Published online Nov 20, 2016. doi: 10.5493/wjem.v6.i4.72
Peer-review started: March 24, 2016
First decision: May 16, 2016
Revised: May 25, 2016
Accepted: July 14, 2016
Article in press: July 16, 2016
Published online: November 20, 2016
To describe the morphogenesis of different neuronal cells from the human embryonic stem cell (hESC) line, SCT-N, under in vitro culture conditions.
The directed neuronal cell line was produced from a single, spare, pre-implantation stage fertilized ovum that was obtained during a natural in vitro fertilization process. The hESCs were cultured and maintained as per our proprietary in-house technology in a Good Manufacturing Practice, Good Laboratory Practice and Good Tissue Practice compliant laboratory. The cell line was derived and incubated in aerobic conditions. The cells were examined daily under a phase contrast microscope for their growth and differentiation.
Different neural progenitor cells (NPCs) and differentiating neurons were observed under the culture conditions. Multipotent NPCs differentiated into all three types of nervous system cells, i.e., neurons, oligodendrocytes and astrocytes. Small projections resembling neurites or dendrites, and protrusion coming out of the cells, were observed. Differentiating cells were observed at day 18 to 20. The differentiating neurons, neuronal bodies, axons, and neuronal tissue were observed on day 21 and day 30 of the culture. On day 25 and day 30, prominent neurons, axons and neuronal tissue were observed under phase contrast microscopy. 4’, 6-diamidino-2-phenylindole staining also indicated the pattern of differentiating neurons, axonal structure and neuronal tissue.
This study describes the generation of different neuronal cells from an hESC line derived from biopsy of blastomeres at the two-cell cleavage stage from a discarded embryo.
Core tip: Human embryonic stem cells (hESCs) have the capability to regenerate and differentiate into a wide variety of cells. In the present study, we described the morphogenesis of different neuronal cells from an hESC line under in vitro culture conditions. The blastomeres were at the two-cell cleavage stage and were taken from a discarded embryo during an in vitro fertilization process. We showed that neuronal axons and tissues were generated by the joining of multiple cells that communicate and transfer signals to each other, thereby forming neuronal cells and tissue.
- Citation: Shroff G. Morphogenesis of human embryonic stem cells into mature neurons under in vitro culture conditions. World J Exp Med 2016; 6(4): 72-79
- URL: https://www.wjgnet.com/2220-315X/full/v6/i4/72.htm
- DOI: https://dx.doi.org/10.5493/wjem.v6.i4.72
Human embryonic stem cells (hESCs) offer an unlimited source of human cells[1]. hESCs have the capability to renew and differentiate into all cell types. Differentiation may occur at all development stages upon exposure to the appropriate signals. These signals act in a hierarchical manner that regulates the development of the embryo, which induces the hESCs to differentiate into specific cell types of the three germ layers[2,3].
The pluripotent characteristic of hESCs is considered as a “double-edged sword”. The determination of how to direct hESCs to a specific progenitor cell type is a major challenge[1]. hESCs are a renewable source of neural cells[4]. Different protocols are used to direct the neural differentiation of hESCs; however, across all culture systems, common signals and stages of differentiation are observed. The three main stages of hESCs’ neural differentiation are neural induction, neural stem/progenitor expansion, and neuronal and glial differentiation. These stages may further be subdivided into multiple events to progress a stem cell through its different progenitor stages[1].
The human neuronal system is ectodermal in origin and contains multipotent stem cells, i.e., neuronal stem cells or neural progenitor cells (NPCs), which give rise to the different cells of the nervous system. Multipotency, the ability to differentiate into different types of neuronal cells, viz. neurons, astrocytes and oligodendrocytes, is the key characteristics of NPCs[5,6]. hESCs can be differentiated into NPCs that exhibit a broad cellular developmental and lineage spectrum[7,8]. According to Wu et al[9], in vitro differentiation of hESCs and in vivo embryonic formation of the neuroectoderm share some similarities.
The present study describes the generation of different neuronal cells from an hESC line (SCT-N) produced from biopsied blastomeres at the two-celled cleavage stage from a discarded embryo during an in vitro fertilization (IVF) process. These cells use defined animal free conditions during derivation and long-term culture, which make them suitable for clinical cell therapy. We showed that neuronal axons and tissues were not generated from single cell differentiation, but were produced by the joining of multiple cells that communicate and transfer signals to each other, thereby forming neuron tissue.
The study protocol was approved by an independent Institutional Ethics Committee (IEC) of Nutech Mediworld. The institutional committee for stem cell research and therapy of Nutech Mediworld, New Delhi, India reported the study to the National Apex Committee for Stem Cell Research and Therapy (NAC-SCRT). The study was conducted according to the Declaration of Helsinki[3].
The directed cell line (neuronal) was obtained from a single, spare, pre-implantation stage fertilized ovum that was obtained during a natural IVF process. The donor gave her consent.
The hESCs were cultured and maintained according to our proprietary in-house technology (United States Granted Patent No US 8592, 208, 52) in a Good Manufacturing Practice (GMP), Good Laboratory Practice (GLP) and Good Tissue Practice (GTP) compliant laboratory. The cell lines were chromosomally stable and free from any animal product. The detailed cell culture and differentiation techniques were described in our previous paper[10].
The embryo was suspended in Roswell Park Memorial Institute (RPMI) medium and broken mechanically. Human beta-chorionic gonadotropin (β-hCG) and progestin were added and the cells were incubated in a CO2 water-jacketed incubator for 24 h in aerobic conditions. The cell suspension was then re-incubated in the same incubator after adding Dulbecco’s Modified Eagle’s Medium (DMEM, Himedia Labs, Mumbai, India) under anaerobic conditions. The details of cell culture and derivation are explained in our previous paper[10].
For this experiment, the above cell line was taken and re-incubated in aerobic conditions after the addition of DMEM. The cells were examined daily for the growth and differentiation of the neural tissue.
Syringes of frozen cells containing 2.5 to 3.5 million cells per milliliter were thawed and raised to body temperature. After this slow thawing process, the cells were characterized for their expression of different markers[11] using reverse transcription polymerase chain reaction RT-PCR on a Bio-Rad T100 (Bio-Rad Laboratories Inc., Hercules, CA, United States), immunofluorescence using a Nikon Ellipse E200 (Nikon corporation, Minato-ku, Tokyo, Japan) and fluorescence-activated cell sorting (FACS).
A quality check for the integrity, viability and microbial contamination was performed for the stored cell batches and the cultured cells obtained from the GLP laboratory.
To investigate the differentiation of hESCs into different NPCs and neuronal tissue, hESCs were cultured for one month (day 0 of inoculation to day 30 of the culture). The cells were observed for differentiation at different days under a phase contrast microscope (Nikon Ellipse E200), which was used as the major instrument to determine neuronal differentiation.
To determine the presence of nuclei and chromatin materials, hESCs were stained with DAPI. The cells were incubated in a DAPI solution at a concentration of 0.1-1 μg/mL for 5 min. DAPI stained cells were mounted in ProLong® Gold Antifade (Life technologies, OR, United States) and were evaluated under a fluorescence microscope.
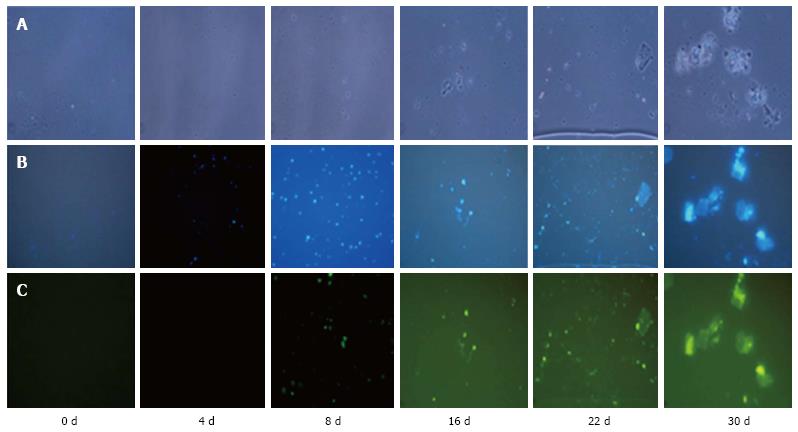
The expression of the NeuN gene was examined in cells cultured for different days (0-30 d). The cultured cells were reacted with an anti-NeuN antibody. The staining was performed at day 4, 8, 16, 22 and 30 of culture.
Cells (1 × 106) were adhered on a cover slide coated with poly-L-lysine, followed by washing in PBS and fixing for 10-20 min in 4% paraformaldehyde (PFA) at room temperature. The cells were permeabilized in Triton-X (0.25%) for 20 min. BSA (2%) and donkey (5%) serum were used to block the PBS washed cells for 2 h.
The treated cells were incubated at 37 °C with a primary antibody against NeuN (1:100, Neuromics) and then with the secondary antibody Alexa fluor©488 donkey (SC2044) anti-mouse IgG (Invitrogen, Life technology, 557782) for 1 h and 30 min respectively. The cells were counterstained with 0.1-1 μg/mL DAPI for 5 min, mounted in ProLong® Gold Antifade (Life technologies, OR, United States) and evaluated under a fluorescence microscope (Nikkon Micro E200).
All the cell batches and the cultured cells were found to be viable and sterile. No microbial contamination was observed.
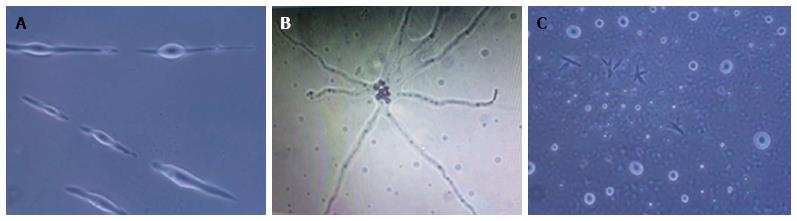
Different NPCs and differentiating neurons were observed in the hESCs culture. Multipotent NPCs differentiated into all three types of cells of the nervous system, i.e., neurons, oligodendrocytes and astrocytes, as observed under a phase contrast microscope (Figure 1).
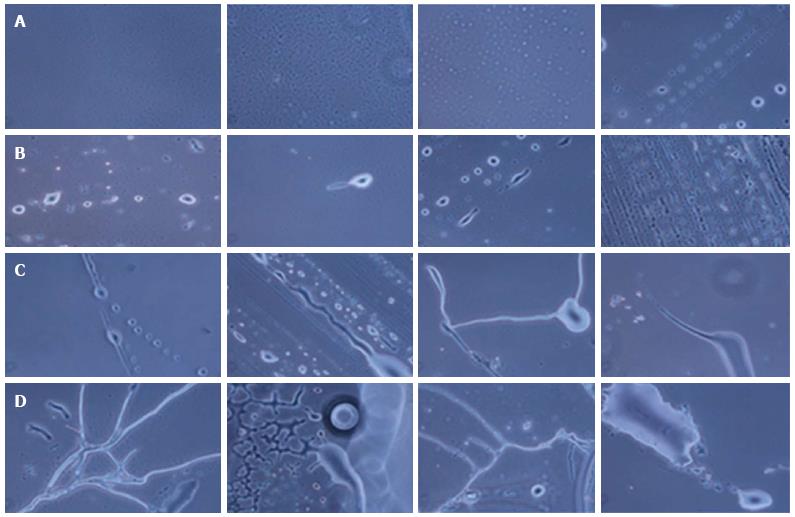
The hESCs were cultured for 30 d and observed at regular interval under a phase contrast microscope. Low cell numbers were observed just after inoculation; however, after 24-48 h of culture, the cell number increased and the cells started to clump together. Further culture led to an increase in cell size and the generation of NPCs (Figure 2). From 6-8 d of culture, we observed increased numbers of NPCs, while some of the cells showed the first signs of differentiation.
We observed small projections, such as those on neurites or dendrites, and protrusions coming out of the cells. Significant differentiation of the cells was observed at 18-20 d of culture.
Differentiating neurons, neuronal bodies, axons and neuronal tissue were observed on day 21 and day 30 of culture. On day 25 and day 30, prominent neurons, axons and neuronal tissue were observed under phase contrast microscopy.
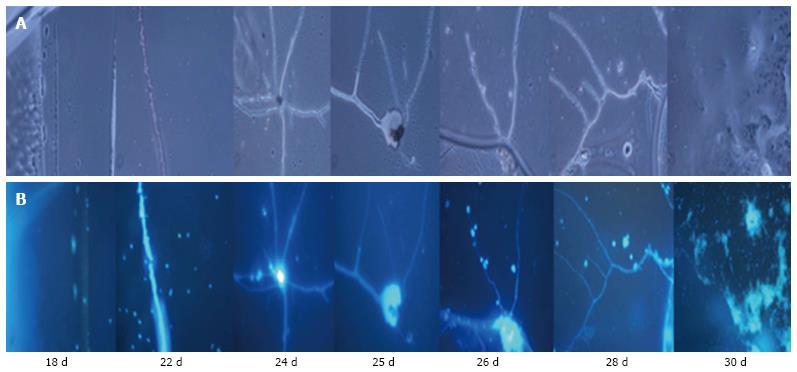
Further validation of the cells’ morphology, differentiation pattern, presence of chromatin material and presence of tissue structure in culture were examined by DAPI (nuclear staining). The DAPI staining confirmed the presence of nuclei inside the cells. DAPI staining was more prominent in smaller cells compared with larger cells, probably because the larger cells were less permeable to DAPI compared with smaller cells (Figure 3).
DAPI staining also indicated the pattern of differentiating neurons, axonal structure and neuronal tissue.
NeuN staining of cells was observed on day 4, 8, 16, 22 and 30 of culture. We observed the staining of the cells from day 4 of culture, which continued on day 8, 16, 22 and 30 of culture. Tissue staining was observed on day 16 onwards (Figure 4).
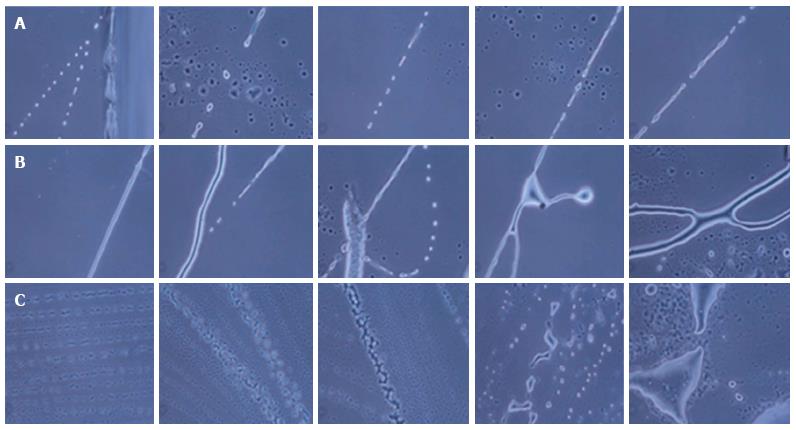
During the formation of neurons, neuronal axons and neuronal tissue, the NPCs were first arranged in a line and then differentiated into neuronal cells with single or multiple projections. Furthermore, these projections joined the progenitor cells and increased in size, giving rise to small single protrusions. This protrusion increased in length and then joined to another cell’s projection. The joining of one cell projection to another might either be direct or via joining to other progenitor cells. In this way, these cells formed a chain and their cytoplasmic materials fused together to form neuronal axons (Figure 5).
Likewise, for the formation of neuronal tissue, the cells were first arranged in a line and then their projections joined together to form a network-like structure. Further growth and development of these joined cells in a network led to the development of the neuronal tissue. We also observed branching of neurons by the joining of single cell progenitor cells.
Under our culture condition, we observed that during differentiation of NPCs, all the cells do not commit towards differentiation at the same time. We hypothesized that among these cells, if one cell commits to differentiation, other surrounding cells will assist these progenitor cells for their growth, development and differentiation.
In this in vitro culture, we could observe single neuronal cell differentiation and the spread of a tissue-like structure. By joining together, these cells communicate and transfer signals to each other.
In the present study, we determined the ability of hESCs to differentiate into several neural cells and tissue. We showed the mechanism of division and differentiation of single NPCs into neurons, axons and neuronal tissue. Our results indicated that differentiation of hESCs towards lineage specific cells or tissue involves the generation of lineage-specific progenitors from hESCs that then undergo differentiation.
The pluripotent nature of hESCs allow them to generate the neuronal and glial cells found in neural tissue[5]. To direct and understand the differentiation process of hESCs into neuronal lineages, we cultured the cells in normal culture media, which was free from feeder layers and animal product. A unique feature of this culture media was that it did not contain any supplements. Under these culture conditions, the differentiation pattern of the stem cells was similar to the accepted model of the adult human and mouse nervous system differentiation process[12,13]. Ying et al[14] cultured embryonic stem cells (ESCs) in defined serum-free and feeder-free conditions, in the absence of bone morphogenetic proteins (BMP) signals, which resulted in efficient neural commitment and differentiation. Our results indicated that our unique culture conditions allow the lineage specific differentiation of neuronal cells without adding supplements.
In our study, hESCs generated NPCs that were capable of differentiating into neural cells and tissue. Microscopy revealed three types of nervous system cells, i.e., neurons, oligodendrocytes and astrocytes. A previous study by Rubinoff et al[4] reported similar results: The proliferating progenitors generated from hESCs were able to differentiate into astrocytes, oligodendrocytes and neurons. Similarly, Zhang et al[15] demonstrated that hESCs-derived neural precursors generated all three types of neuronal cells in vitro.
After establishing the differentiation of hESCs in our culture conditions, we studied how hESCs were differentiated into neurons and neuronal tissue and the time course of differentiation. The differentiation stages of hESCs were followed at different days (0 day to 30th day). The cells started growing and were arranged in a line. Arranging of the cells in a line was identified as a characteristic of neuronal differentiation in our culture conditions. After culturing hESCs for a week, small projections started to come out of the cells. The multipotent NPCs underwent asymmetrical cell division, which gave rise to smaller, undifferentiated multipotent stem cells and differentiated neuron cells. Hence, our results confirm the potential of hESCs to differentiate into neural cells. These results are consistent with previous studies[16]. In a study by Zhang et al[15], hESCs differentiated to form neural tube-like rosette structures by day 7 of cell culture in a defined medium. Rubinoff et al[4] cultured hESCs for 3 to 4 wk to induce differentiation and derivation of NPCs. Early differentiation started after a week of culture, as indicated by changes in the cell morphology observed at the center of the colonies under phase contrast microscopy. Differentiation accelerated during next two weeks. Abranches et al[12] cultured ESCs for several days and observed that the cells developed very long cellular projections similar to those of radial glia, which then differentiated into all three neural lineages.
Differentiation of hESCs towards neuronal lineages leads to expression of neuronal-specific markers. Therefore, after establishing the differentiation of neurons and neuronal tissue in our culture conditions, we also confirmed the expression of neuronal system specific markers by immunofluorescence microscopy. NeuN is a specific marker that is expressed in neuronal differentiated cells and tissues[17]. Expression of this neuron-specific nuclear protein is a reliable marker of proliferative capacity that might indicate the physiological status of a post mitotic neuron[18]. In our previous study, we observed the expression of neuronal lineage-specific markers like nestin (neuronal progenital cells, NPCs) and NeuN (neuronal marker of differentiated cells) in hESCs at the mRNA level[10]. On the 8th day of culture, we observed staining of smaller cells with the anti-NeuN antibody, which indicated that the cells might be entering differentiating mode. On day 30 of culture, we observed the staining of differentiated cells and differentiated tissue, which indicated that the later stage of the culture contained both differentiating cells and tissues. The spontaneous expression of neural markers may be considered as an evidence of the hESCs’ differentiation towards neural lineages.
We also determined the mechanism of neuronal axons and tissue formation under in vitro culture conditions. Most of the studies on neurons and neuronal tissues differentiation have been done with either stem cells isolated from the pluripotent blastocyst stage or multipotent adult stem cells. These cells were found to account for the morphology and antigenic properties of neurons and astrocytes after their division and differentiation into neurons and astrocytes, respectively[19,20]. Reynolds et al[20] demonstrated the induced in vitro proliferation of striatum-isolated cells via epidermal growth factor. As far as we know, this is the first study to explain the mechanism of neuron network formation, axon formation and neuronal tissue differentiation of hESCs. Based on the principle that a brain-like structure can emerge as a self-organizing cytoarchitecture from stem cells in vitro[19], we were able to show the mechanism of differentiation, extension of axons, cell joining and tissue differentiation from totipotent hESCs during in vitro culture. In our culture conditions, the pattern of aligning in a row of hESCs was found to be a characteristic feature of neuronal differentiating cells. We also observed that all the cells do not differentiate simultaneously, rather multiple single cells first align together and then join together through small protrusions, thereby forming neuronal cells, axon and tissue.
This study provided a system for generation of NPCs from hESCs. Moreover, the resulting NPCs from hESCs could serve as an expandable source for neuron production, which could be applied for many purposes, such as the treatment of neurodegenerative diseases. Recently, clinical trials using hESC derivatives to treat several neurodegenerative diseases have been approved, which led to the derivation of specific cell types from hESCs[6]. The development of neuronal tissue differentiation protocols will expand the application of hESCs-derived NPCs to neurological diseases. The stem cells used in our study have been injected into patients suffering from various neurodegenerative diseases such as cerebral palsy, spinal cord injury and Friedreich’s ataxia and have shown successful results[11,21-23].
To understand how to manipulate stem cells, and to restore and repair neural circuitry, it is important to identify the signals and mechanisms involved at each of these stages[1]. In view of the importance of understanding self-renewal and differentiation of hESCs at the transcriptional level, further studies are needed. This study will have a significant impact on the emerging field of regenerative medicine.
The author acknowledges Knowledge Isotopes Pvt. Ltd. (http://www.knowledgeisotopes.com) for their assistance with writing the paper.
Human embryonic stem cells (hESCs) offer an unlimited source of cells. They have the capability to renew and differentiate into all cell types. Differentiation may occur at all development stages upon exposure to appropriate signals. These signals act in a hierarchical manner that regulates the development of the embryo, which induces the hESCs to differentiate into specific cell types of three germ layers.
The study describes the generation of different neuronal cells from the cell line SCT-N produced using hESCs from biopsy of blastomeres at the two-celled cleavage stage from a discarded embryo during an in vitro fertilization process.
The hESC line uses defined animal free conditions during its derivation and long-term culture, which makes it suitable for clinical cell therapy. The author showed that neuronal axons and tissues were not generated from single cell differentiation but by the joining of multiple cells that communicate and transfer signals to each other, thereby forming neuronal tissue.
The study provided a system to generate neural progenitor cells (NPCs) from hESCs. Moreover, the resulting NPCs could serve as an expandable source for neurons production, which could be applied for many purposes, such as the treatment of neurodegenerative diseases.
NPCs are multipotent, self-renewable cells that are responsible for forming the main phenotype of the nervous system.
The paper “Morphogenesis of human embryonic stem cells into mature neurons under in vitro culture conditions” by Shroff, describes the differentiation of hESCs into neurons. It is a rapidly growing field of translational research that combines high expectations and safety concerns. Maintaining hESCs in defined conditions under strict GMP regulations is a very important part of the research, together with detailed step-by-step descriptions of differentiating cells and their characterization.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Bártová E, Kiselev SL, Tanabe S S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Denham M, Dottori M. Signals involved in neural differentiation of human embryonic stem cells. Neurosignals. 2009;17:234-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Hay DC, Zhao D, Fletcher J, Hewitt ZA, McLean D, Urruticoechea-Uriguen A, Black JR, Elcombe C, Ross JA, Wolf R. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells. 2008;26:894-902. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-1147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11399] [Cited by in F6Publishing: 10061] [Article Influence: 387.0] [Reference Citation Analysis (0)] |
| 4. | Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben-Hur T. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19:1134-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 871] [Cited by in F6Publishing: 749] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 5. | Willerth SM. Neural tissue engineering using embryonic and induced pluripotent stem cells. Stem Cell Res Ther. 2011;2:17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Noisa P, Raivio T, Cui W. Neural Progenitor Cells Derived from Human Embryonic Stem Cells as an Origin of Dopaminergic Neurons. Stem Cells Int. 2015;2015:647437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2417] [Cited by in F6Publishing: 2466] [Article Influence: 164.4] [Reference Citation Analysis (0)] |
| 8. | Gerrard L, Rodgers L, Cui W. Differentiation of human embryonic stem cells to neural lineages in adherent culture by blocking bone morphogenetic protein signaling. Stem Cells. 2005;23:1234-1241. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Wu JQ, Habegger L, Noisa P, Szekely A, Qiu C, Hutchison S, Raha D, Egholm M, Lin H, Weissman S. Dynamic transcriptomes during neural differentiation of human embryonic stem cells revealed by short, long, and paired-end sequencing. Proc Natl Acad Sci USA. 2010;107:5254-5259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Shroff G. Establishment and characterization of a neuronal cell line derived from a 2-cell stage human embryo: clinically tested cell-based therapy for neurological disorders. Int J Recent Sci Res. 2015;6:3730-3738. [Cited in This Article: ] |
| 11. | Shroff G, Gupta A, Barthakur JK. Therapeutic potential of human embryonic stem cell transplantation in patients with cerebral palsy. J Transl Med. 2014;12:318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Abranches E, Silva M, Pradier L, Schulz H, Hummel O, Henrique D, Bekman E. Neural differentiation of embryonic stem cells in vitro: a road map to neurogenesis in the embryo. PLoS One. 2009;4:e6286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | Oikari LE, Griffiths LR, Haupt LM. The current state of play in human neural stem cell models: what we have learnt from the rodent. OA Stem Cells. 2014;2:7. [Cited in This Article: ] |
| 14. | Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1178] [Cited by in F6Publishing: 1099] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 15. | Zhang SC, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129-1133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1451] [Cited by in F6Publishing: 1307] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 16. | Ahmad R, Wolber W, Eckardt S, Koch P, Schmitt J, Semechkin R, Geis C, Heckmann M, Brüstle O, McLaughlin JK. Functional neuronal cells generated by human parthenogenetic stem cells. PLoS One. 2012;7:e42800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201-211. [PubMed] [Cited in This Article: ] |
| 18. | Weyer A, Schilling K. Developmental and cell type-specific expression of the neuronal marker NeuN in the murine cerebellum. J Neurosci Res. 2003;73:400-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 208] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Gaspard N, Vanderhaeghen P. Mechanisms of neural specification from embryonic stem cells. Curr Opin Neurobiol. 2010;20:37-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707-1710. [PubMed] [Cited in This Article: ] |
| 21. | Shroff G. A novel approach of human embryonic stem cells therapy in treatment of Friedreich’s ataxia. Int J Case Rep Images. 2015;6:261-266. [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Shroff G, Das L. Human embryonic stem cell therapy in cerebral palsy children with cortical visual impairment: a case series of 40 patients. J Cell Sci Ther. 2014;5:189. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Shroff G, Gupta R. Human embryonic stem cells in the treatment of patients with spinal cord injury. Ann Neurosci. 2015;22:208-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |