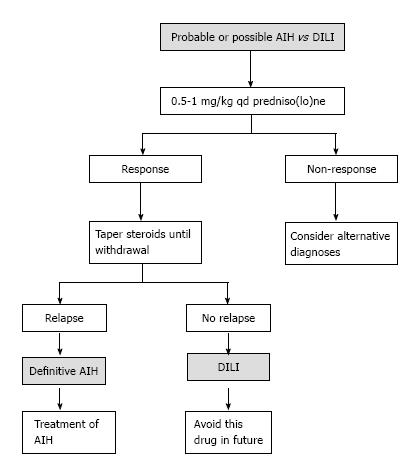
As an outpatient, there was a gradual reduction of the prednisolone dose, until leaving a minimum dose of 10 mg/qd. With normal liver profile, cART treatment was restarted, replacing efavirenz with raltegravir and continuing Tenofovir disoproxil fumarate/Emtricitabine. A month later, the patient went again to the emergency room for recurrence of jaundice; increased serum transaminase levels greater than 2000 mg/dL were documented as well as bilirubin of 18 mg/dL at the expense of the direct bilirubin and the prolongation of prothrombin time and INR. He was hospitalized, cART therapy was once again suspended and doses of prednisolone of 1 mg/kg qd, were started, adding Azathioprine 25 mg/qd, and achieving a progressive reduction of transaminase and bilirubin. There was confusion to determine if the worsening of the hepatitis was due to the decrease of prednisolone (due to autoimmune hepatitis) or when the cART started (due to DILI). A Medical Board was held between the services of gastroenterology, internal medicine and infectious diseases, and based on the current guidelines for the diagnosis of autoimmune hepatitis and their differentiation with hepatitis due to medications (Table 1 and Figure 3), autoimmune hepatitis was defined as the definitive diagnosis[19]. To completely rule out DILI, in a hospital environment, antiretroviral therapy was restarted and the liver profile monitored, which continued to improve until it was normal and the patient discharged.
| Parameter | Discriminator | Score |
| ANA or ASMA+ | ≥ 1:40 | 1 |
| ANA or ASMA+ | ≥ 1:80 | 2 |
| ANA or LKM+ | ≥ 1:40 | 2 |
| ANA or SLA/LP+ | Any title | 2 |
| IgG or gamma globulins | > ULN | 1 |
| > 1.1 × ULN | 2 | |
| Liver biopsy | Compatible with AIH | 1 |
| Typical AIH | 2 | |
| Atypical | 0 | |
| Absence of viral hepatitis | No | 0 |
| Yes | 2 |
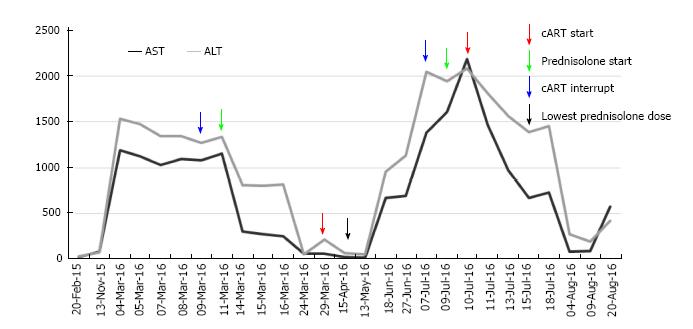
After six months of follow-up, the patient was asymptomatic, receiving maintenance therapy for AIH only with azathioprine 100 mg/qd, HIV treatment and replacement hormone therapy with levothyroxine. The liver profile has remained unchanged. Figure 4, shows the evolution of the liver profile before and during treatment.
A case of autoimmune hepatitis in a patient with HIV infection has been presented. The coexistence of these diseases is rare: Three independent reviewers conducted the literature search in different electronic databases (PubMed, Science Direct, EBSCO and ProQuest), the search terms used were a combination of keywords and MeSH terms and included “HIV”, “AIDS”, “Human immunodeficiency virus”, “Autoimmune disease” “Autoimmunity”, “Autoimmune Hepatitis”, “Anti-HIV Agents”, “Anti-Retroviral Therapy” and “Drug-Induced Liver Injury” in the title, abstract, or keywords with no limit in dates, types of publication or language. Nine reports with only 22 cases of patients with both entities were found, none of them described in Latin America[10-18]; their characteristics can be seen in Table 2. However, in patients with HIV, AIH can be under-diagnosed because of the complexity of the diagnosis and the confounding factors present in patients with HIV (adverse effects to medications, hidden viral hepatitis, opportunistic infections, neoplasms)[14,16,20].
| Author | Vasilios | Wan | Daas | Coriat | Puius | O'Leary | Hagel | Cazanave | Murunga |
| Patients reported (n) | 1 | 4 | 1 | 1 | 3 | 1 | 1 | 1 | 9 |
| Age (min/max) (yr) | 38 | 49-56 | 42 | 48 | 29-65 | 44 | 52 | 43 | 23-45 |
| Gender (M/F) | M | 1-3 | F | F | 1-3 | F | M | F | 1-8 |
| CD4+ cell count (cells/μL) (min/max) | 216 | 174-357 | 157 | 250 | 200-259 | 269 | 641 | 500 | 253-876 |
| HIV viral load at presentation (min/max) (copies/mL) | 81000 | < 50-232734 | 120000 | No data | < 75-8687 | < 50 | UD | < 1000 | UD-8 |
| AST (min/max) (IU/L) | 120 | 45-186 | 1526 | No data | 20-500 | NO DATA | 343 | No data | 13-34 |
| ALT (min/max) (IU/L) | 274 | 45-167 | 777 | 355 | 12-641 | 26-940 | 192 | 500 | 10-39 |
| cART | Z, L, Nelfinavir | No data | F, T, Etravirine | R, E, Atazanavir | L (2), Z, E (3), F (1), T (1), stavudine | T, F, E | Didanosine, stavudine, E | L,Stavudine, E | E (7) F (6) T (8) L (3) N (1) Z (1) L/R (1) |
| Positive autoantibodies (n) dils | ANA 1:320 ASMA 1:40 | ANA 1:20-40 ASMA(1) | ANA: 1:1280 ASMA(-) | ANA: 1:80 LKM: 1:320 | ANA (2) 1:80-640 ASMA (1) | ANA 1:160 | ANA: 1:2560 | ANA: 1:8000 ASMA: 1: 4000 | ANA (4), ASMA (6), ALKM (1) |
| Inmunoglobulin G (g/L) | 29.25 | 639-2020 | 46 | 30 | 3050-7500 | 2640 | 39 | Normal | 16.5-55.2 |
| Fibrosis | No | No | Yes | No | Yes | No | No | Yes | Yes |
| International AIH group score (min/max) | 15 | 10-18 | 19 | No data | 10-15 | 22 | 15 | No data | 12-20 |
| Treatment | No data | Prednisolone (4) Azathioprine (3) | Prednisone | Prednisolone | Prednisolone, Azathioprine | Prednisolone, Azathioprine | Prednisolone | Prednisolone, Azathioprine | Prednisolone |
| Publication Year | 2005 | 2009 | 2011 | 2008 | 2008 | 2008 | 2012 | 2006 | 2016 |
| Ref. | [10] | [11] | [12] | [13] | [14] | [15] | [16] | [17] | [18] |
The exact mechanisms by which patients with HIV may present AIH are unknown. However, an HIV infection is related to various autoimmunity phenomena[5,9]. The direct damage caused by the virus, the molecular mimicry, the deregulation of T/B cells, the generation of immune complexes and auto-antibodies can trigger damage to its own tissues[8,21]. AIH stands out due to the presence of autoreactive CD4+ and CD8+ T lymphocytes, and a cellular response mediated by antibodies with a particular increase in the Th17 subtype and IL17 release. This can be silent in stages of deep immunosuppression, with similar clinical and paraclinical reactivations similar to the immune reconstitution inflammatory syndrome (IRIS-like) that occurs with the immunological recovery associated with the effective antiretroviral therapy[18]. Four immunological stages have been described in HIV infection[9,10], the acute infection-latency (stage I-II) stage and the immune restoration related to antiretroviral therapy (stage IV) are the most likely to develop autoimmune disorders, furthermore, some disorders mediated by CD8+ cytotoxicity are present in the AIDS phase (stage III)[8].
Based on the hypotheses set forth, it is considered that, in the case described above, the immune restoration by antiretroviral therapy, in addition to the predisposition to autoimmune disorders, were the triggers of the hepatitis, the thrombocytopenia, and Hashimoto’s thyroiditis. This is consistent with the reports described in literature that supports the association between AIH and other autoimmune diseases, mainly Hashimoto’s thyroiditis[14].
The AIH is a disease common in women, however, it can occur in males, as has been reported in other cases (Table 2), and is characterized by an injury with hepatocellular pattern, circulating autoantibodies (anti-nuclear, anti-smooth muscle, or anti-microsomal liver-kidney), elevated levels of immunoglobulin G and a consistent liver biopsy. Histologically, there are characteristic changes (non pathognomonic) that include interface hepatitis, plasmacytic infiltration and regenerative hepatocyte rosettes[14].
The diagnosis is established by combining criteria and discarding other entities that cause hepatitis (Table 2)[17,19]. The acute case this patient developed occurs only in 25% of AIH, and makes diagnosis difficult, being that the negativity of the anti-smooth muscle antibodies is frequent[17]. In this patient, the diagnosis of AIH was achieved by the combination of clinical, paraclinical and histological findings, the good response to immunomodulatory therapy and the exclusion of other causes.
Once there is suspicion of an AIH diagnosis in a patient with HIV, they should be treated in a similar manner to immune competent patients. The first line includes prednisone or prednisolone (0.5-1 mg/kg per day), and azathioprine (1-2 mg/kg per day), gradually decreasing steroid doses and continuing maintenance treatment based on azathioprine. It is essential to continue antiretroviral therapy[19].
As in this case, in all reported AIH and HIV cases in literature, the development of hepatitis generated a suspicion of toxicity caused by antiretroviral therapy. In the recent AIH guide[19], a protocol was proposed in order to differentiate it from DILI (Figure 3). Typically, the antiretroviral therapy has a pattern of hepatocellular or cytolytic injury[13,14,22]. The most common histologic findings in hepatotoxicity related to drugs are microvesicular steatosis, acute hepatitis, eosinophilic infiltration and cholestatic injury[23]. DILI associated with antiretroviral therapy is a major problem; some of the nucleoside reverse transcriptase inhibitors (zidovudine, didanosine, stavudine, abacavir) are associated with mitochondrial toxicity and hepatic steatosis. The non-nucleoside inhibitors, such as Efavirenz and Nevirapine are associated with an increase of liver enzymes, and the latter to hypersensitivity reactions. Protease inhibitors (Indinavir, Saquinavir, Nelfinavir, Ritonavir, Lopinavir-ritonavir, Fosamprenavir, Atazanavir and Tipranavir) are associated with an increase of transaminases, and some, such as Indinavir and Atazanavir to indirect hyperbilirubinemia[24].
Upon suspicion of DILI, suspicious medication should be removed and the liver profile monitored until recovery, later each potentially involved medication reapplied. There are frequent difficulties in identifying the medications that are responsible, since several suspects can coexist, as happens with antituberculosis drugs (Rifampin, Isoniazid, Pyrazinamide), antiretroviral drugs (Efavirenz, Nevirapine, protease inhibitors) and other therapies such as Trimetoprim-sulfamethoxazole. If necessary, an attempt should be made to reintroduce the medications, avoiding the more suspicious agent, as there is a possibility of DILI with greater severity[25].
This is a case in point that does not fully represent the behavior of the autoimmune hepatitis in HIV patients. Therefore, more studies are needed in this population in order to achieve a better understanding of these illnesses.
In conclusion, autoimmune diseases should be taken into consideration in patients with HIV infection, especially those who receive cART therapy, as the immunological recovery can be unleashing them. Likewise, for patients with HIV who show elevated aminotransferase levels, autoimmune hepatitis should be taken into account as one of the possible causes and perform a diagnostic approach to differentiate it from other etiologies. This allows the appropriate treatment and avoids the prolonged suspension of antiretrovirals, and the complications arising from poor virologic control.
A 26-year-old male, diagnosed with human immunodeficiency virus (HIV) infection and treated with Efavirenz/Tenofovir disoproxil fumarate/Emtricitabine, was admitted to the emergency room due to jaundice, anti-retroviral treatment was suspended.
Jaundice without right upper quadrant pain or hepatomegaly.
Drug-induced liver injury, viral hepatitis, alcoholic liver disease, neoplasm, acquired immune deficiency syndrome cholangiopathy.
Hyperbilirubinemia with a predominance of direct bilirubin, severe elevation of transaminases and prolongation of the prothrombin time. The serology for hepatotropic viruses was negative (A, B, C and E), viral loads for virus B viral hepatitis, C viral hepatitis, Epstein Bar and cytomegalovirus were undetectable. The antinuclear antibodies were positive with mottled pattern, negative anti-mitochondrial and anti-muscle antibodies and high levels of immunoglobulin G.
The hepato-biliary ultrasound and portal doppler were normal.
Lymphoplasmacytic inflammatory infiltration with eosinophils and severe interface activity, hepatocytes with peri-central inflammation and focal necrosis (“compatible with autoimmune hepatitis”).
Prednisolone of 1 mg/kg per day following tapered doses, Azathioprine 100 mg/qd and cART (Tenofovir disoproxil fumarate/Emtricitabine and Raltegravir).
After literature search, nine reports with only 22 cases of patients with both entities were found, none of them described in Latin America.
Autoimmune hepatitis is a chronic inflammation of the liver of unknown cause, pathogenesis includes environmental triggers, failure of immune tolerance mechanisms, and a genetic predisposition that induce a T cell-mediated immune attack characterized with continuing hepatocellular necroinflammatory and fibrotic process. The diagnosis is based on histologic abnormalities, clinical and laboratory findings, abnormal levels of immunoglobulin G, and one or more characteristic autoantibodies.
Autoimmunity in patients with HIV infection on cART is uncommon, nevertheless in some clinical scenarios should be considered. The differentiation among autoimmune hepatitis (AIH), drug induced liver injury or infectious hepatitis can be challenging and needs an extensive work-up.
To Jaqcueline Mugnier MD pathologist of the Fundación Cardioinfantil (Bogotá, Colombia) for the histopathology images of the case, and to Ximena Castaneda MD of the Infectious Disease service of the Fundación Cardioinfantil (Bogotá, Colombia) for helping us to collect part of the information from the first hospitalization of the case.
Manuscript source: Unsolicited manuscript
Specialty type: Infectious diseases
Country of origin: Colombia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Beloukas AI, Maggi F S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | GBD 2015 HIV Collaborators, Wang H, Wolock TM, Carter A, Nguyen G, Kyu HH, Gakidou E, Hay SI, Mills EJ, Trickey A, Msemburi W, Coates MM, Mooney MD, Fraser MS, Sligar A, Salomon J, Larson HJ, Friedman J, Abajobir AA, Abate KH, Abbas KM, Razek MM, Abd-Allah F, Abdulle AM, Abera SF, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NM, Abyu GY, Adebiyi AO, Adedeji IA, Adelekan AL, Adofo K, Adou AK, Ajala ON, Akinyemiju TF, Akseer N, Lami FH, Al-Aly Z, Alam K, Alam NK, Alasfoor D, Aldhahri SF, Aldridge RW, Alegretti MA, Aleman AV, Alemu ZA, Alfonso-Cristancho R, Ali R, Alkerwi A, Alla F, Mohammad R, Al-Raddadi S, Alsharif U, Alvarez E, Alvis-Guzman N, Amare AT, Amberbir A, Amegah AK, Ammar W, Amrock SM, Antonio CA, Anwari P, Ärnlöv J, Artaman A, Asayesh H, Asghar RJ, Assadi R, Atique S, Atkins LS, Avokpaho EF, Awasthi A, Quintanilla BP, Bacha U, Badawi A, Barac A, Bärnighausen T, Basu A, Bayou TA, Bayou YT, Bazargan-Hejazi S, Beardsley J, Bedi N, Bennett DA, Bensenor IM, Betsu BD, Beyene AS, Bhatia E, Bhutta ZA, Biadgilign S, Bikbov B, Birlik SM, Bisanzio D, Brainin M, Brazinova A, Breitborde NJ, Brown A, Burch M, Butt ZA, Campuzano JC, Cárdenas R, Carrero JJ, Castañeda-Orjuela CA, Rivas JC, Catalá-López F, Chang HY, Chang JC, Chavan L, Chen W, Chiang PP, Chibalabala M, Chisumpa VH, Choi JY, Christopher DJ, Ciobanu LG, Cooper C, Dahiru T, Damtew SA, Dandona L, Dandona R, das Neves J, de Jager P, De Leo D, Degenhardt L, Dellavalle RP, Deribe K, Deribew A, Des Jarlais DC, Dharmaratne SD, Ding EL, Doshi PP, Driscoll TR, Dubey M, Elshrek YM, Elyazar I, Endries AY, Ermakov SP, Eshrati B, Esteghamati A, Faghmous ID, Farinha CS, Faro A, Farvid MS, Farzadfar F, Fereshtehnejad SM, Fernandes JC, Fischer F, Fitchett JR, Foigt N, Fullman N, Fürst T, Gankpé FG, Gebre T, Gebremedhin AT, Gebru AA, Geleijnse JM, Gessner BD, Gething PW, Ghiwot TT, Giroud M, Gishu MD, Glaser E, Goenka S, Goodridge A, Gopalani SV, Goto A, Gugnani HC, Guimaraes MD, Gupta R, Gupta R, Gupta V, Haagsma J, Hafezi-Nejad N, Hagan H, Hailu GB, Hamadeh RR, Hamidi S, Hammami M, Hankey GJ, Hao Y, Harb HL, Harikrishnan S, Haro JM, Harun KM, Havmoeller R, Hedayati MT, Heredia-Pi IB, Hoek HW, Horino M, Horita N, Hosgood HD, Hoy DG, Hsairi M, Hu G, Huang H, Huang JJ, Iburg KM, Idrisov BT, Innos K, Iyer VJ, Jacobsen KH, Jahanmehr N, Jakovljevic MB, Javanbakht M, Jayatilleke AU, Jeemon P, Jha V, Jiang G, Jiang Y, Jibat T, Jonas JB, Kabir Z, Kamal R, Kan H, Karch A, Karema CK, Karletsos D, Kasaeian A, Kaul A, Kawakami N, Kayibanda JF, Keiyoro PN, Kemp AH, Kengne AP, Kesavachandran CN, Khader YS, Khalil I, Khan AR, Khan EA, Khang YH, Khubchandani J, Kim YJ, Kinfu Y, Kivipelto M, Kokubo Y, Kosen S, Koul PA, Koyanagi A, Defo BK, Bicer BK, Kulkarni VS, Kumar GA, Lal DK, Lam H, Lam JO, Langan SM, Lansingh VC, Larsson A, Leigh J, Leung R, Li Y, Lim SS, Lipshultz SE, Liu S, Lloyd BK, Logroscino G, Lotufo PA, Lunevicius R, Razek HM, Mahdavi M, Majdan M, Majeed A, Makhlouf C, Malekzadeh R, Mapoma CC, Marcenes W, Martinez-Raga J, Marzan MB, Masiye F, Mason-Jones AJ, Mayosi BM, McKee M, Meaney PA, Mehndiratta MM, Mekonnen AB, Melaku YA, Memiah P, Memish ZA, Mendoza W, Meretoja A, Meretoja TJ, Mhimbira FA, Miller TR, Mikesell J, Mirarefin M, Mohammad KA, Mohammed S, Mokdad AH, Monasta L, Moradi-Lakeh M, Mori R, Mueller UO, Murimira B, Murthy GV, Naheed A, Naldi L, Nangia V, Nash D, Nawaz H, Nejjari C, Ngalesoni FN, de Dieu Ngirabega J, Nguyen QL, Nisar MI, Norheim OF, Norman RE, Nyakarahuka L, Ogbo FA, Oh IH, Ojelabi FA, Olusanya BO, Olusanya JO, Opio JN, Oren E, Ota E, Padukudru MA, Park HY, Park JH, Patil ST, Patten SB, Paul VK, Pearson K, Peprah EK, Pereira CC, Perico N, Pesudovs K, Petzold M, Phillips MR, Pillay JD, Plass D, Polinder S, Pourmalek F, Prokop DM, Qorbani M, Rafay A, Rahimi K, Rahimi-Movaghar V, Rahman M, Rahman MH, Rahman SU, Rai RK, Rajsic S, Ram U, Rana SM, Rao PV, Remuzzi G, Rojas-Rueda D, Ronfani L, Roshandel G, Roy A, Ruhago GM, Saeedi MY, Sagar R, Saleh MM, Sanabria JR, Santos IS, Sarmiento-Suarez R, Sartorius B, Sawhney M, Schutte AE, Schwebel DC, Seedat S, Sepanlou SG, Servan-Mori EE, Shaikh MA, Sharma R, She J, Sheikhbahaei S, Shen J, Shibuya K, Shin HH, Sigfusdottir ID, Silpakit N, Silva DA, Silveira DG, Simard EP, Sindi S, Singh JA, Singh OP, Singh PK, Skirbekk V, Sliwa K, Soneji S, Sorensen RJ, Soriano JB, Soti DO, Sreeramareddy CT, Stathopoulou V, Steel N, Sunguya BF, Swaminathan S, Sykes BL, Tabarés-Seisdedos R, Talongwa RT, Tavakkoli M, Taye B, Tedla BA, Tekle T, Shifa GT, Temesgen AM, Terkawi AS, Tesfay FH, Tessema GA, Thapa K, Thomson AJ, Thorne-Lyman AL, Tobe-Gai R, Topor-Madry R, Towbin JA, Tran BX, Dimbuene ZT, Tsilimparis N, Tura AK, Ukwaja KN, Uneke CJ, Uthman OA, Venketasubramanian N, Vladimirov SK, Vlassov VV, Vollset SE, Wang L, Weiderpass E, Weintraub RG, Werdecker A, Westerman R, Wijeratne T, Wilkinson JD, Wiysonge CS, Wolfe CD, Won S, Wong JQ, Xu G, Yadav AK, Yakob B, Yalew AZ, Yano Y, Yaseri M, Yebyo HG, Yip P, Yonemoto N, Yoon SJ, Younis MZ, Yu C, Yu S, Zaidi Z, Zaki Mel S, Zeeb H, Zhang H, Zhao Y, Zodpey S, Zoeckler L, Zuhlke LJ, Lopez AD, Murray CJ. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980-2015: the Global Burden of Disease Study 2015. Lancet HIV. 2016;3:e361-e387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 396] [Cited by in F6Publishing: 405] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 2. | INSIGHT START Study Group, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, Avihingsanon A, Cooper DA, Fätkenheuer G, Llibre JM, Molina JM, Munderi P, Schechter M, Wood R, Klingman KL, Collins S, Lane HC, Phillips AN, Neaton JD. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373:795-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1887] [Cited by in F6Publishing: 1962] [Article Influence: 218.0] [Reference Citation Analysis (0)] |
| 3. | Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, Corbelli GM, Estrada V, Geretti AM, Beloukas A. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA. 2016;316:171-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 905] [Cited by in F6Publishing: 915] [Article Influence: 114.4] [Reference Citation Analysis (0)] |
| 4. | Núñez M. Hepatotoxicity of antiretrovirals: incidence, mechanisms and management. J Hepatol. 2006;44:S132-S139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Reveille JD, Williams FM. Infection and musculoskeletal conditions: Rheumatologic complications of HIV infection. Best Pract Res Clin Rheumatol. 2006;20:1159-1179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014;384:258-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in F6Publishing: 484] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 7. | Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329-337. [PubMed] [Cited in This Article: ] |
| 8. | Iordache L, Launay O, Bouchaud O, Jeantils V, Goujard C, Boue F, Cacoub P, Hanslik T, Mahr A, Lambotte O. Autoimmune diseases in HIV-infected patients: 52 cases and literature review. Autoimmun Rev. 2014;13:850-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Walker UA, Tyndall A, Daikeler T. Rheumatic conditions in human immunodeficiency virus infection. Rheumatology (Oxford). 2008;47:952-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | German V, Vassiloyanakopoulos A, Sampaziotis D, Giannakos G. Autoimmune hepatitis in an HIV infected patient that responded to antiretroviral therapy. Scand J Infect Dis. 2005;37:148-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Wan DW, Marks K, Yantiss RK, Talal AH. Autoimmune hepatitis in the HIV-infected patient: a therapeutic dilemma. AIDS Patient Care STDS. 2009;23:407-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Daas H, Khatib R, Nasser H, Kamran F, Higgins M, Saravolatz L. Human immunodeficiency virus infection and autoimmune hepatitis during highly active anti-retroviral treatment: a case report and review of the literature. J Med Case Rep. 2011;5:233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Coriat R, Podevin P. Fulminant autoimmune hepatitis after successful interferon treatment in an HIV-HCV co-infected patient. Int J STD AIDS. 2008;19:208-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Puius YA, Dove LM, Brust DG, Shah DP, Lefkowitch JH. Three cases of autoimmune hepatitis in HIV-infected patients. J Clin Gastroenterol. 2008;42:425-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | O’Leary JG, Zachary K, Misdraji J, Chung RT. De novo autoimmune hepatitis during immune reconstitution in an HIV-infected patient receiving highly active antiretroviral therapy. Clin Infect Dis. 2008;46:e12-e14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Hagel S, Bruns T, Herrmann A, Tannapfel A, Stallmach A. Autoimmune hepatitis in an HIV-infected patient: an intriguing association. Int J STD AIDS. 2012;23:448-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Cazanave C, Rakotondravelo S, Morlat P, Blanco P, Bonnet F, Beylot J. [Autoimmune hepatitis in a HIV-HCV co-infected patient: diagnostic ant therapeutic difficulties]. Rev Med Interne. 2006;27:414-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Murunga E, Andersson M, Rensburg Cv. Autoimmune hepatitis: a manifestation of immune reconstitution inflammatory syndrome in HIV infected patients? Scand J Gastroenterol. 2016;51:814-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971-1004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 659] [Cited by in F6Publishing: 704] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 20. | Caplan M, Trivedi A, McLaughlin M, Hebou A, Kleiner DE, Heller T, Morse CG. Primary Biliary Cirrhosis Overlapping with Autoimmune Hepatitis in an HIV-Infected Patient on Antiretroviral Therapy. J Interdiscip Histopathol. 2013;1:270-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Stratton R, Slapak G, Mahungu T, Kinloch-de Loes S. Autoimmunity and HIV. Curr Opin Infect Dis. 2009;22:49-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Amacher DE. Female gender as a susceptibility factor for drug-induced liver injury. Hum Exp Toxicol. 2014;33:928-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354:731-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 690] [Cited by in F6Publishing: 611] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 24. | Jain MK. Drug-induced liver injury associated with HIV medications. Clin Liver Dis. 2007;11:615-639, vii-viii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Chughlay M, Blockman M, Cohen K. A clinical approach to drug-induced liver injury. Curr Allergy Clin Immunol. 2015;28:252-256. [Cited in This Article: ] |