Published online May 20, 2015. doi: 10.5493/wjem.v5.i2.130
Peer-review started: November 9, 2014
First decision: December 12, 2014
Revised: January 5, 2015
Accepted: January 18, 2015
Article in press: January 20, 2015
Published online: May 20, 2015
One of the most common and serious complications of diabetes mellitus is ulceration of the foot. Among persons with diabetes, 12%-25% will present to a healthcare institution for a foot disorder during their lifespan. Despite currently available medical and surgical treatments, these are still the most common diabetes-related cause of hospitalization and of lower extremity amputations. Thus, many adjunctive and complementary treatments have been developed in an attempt to improve outcomes. We herein review the available literature on the effectiveness of several treatments, including superficial and deep heaters, electro-therapy procedures, prophylactic methods, exercise and shoe modifications, on diabetic foot wounds. Overall, although physical therapy modalities seem to be useful in the treatment of diabetic foot wounds, further randomized clinical studies are required.
Core tip: People with diabetes are prone to frequent and often have severe foot problems. Treatments for diabetic foot ulcer (DFU) include surgical debridement and drainage, antimicrobial therapy for infected wounds, pressure off-loading methods and advanced wound dressings. Thus, many clinicians and researchers have made efforts to develop adjunctive or complementary treatments to improve the outcome of DFUs. This paper presents a review of the epidemiology, pathogenesis and clinical manifestations of DFUs, and a discussion of the data available on relevant physical therapies and rehabilitation methods.
- Citation: Turan Y, Ertugrul BM, Lipsky BA, Bayraktar K. Does physical therapy and rehabilitation improve outcomes for diabetic foot ulcers? World J Exp Med 2015; 5(2): 130-139
- URL: https://www.wjgnet.com/2220-315X/full/v5/i2/130.htm
- DOI: https://dx.doi.org/10.5493/wjem.v5.i2.130
Diabetes mellitus is a growing problem worldwide and now affects about 5%-15% of the entire population in many developed and developing countries[1]. While diabetes can lead to complications in many organ systems and ulceration of the foot is now among the most common and serious complications[2]. An estimated 12%-25% of persons with diabetes will present to a healthcare institution for some type of foot disorder during their lifetime[3-5]. Most often these are ulcers that arise largely as a consequence of peripheral neuropathy and, to a lesser degree, peripheral arterial disease. Diabetic foot ulcers (DFUs) often result in severely adverse outcomes, such as serious infections, the need for hospitalization and lower extremity amputations, that are associated with a five-year mortality of about 50%[6-8].
Treatments for DFU include surgical debridement and drainage, antimicrobial therapy for infected wounds, pressure off-loading methods and advanced wound dressings. Despite these treatments, lower extremity amputations in diabetic patients occur at a rate 17 to 40-fold higher than in non-diabetic individuals[9]. The most frequent precipitating cause for non-traumatic foot amputations worldwide is a DFU with secondary infection[10-13]. Today, it is estimated that worldwide a foot is lost due to complications of diabetes every 30 s[6]. Thus, many clinicians and researchers have made efforts to develop adjunctive or complementary treatments to improve the outcome of DFUs. This paper presents a review of the epidemiology, pathogenesis and clinical manifestations of DFUs, and a discussion of the data available on relevant physical therapies and rehabilitation methods.
While it is difficult to obtain firm data on the prevalence and incidence of diabetic foot ulcers, published trials suggest that approximately 15% of diabetic patients will develop a foot ulcer complication during their lifetime[14]. In one study in 37 primary care centers in the United Kingdom 5.3% of 811 type 2 diabetic patient were reported to have a diabetic ulcer at some point in their life[15]. In the United States, while the rate of hospitalization for diabetic foot ulcer was 5.4 per 100 diabetic patients in 1980, this figure increased to 6.9 in 2003[16].
The review of patients with diabetic foot ulcers shows that there are more male patients than female patients[17]. One retrospective review from Turkey of 142 cases found that 65% were males[18]. Diabetic foot ulcers occur in both in the type 1 and type 2 forms of diabetes, but there are far more people with type 2 disease. The average age of patients with a diabetic foot ulcer is about 60 years. The development of diabetic foot ulcer is more strongly associated with the duration of the disease rather than the age at disease onset[2].
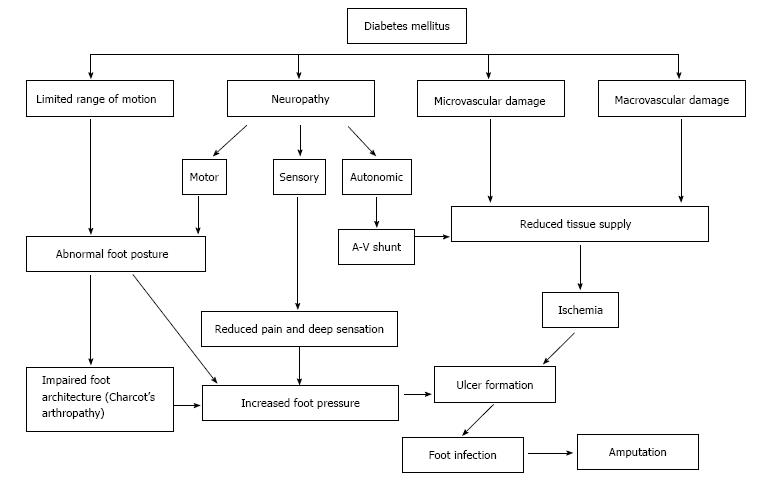
Several factors are involved in the development of DFUs but the two main causes can be divided into primary and secondary pathologies. Primary etiologies involve peripheral neuropathy and vasculopathy, while secondary causes relate to complications from hyperglycemia[19]. Diabetic foot ulcers may be completely neuropathic (35%), completely ischemic (15%) or a mixed neuro-ischemic (50%)[6]. Injury to neural cells related to poorly controlled blood sugar levels cause motor, sensory and autonomic neuropathy, leading to altered foot anatomy (such as claw toe and hammer toe deformity), impaired sensory perception and formation of skin cracks[10,20-22]. Microvascular dysfunction and macrovascular (atherosclerotic) disease leads to ischemia through impaired blood supply to the feet (Figure 1). Ulcers usually occur in the feet due to physical, thermal or chemical trauma.
Musculoskeletal complications involving the foot, most commonly seen in patients with a longstanding history of diabetes, put patients at risk for developing ulcers. Major underlying causes include increased glycosylation of collagen in the skin and periarticular tissue (tendon, ligaments and the joint capsule), decreased collagen degradation, and diabetic microangiopathy[23]. Alterations in the structure of the foot, including loss of flexibility and limited joint mobility, impair the ability of the foot to absorb and redistribute forces related to impact with the ground while walking. The development of foot deformities is a major factor in creasing plantar pedal pressure. Among the foot joints, the metatarsophalangeal and subtalar joints are exposed to the highest level of pressure[23], which lead to the development of foot ulceration[14]. The factors discussed in this section are summarized in Table 1[10].
| Risk factor | Cause/effect of the risk factor |
| Peripheral motor neuropathy | Abnormal foot anatomy and bio-mechanics, manifesting with paw feet, high foot arch, subluxed metatarsophalangeal joints, increased foot pressure and callus formation |
| Peripheral sensory neuropathy | Minor chronic injuries secondary to heat, mechanical or high pressure as a result of deficiency of protective pain sensation |
| Peripheral autonomic neuropathy | Cracks on the dry skin due to reduced moisture |
| Neuroosteoarthropodic deformities | Abnormal foot anatomy and bio-mechanics secondary to increased foot pressure (particularly in the midplantar region) |
| Vascular failure | Reduced neutrophil migration, loss of the tissue viability and delayed wound healing |
| Uncontrolled blood sugar and other metabolic imbalances | Deficient immune system (particularly in the neutrophil functions), wound healing and collagen production |
| Patient characteristics | Loss of vision, limited motion, previous amputation(s) |
| Incompliant patient attitude | incompliance with the hygiene rules, foot care, prophylactic measures and healthcare principles (excessive weight gain, etc.) |
| Inadequacy of the healthcare system | Inadequate patient training on foot care, blood sugar control etc.; insufficient treatment centers that provide one-to-one patient care and/or insufficient bed counts; deficiency of a multi-disciplinary approach |
When assessing a patient with a diabetic foot wound, formulating a treatment approach requires understanding how to characterize any foot infection present as well as the patient’s overall status. While there are now many schemes for assessment of diabetic foot pathology, the most commonly used classifications include those by Wagner, the University of Texas, and the International Working Group on the Diabetic Foot PEDIS system (the “infection” part of which is very similar to that proposed by the Infectious Diseases Society of America)[24-26]. While the Wagner scheme can predict clinical outcome, it is mainly useful for vascular and surgical classification and not for assessing infection[27]. The University of Texas classification is now more widely used, but it classifies infection only as present (which it does not define) or absent. On the other hand, the PEDIS classification is a research tool for assessing for circulatory impairment (perfusion), the extent and depth of the ulcer and neuropathy (sensation) as well as for the presence and severity of infection[25].
Evaluating a patient with a diabetic foot wound requires assessing the dermatologic, vascular, neurologic and musculoskeletal findings[2]. Important findings include any redness, swelling, increased warmth, pain or tenderness, numbness, skin breaks, blisters, peeling, ingrown toenails or other nail deformity, callus, or skin dryness[28]. The clinician should examine the dorsal, plantar, medial, lateral and posterior surfaces of the feet, along with the nails, comparing them to the upper extremity[28]. Assessment for peripheral vascular disease includes palpating the dorsalis pedis, tibialis posterior, popliteal and superficial femoral arteries checking the skin for paleness and coolness compared to more proximal regions of the lower extremity[28].
While the probability of having peripheral neuropathy is 30%-40% in patients with a 10-year diabetes history, and up to 60%-70% of those with a diabetes over 25 years, the rate is over 80% in those with a foot ulcer[14]. The neuropathy is a distal, symmetrical, sensorimotor type that may be asymptomatic or manifest with pain, allodynia, numbness or a burning sensation[29]. Because the small diameter fibers are involved early, pain and heat sensation disappear first. Subsequently, with large-diameter motor neuron damage there is a reduction in deep tendon reflexes, vibration and pressure sensation[2]. The vibration sensation can most easily be measured with a tuning fork, with the normal time of vibration perception approximately 20 s. Superficial sensation can be examined by touching with cotton, two point discrimination by using the blunted end of a compass placed on the dorsal foot. The most commonly used and well-validated test is checking for presence of pressure sensation with a monofilament. Absence of the ability to feel a 5.07-size monofilament at selected sites on the foot means the patient lacks protective sensation, which greatly increasing the risk of a diabetic foot ulcer[29]. Foot deformities related to motor neuropathy include pes cavus, pes planus, hallux valgus, hallux malleus, claw toe, and Charcot’s arthropathy[23]. This impairs the architecture of the foot leads to loading excessive pressure onto certain points, increasing the risk of ulceration.
Outcomes have consistently been shown to be better when patients with a diabetic foot ulcer are cared for a multi- (or, more accurately, inter-) disciplinary approach. Disciplines involved should optimally include a diabetologist, infectious diseases/microbiology expert, plastic, orthopedic or podiatric surgeon, angiologist or vascular surgeon, physical therapist, wound care nurse, orthotist and rehabilitation expert. The treatment plan should be established by joint council, optimally with as many team members in the same room with the patient as possible.
Diabetic foot ulcers prolong the duration of hospitalization to a greater extent than almost all other diabetes-related complications[30]. And, despite great advances in the treatment of diabetic foot ulcers, a substantial minority of patients still undergoes a lower extremity amputation. In fact, complications of diabetes are the leading cause of non-traumatic lower leg amputations worldwide. These amputations are associated with major morbidity, worsening of quality of life and financial costs, but perhaps most importantly, the five year post-amputation patient survival is only 50%. Therefore, every means possible should be used to try to heal a diabetic foot ulcer, including exercise therapy, footwear modifications physical therapy and rehabilitation methods. Currently, there is some published literature on the value of physical therapy and rehabilitation methods for diabetic foot ulcers, but no Cochrane Database of Systematic Reviews, National Guidelines or consensus declarations.
The physical treatment modalities used in diabetic polyneuropathy and diabetic foot ulcers are summarized in Table 2.
| Heating agents | Electrotherapy methods |
| Superficial heaters: Infrared treatment, global heat treatment | Electrical stimulation |
| Deep heaters: Ultrasound treatment | Shock wave therapy (ESWT) |
| Laser treatment | |
| Magnetic field treatment | |
| Galvanic current treatment |
Thermotherapy: Heat, a form of energy, is a commonly used physical treatment agent. When a hot substance contacts a cold one, it transfers of heat. During superficial heat treatment the heated therapeutic agents transfer heat to the body[31].
Heat treatment increases blood flow to the area as a result of inducing vasodilatation, which is thought to contributes to wound healing[31]. There is, however, only one study evaluating the benefits of this treatment for chronic wounds in persons[32]. In this study, patients who had previously received electrical stimulation were treated with either global heat treatment or local heat treatment. Global heat treatment was administered by keeping the patients in rooms at 32 °C of temperature for 20 min after establishing a heater and a fan system in a room. Local therapy was administered using an infrared heat lamp heated to 37 °C that was directed on the wound site for 20 min. The control subjects only received treatment with electric stimulation, which was administered at 30-Hz frequency and 20-mA ampere with 2-cm × 2-cm-diameter carbonized electrodes. Wound healing was calculated by measuring the length of the wound with a digital machine, the depth with a metric ruler and the blood flow with laser Doppler ultrasound. Wound healing rates were significantly higher (by 20%) with global heat treatment compared to the local heat therapy. With local heat therapy the blood flow measured by laser Doppler ultrasound had increased, but it was relatively lower than that in global heat treatment. Wound healing in the control (electric stimulation) group was less than in the two heat treated groups[32]. Although local heat therapy is more practical to administer, global heat treatment was superior for improving wound healing.
Sound waves turn into heat energy while passing through a homogenous environment. Ultrasound involves sound waves with a frequency higher than what the human can hear. Deep-heating agents such as ultrasound can effectively heat the skin as deep as > 1 cm. The sound frequencies used in ultrasound therapy are typically 1.0 to 3.0 MHz (1 MHz = 1 million cycles per second) at amplitude densities of 0.1 to 3 w/cm2[33] (see Figure 2). The presumed modes of action of ultrasound include causing vasodilatation, increasing cell metabolism, enhancing cell permeability, inducing fibroblast proliferation, releasing vascular endothelial growth factor, increasing collagen’s flexibility, and reducing edema by increasing the interstitial fluid flow. The result of all these effects is to improve wound healing[33].
Contraindications to ultrasound treatment include directing acoustic energy over a malignant lesion, pregnant abdomen, or a plastic or metal implant. Additionally, this therapeutic mode should not be used in patients with decompensated cardiac failure, cardiac pacemaker, acute infection, hemorrhagic diathesis[33]. Ultrasound treatments are categorized by their frequency, intensity, pulsed or continuous administration, and tissue-contact or contact-free administration[34]. When ultrasound is used for debridement it creates cavitation effects in the tissue; at high doses this may result in a level of cavitation that is not stable. Low-frequency contact, or contact-free ultrasound treatment, activates the cell membrane and induction of fibroblast proliferation with DNA synthesis, elicitation of vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) in osteoblasts[35]. When ultrasound therapy is used in musculoskeletal diseases at low frequencies (such as 20-40 kHz) its thermal effect is reduced and the debridement effect becomes more prominent; concomitant bactericidal and wound healing effects have also been observed[34].
Ennis et al[36] conducted a multicenter, double-blind randomized controlled trial that compared active 40-Khz ultrasound treatment to sham ultrasound treatment in 3 sessions per week for 12 wk in patients with a chronic diabetic foot ulcer. In both groups the rates of wound healing were increased after treatment compared to pre-treatment rates. In neither group was there any statistically significant improvement in wound exudation or wound closure.
A newly discovered ultrasound modality called contact-free low-frequency ultrasound is thought to improve wound healing by inducing fibroblast proliferation and releasing VEGF and IL-8, thereby reducing growth of bacterial in chronic wounds[37]. In a study performed by Yao et al[37], 12 patients were grouped into those who received: (1) contact-free low-frequency ultrasound at 40 kHz three times a week; (2) contact-free low-frequency ultrasound at 40 kHz once a week, both in addition to standard treatment; and (3) only standard treatment for 4 wk. The group receiving contact-free low-frequency ultrasound three times a week had significantly higher rates of reduction in the size of the wound than the two other groups, while there was no significant difference between the second and the third groups. Thus, contact-free low-frequency ultrasound administered three times a week appeared to be most effective in reducing the area of the wound[37].
Voight et al[34] conducted a systematic review and meta-analysis to examine studies of low-frequency (20-30 kHz) ultrasound delivered at either low or high intensity. They found 8 randomized controlled trials, in five of which both groups received standard treatment modalities (debridement, wound healing) but only one group also received active to low-intensity contact-free ultrasound, while the control group received sham ultrasound therapy. In the other three trials either low-frequency or high-intensity contact therapeutic ultrasound treatment was administered. The results of these trials demonstrated that early healing (at ≤ 5 mo) in patients with diabetic foot ulcers was favorably influenced by both high- and low- intensity ultrasound delivered at a low frequency, either via contact or noncontact techniques. The authors concluded that low-frequency, low-intensity, noncontact ultrasound is more effective at producing complete healing than standard wound care[34].
Electrotherapy methods: Electrical stimulation is normally used in physical therapy to strengthen paralytic muscles, but there are also clinical trials that suggest it is beneficial in treating diabetic foot ulcers. Electrical stimulation wound therapy produces short pulse electrical stimuli intended to mimic the body’s natural electrical system and stimulate wound repair[38]. The mechanism of action appears to involve an effect on calcium channels in the cell membrane that increases the intra-cellular calcium permeability, which stimulates the production of nitric oxide (NO) by increasing the nitric oxide synthase (NOS). NO, a strong vasodilator enhances the blood flow, thereby potentially accelerating wound healing. It also can change form to create peroxynitrate which has strong bactericidal effects. In addition, NO enhances the transfer of glucose into cells and increases epithelization and collagen storage[39]. Electrical stimulation may also stimulate the migration of various wound-modifying cells including keratinocytes, fibroblasts, macrophages, and neutrophils via various signaling mechanisms[40]. Electrical stimulation is typically administered at 30-Hz frequency, at a pulse every 250 microseconds, and 20-milliampere current, using 5 cm × 5 cm disposable carbonized electrodes, for 30 min three times weekly[41]. This treatment modality should not be used in patients with a cardiac pacemaker[39].
Decreased local tissue perfusion and the subsequent tissue hypoxia contribute to the occurrence, and failure to heal, of a foot ulcer in many diabetic patients[6]. Some clinical trials suggest that the tissue perfusion in chronic ulcers is increased following electrical stimulation and this is associated with wound healing[42]. One evaluation following electrical stimulation, using laser Doppler flowmetry, demonstrated a significant increase in tissue perfusion achieved in patients with diabetes and peripheral vascular disease[43]. In another trial, the transcutaneous oxygen pressure levels were significantly increased within the first 5 min following electrical stimulation in diabetic patients with peripheral vascular disease[44].
Studies in the literature used different protocols of electrical stimulation for diabetic foot ulcers since. In two, treatment with electrical stimulation was compared to sham treatment[45,46] while in another two studies electrical stimulation was compared to infrared heat lamp[32,41]. Another study compared electrical stimulation to two different control groups, one receiving electrical current at a very low dose (4-mA intensity) and the other not receiving electrical stimulation[47]. While all these studies investigated the effect of electrical stimulation the authors did not provide any data on calculation of the sample size and streaming[41,45,46] and the electrical current was administered as symmetrical biphasic, monophasic or square wave current. In all 5 trials the rates of healing, measured by the diameter of the wound, were the main result; in 4 the rates of healing were significantly higher in the study group compared to the control group[32,41,45-47]. Another randomized study by Peters et al[46] enrolled a total of 40 patients with diabetic foot ulcer. Twenty patients were randomized into each of the control group and the study group. The study group received electrical current via a micro-computer each night for 8 h, while the control group received no current. The patients were followed up for 12 wk, or until at least one patient achieved healing, and the healing rates were not significantly different for the two groups.
Extracorporeal shock wave therapy (ESWT) is a non-invasive method of treating certain soft tissue injuries. It focuses strong sound waves on affected site using an ellipsoid-shaped steel probe. This device can administer an amount of energy 10-fold higher than delivered by ultrasonic devices within 1 microsecond. While ESWT is non-invasive, it delivers high-amplitude, short, single, pulsatile, acoustic waves that distribute their mechanical energy into the environment while passing from the soft tissue to the bone. If the delivery is < 0.1 mJ/mm2 it is classified as low-energy, if 0.1-0.2 mJ/mm2 it is middle-energy and if > 0.2 mJ/mm2 it is high-energy. Recommendations for the treatment of diabetic foot ulcer are generally administration at an energy level of 0.03 mjoul/mm2 twice weekly for a total of 6 applications, to achieve 100 pulses/cm2. The treatment may take up to 30 min per foot[48].
ESWT has been extensively studied over the past 20 years and been found to be effective for the treatment of various musculoskeletal diseases (plantar fasciitis, calcific tendonitis of the shoulder, tennis elbow, pseudo-arthrosis and patellar tendinitis). The mechanism of action involves the stimulation of tissue healing, reduction of calcifications and inhibition of the pain receptors[49]. It appears to induce the early expression of angiogenesis-associated growth factors, endothelial NO synthetase and vascular endothelial growth factors, thus increasing cell proliferation and accelerating tissue regeneration and healing[50]. Our literature search identified two clinical trials investigating ESWT for foot ulcers. One enrolled 30 patients with diabetic foot ulcer, all of whom received standard treatment (wound debridement, infection treatment, adequate pressure transfer), but the study group also received ESWT. They found significantly better results in complete wound closure, healing time, and re-epithelization indices following a 20 wk of treatment in the ESWT group[48]. In another trial, 32 wound in 30 patients who were unresponsive to conservative or advanced dressing treatment received ESWT, while control group of 10 patients received standard treatment (wound debridement, infection treatment and adequate pressure transfer). Complete wound closure was achieved after 6 sessions of ESWT in 16 patients the study group. In wounds with incomplete healing exudate was reduced, granulation tissue increased and the size of the wound decreased significantly after 4-6 sessions of ESWT. The study group also had a significant reduction in pain and a significant increase in wound healing compared to the control group[51]. These limited results suggest that ESWT may be beneficial and safe in the treatment of diabetic foot ulcers. This modality is contraindicated in patients with previous cardiac bypass operation, active pregnancy, major cancer or coagulation disorder.
There are three types of laser treatment, based on their potency (see Table 3)[52]. At physical or occupational therapy units low level lasers (typically between 5 mW to 500 mW of output power) are typically used in treatment. Low-level laser light has been reported to be effective in the treatment of impaired microcirculation (thought to be a relatively common problem in patients with a diabetic foot ulcer), wound healing and pain syndromes. The mechanism of action is thought to involve enhancement of the blood circulation and stimulation of the neo-angiogenesis through an increase in skin heat[52].
| Low-level lasers (cold laser) | Helium and neon |
| Moderate-level lasers | Gallium, aluminum-arsenide |
| Potent lasers | Argon-CO-Yttrium aluminum oxide |
In a double-blind, placebo-controlled clinical trial in patients with diabetic microangiopathy, 15 received low-level laser therapy at a dose of 30 J/cm2, while 15 patients received a sham beam[53]. The results revealed a significant increase in the skin heat 15 min, following laser treatment compared to the control group. Presumably this was related to the increased skin blood circulation and contributed to improved healing[53]. In another randomized controlled trial of patients with a diabetic foot ulcer, 13 received low-level laser therapy and 10 patients were in a placebo group[54]. Wound size was significantly reduced at week 4 in the low-level laser therapy compared with the placebo group, and at week 20 eight patients in the treatment group and only three in the placebo group achieved complete wound healing. The mean duration until complete wound healing was not statistically significant: week 11 in the treatment group compared to week 14 in the control group[54]. These limited data suggest there may be a role for low-level laser therapy in accelerating healing of diabetic foot ulcers wound.
Magnetic field treatment use magnets or magnetism. Magnetotherapy is believed to have positive impact on immunological condition of the patient and causes dilation of blood vessels secondary to changes in autonomic nervous system, thereby removing toxins causing pain. Additionally, it is may increase the permeability of neuronal membranes and promoting release of hormones with analgesic action such as endorphin[55].
For the treatment of a diabetic foot ulcer, magnetic field treatment is generally administered for one hour daily for 10 d, at low intensity (30 Gauss). Using this magnetic field device, the foot is placed in a solenoid coil. During the procedure magnetic field depth of penetration is 20 mm[56].
Another trial investigating the efficacy of magnetic field treatment randomized 375 diabetic patients with symptomatic polyneuropathy to either wearing 450 G magnetic insoles (study group) or non-magnetic insoles (control group) for 4 mo. The study group had a greater reduction than the control group in pain, numbness and burning and this improvement was maintained for 4 mo[56].
The limited published data suggest that magnetic field treatment may be effective modality in reducing symptoms of diabetic polyneuropathy. This physical therapy modality is contraindicated in patients with pregnancy, implanted inner ear haring device and other small metallic implants[55].
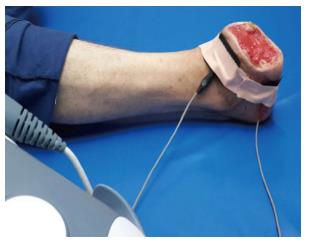
Galvanic (direct) current treatment uses zero-frequency electrical current. The current flows in one direction and has polarity current. Through stimulation of the myelin-free pain fibers, this current achieves paresthesia of both the superficial and the deep skin layers[57]. It is administered by carbon silicon surface electrodes with a current intensity of 1 Ma for duration of 20 min[57] in the treatment of a diabetic foot ulcer (Figure 3).
To our knowledge, there is only one published study in the literature that investigated the efficacy of treatment of diabetic ulcer wounds with galvanic stimulation. In this study 11 diabetic patients with impaired peripheral perfusion pressure (transcutaneous oxygen tension of < 40 mmHg) received galvanic stimulation treatment for 60 min on each of 2 d. Measurements were then performed of transcutaneous oxygen in the dorsum of both feet via and of skin blood flow via laser Doppler flowmetry. The authors reported a significant increase in perfusion in the patients who received galvanic stimulation within the first 5 min, compared to the control group[44]. This modality is contraindicated in pregnant women and in patients with cardiac pacemaker[57].
Exercise treatment: Recent studies suggested that exercise, may be an effective therapeutic modality for patients with a diabetic foot ulcer. Range of joint motion, stretching exercises, Buerger-Allen exercise, and proprioception and balance exercises may be helpful in patients with, or predisposition to, a diabetic foot ulcer[58-60]. Exercises involving range of joint motion or stretching in all directions may increase the blood flow to the feet. Proprioception exercises increase the sensory input in patients with diabetic polyneuropathy, thereby enhancing their perception capacity and ability to protect the extremity. These exercises may lead to a reduced risk of falling related to improved balance and coordination. In addition, as well as these exercises, balance and coordination exercises may lead to a reduced risk of falling related to improved.
Using the Buerger-Allen exercises may enhance the blood supply to the extremity, potentially leading to formation of new vascular structures. While performing this exercise the patient should lie in supine position for 3 min, lifting his/her feet to a higher ground. Then, he/she should sit and keep both feet in the following positions for three minutes each: flexion, extension, pronation and supination. The feet should turn pink (related to improved blood flow) upon practicing these movements; if they become blue or painful, the patient should lift his/her feet to a higher ground again and rest, as needed. At the end of the exercise, the patient should lie in a supine position for 5 min, keeping the feet warm by wrapping them up with a blanket.
In a study by Goldsmith et al[60] investigating the efficacy of range-of-motion exercises in diabetic foot patients, they asked the patients to draw letters of the alphabet with their feet and also practiced passive and active dorsiflexion of the ankles and metatarsophalangeal joints, plantar flexion, active subtalar joint pronation and supination, stretching of the gastrocnemius and soleus muscles. The authors reported that these exercises resulted in a reduction in the joint limitation and foot plantar pressure during walking[60]. In patients with a diabetic foot ulcer, Flahr et al[59] assessed the effects of 10-repetition foot exercises, consisting of the active inversion, eversion, dorsiflexion and plantar flexion of the feet and ankles, practiced twice daily. In this prospective, quasi-experimental pilot study, following a 12-wk exercise program the patients practicing the exercise were reported to have faster ulcer healing compared to those who did not participate in the exercises. The mechanism of action of these foot exercise is believed to involve increase of the blood supply to the region, the resulting in improved wound healing[59].
Shoe modification: Wearing inappropriate (i.e., poorly fitting) shoes may lead to formation of callus, redness, blisters and eventually various deformities. As diabetic patients, especially those with peripheral neuropathy or foot deformity, are especially prone to these problems they may need appropriate shoe modifications to avoid foot ulcers. Such patients should use specially manufactured shoes with large and high finger toe box and rocker bars, made from soft and flexible leather. Shoes with rocker bars reduce the ground reaction force and facilitate the push-off phase of walking. Rigid insoles should be avoided[61] Using plastazote insoles may help ensure a homogenous distribution of the load. The heels of the shoe should be supported with a soft pad and made from at least two materials of different densities, with a robust edge and a capacity to absorb light shocks. The soles should be renewed every 6 to 12 mo.
Prophylactic methods for preventing foot ulcers development: After a foot ulcer, the rate of recurrent ulceration has been reported to be 28% in the first year, reaching up to 100% at 40 mo in diabetic patients ulcer is the precursor in more than 85% of foot amputations[61]. Thus, it is crucial to apply prophylactic methods to these high risk patients. Every professional who treats patients with diabetes must receive continuing professional education and clinicians should provide regular foot examination for diabetic patients. Patients should also be informed about their disease, the potential related foot complications, and the importance of foot inspection and care and glycemic control. In addition to regular professional foot examinations patients should be instructed to check their feet every day (including with a mirror to see the bottoms) for the presence of skin breaks, redness, swelling, callus or other problems. The patient should also be instructed to wash the feet with warm water (checking the temperature with the hand, not the foot) and to dry them with a soft towel every day, then apply an appropriate moisturizer. The nails should be cut carefully with a good quality nipper, with a straight border; this may need to be done by a medical professional and patients should avoid pedicures. Many patients view their own homes as “safe”, but they should be advised that there, and elsewhere, they should avoid walking on bare foot, or using flimsy or poorly fitting sandals or slippers. It is best to alternate footwear during the week to avoid excess pressure in specific areas and to allow the perspiration in the shoes and insoles to evaporate. The patients socks should be made from cotton and be seam-free. They also should be warned against smoking to avoid worsening blood circulation[62].
In summary, the treatment of diabetic foot wounds requires a multidisciplinary approach. It may include physical therapy and rehabilitation methods. Unfortunately, studies on this topic were conducted with moderate quality of evidence. It is required to be supported by larger randomized trials.
P- Reviewer: Pedro XE, Puntel RL, Siu PM, Sugawara I S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
| 1. | International Diabetes Federation. Diabetes Atlas. 6th ed. Brussels, Belgium: International Diabetes Federation 2014; Available from: http: //www.idf.org/sites/default/files/Atlas-poster-2014_EN.pdf. [Cited in This Article: ] |
| 2. | Fard AS, Esmaelzadeh M, Larijani B. Assessment and treatment of diabetic foot ulcer. Int J Clin Pract. 2007;61:1931-1938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 75] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 3. | Cavanagh PR, Lipsky BA, Bradbury AW, Botek G. Treatment for diabetic foot ulcers. Lancet. 2005;366:1725-1735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 297] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 4. | Lipsky BA, Berendt AR, Embil J, De Lalla F. Diagnosing and treating diabetic foot infections. Diabetes Metab Res Rev. 2004;20 Suppl 1:S56-S64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1841] [Cited by in F6Publishing: 1670] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 6. | Armstrong DG, Cohen K, Courric S, Bharara M, Marston W. Diabetic foot ulcers and vascular insufficiency: our population has changed, but our methods have not. J Diabetes Sci Technol. 2011;5:1591-1595. [PubMed] [Cited in This Article: ] |
| 7. | Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, Deery HG, Embil JM, Joseph WS, Karchmer AW. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1015] [Cited by in F6Publishing: 1051] [Article Influence: 87.6] [Reference Citation Analysis (0)] |
| 8. | Most RS, Sinnock P. The epidemiology of lower extremity amputations in diabetic individuals. Diabetes Care. 1983;6:87-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 400] [Cited by in F6Publishing: 338] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Humphrey LL, Palumbo PJ, Butters MA, Hallett JW, Chu CP, O’Fallon WM, Ballard DJ. The contribution of non-insulin-dependent diabetes to lower-extremity amputation in the community. Arch Intern Med. 1994;154:885-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, LeFrock JL, Lew DP, Mader JT, Norden C. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004;39:885-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 11. | Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13:513-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1015] [Cited by in F6Publishing: 903] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 12. | Reiber GE, Vileikyte L, Boyko EJ, del Aguila M, Smith DG, Lavery LA, Boulton AJ. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care. 1999;22:157-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 700] [Cited by in F6Publishing: 607] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 13. | Trautner C, Haastert B, Giani G, Berger M. Incidence of lower limb amputations and diabetes. Diabetes Care. 1996;19:1006-1009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 113] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Frykberg RG, Lavery LA, Pham H, Harvey C, Harkless L, Veves A. Role of neuropathy and high foot pressures in diabetic foot ulceration. Diabetes Care. 1998;21:1714-1719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 232] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | Kumar S, Ashe HA, Parnell LN, Fernando DJ, Tsigos C, Young RJ, Ward JD, Boulton AJ. The prevalence of foot ulceration and its correlates in type 2 diabetic patients: a population-based study. Diabet Med. 1994;11:480-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 246] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg. 2010;52:17S-22S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 17. | Leone S, Pascale R, Vitale M, Esposito S. [Epidemiology of diabetic foot]. Infez Med. 2012;20 Suppl 1:8-13. [PubMed] [Cited in This Article: ] |
| 18. | Ozkan Y, Colak R, Demirdag K, Yildirim AM, Ozalp G, Koca SS. Retrospective evulation of 142 case with diabetic foot syndrome. Turkiye Klinikleri J Endocrin. 2004;2:191-195. [Cited in This Article: ] |
| 19. | Ertugrul MB. Diabetic foot infections. Türkiye Klinikleri J Gen Surg-Special Topics. 2010;3:46-56. [Cited in This Article: ] |
| 20. | Ertugrul MB, Baktiroglu S, Aksoy M, Calangu S. Diabetic foot infection. J Klimik. 2004;17:3-12. [Cited in This Article: ] |
| 21. | Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care. 2006;29:1288-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 467] [Cited by in F6Publishing: 416] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 22. | Lavery LA, Peters EJ, Williams JR, Murdoch DP, Hudson A, Lavery DC. Reevaluating the way we classify the diabetic foot: restructuring the diabetic foot risk classification system of the International Working Group on the Diabetic Foot. Diabetes Care. 2008;31:154-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Crisp AJ, Heathcote JG. Connective tissue abnormalities in diabetes mellitus. J R Coll Physicians Lond. 1984;18:132-141. [PubMed] [Cited in This Article: ] |
| 24. | Lavery LA, Armstrong DG, Harkless LB. Classification of diabetic foot wounds. J Foot Ankle Surg. 1996;35:528-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 287] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 25. | Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metab Res Rev. 2004;20 Suppl 1:S90-S95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 323] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 26. | Wagner FW. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle. 1981;2:64-122. [PubMed] [Cited in This Article: ] |
| 27. | Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Harkless LB, Boulton AJ. A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems. Diabetes Care. 2001;24:84-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 323] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 28. | Kravitz SR, McGuire J, Shanahan SD. Physical assessment of the diabetic foot. Adv Skin Wound Care. 2003;16:68-75; quiz A022-3. [PubMed] [Cited in This Article: ] |
| 29. | Caputo GM, Cavanagh PR, Ulbrecht JS, Gibbons GW, Karchmer AW. Assessment and management of foot disease in patients with diabetes. N Engl J Med. 1994;331:854-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 313] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 30. | Pliskin MA, Todd WF, Edelson GW. Presentations of diabetic feet. Arch Fam Med. 1994;3:273-279. [PubMed] [Cited in This Article: ] |
| 31. | Aksit R. Warm and cold in treatment. Medical Rehabilitation. İstanbul: Nobel Tıp Kitapevi 1995; 179-198. [Cited in This Article: ] |
| 32. | Petrofsky JS, Lawson D, Suh HJ, Rossi C, Zapata K, Broadwell E, Littleton L. The influence of local versus global heat on the healing of chronic wounds in patients with diabetes. Diabetes Technol Ther. 2007;9:535-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Karamehmetoglu S. Deep heaters. Physical Therapy Methods in the Movement System Diseases. İstanbul: Nobel Tıp Kitapevi 2002; 51-60. [Cited in This Article: ] |
| 34. | Voigt J, Wendelken M, Driver V, Alvarez OM. Low-frequency ultrasound (20-40 kHz) as an adjunctive therapy for chronic wound healing: a systematic review of the literature and meta-analysis of eight randomized controlled trials. Int J Low Extrem Wounds. 2011;10:190-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Dinno MA, Dyson M, Young SR, Mortimer AJ, Hart J, Crum LA. The significance of membrane changes in the safe and effective use of therapeutic and diagnostic ultrasound. Phys Med Biol. 1989;34:1543-1552. [PubMed] [Cited in This Article: ] |
| 36. | Ennis WJ, Foremann P, Mozen N, Massey J, Conner-Kerr T, Meneses P. Ultrasound therapy for recalcitrant diabetic foot ulcers: results of a randomized, double-blind, controlled, multicenter study. Ostomy Wound Manage. 2005;51:24-39. [PubMed] [Cited in This Article: ] |
| 37. | Yao M, Hasturk H, Kantarci A, Gu G, Garcia-Lavin S, Fabbi M, Park N, Hayashi H, Attala K, French MA. A pilot study evaluating non-contact low-frequency ultrasound and underlying molecular mechanism on diabetic foot ulcers. Int Wound J. 2014;11:586-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Braun LR, Fisk WA, Lev-Tov H, Kirsner RS, Isseroff RR. Diabetic foot ulcer: an evidence-based treatment update. Am J Clin Dermatol. 2014;15:267-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Koyuncu H. Low-frequency ultrasound. Physical Therapy Methods in the Movement System Diseases. İstanbul: Nobel Tıp Kitapevi 2002; 27-37. [Cited in This Article: ] |
| 40. | Thakral G, Lafontaine J, Najafi B, Talal TK, Kim P, Lavery LA. Electrical stimulation to accelerate wound healing. Diabet Foot Ankle. 2013;4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 41. | Petrofsky JS, Lawson D, Berk L, Suh H. Enhanced healing of diabetic foot ulcers using local heat and electrical stimulation for 30 min three times per week. J Diabetes. 2010;2:41-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Feedar JA, Kloth LC, Gentzkow GD. Chronic dermal ulcer healing enhanced with monophasic pulsed electrical stimulation. Phys Ther. 1991;71:639-649. [PubMed] [Cited in This Article: ] |
| 43. | Forst T, Pfützner A, Bauersachs R, Arin M, Bach B, Biehlmaier H, Küstner E, Beyer J. Comparison of the microvascular response to transcutaneous electrical nerve stimulation and postocclusive ischemia in the diabetic foot. J Diabetes Complications. 1997;11:291-297. [PubMed] [DOI] [Cited in This Article: ] |
| 44. | Peters EJ, Armstrong DG, Wunderlich RP, Bosma J, Stacpoole-Shea S, Lavery LA. The benefit of electrical stimulation to enhance perfusion in persons with diabetes mellitus. J Foot Ankle Surg. 1998;37:396-400; discussion 447-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 45. | Lundeberg TC, Eriksson SV, Malm M. Electrical nerve stimulation improves healing of diabetic ulcers. Ann Plast Surg. 1992;29:328-331. [PubMed] [Cited in This Article: ] |
| 46. | Peters EJ, Lavery LA, Armstrong DG, Fleischli JG. Electric stimulation as an adjunct to heal diabetic foot ulcers: a randomized clinical trial. Arch Phys Med Rehabil. 2001;82:721-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Baker LL, Chambers R, DeMuth SK, Villar F. Effects of electrical stimulation on wound healing in patients with diabetic ulcers. Diabetes Care. 1997;20:405-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 133] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 48. | Moretti B, Notarnicola A, Maggio G, Moretti L, Pascone M, Tafuri S, Patella V. The management of neuropathic ulcers of the foot in diabetes by shock wave therapy. BMC Musculoskelet Disord. 2009;10:54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Rompe JD, Hopf C, Nafe B, Burger R. Low-energy extracorporeal shock wave therapy for painful heel: a prospective controlled single-blind study. Arch Orthop Trauma Surg. 1996;115:75-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 143] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 50. | Wang CJ. An overview of shock wave therapy in musculoskeletal disorders. Chang Gung Med J. 2003;26:220-232. [PubMed] [Cited in This Article: ] |
| 51. | Saggini R, Figus A, Troccola A, Cocco V, Saggini A, Scuderi N. Extracorporeal shock wave therapy for management of chronic ulcers in the lower extremities. Ultrasound Med Biol. 2008;34:1261-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Akgun K. Laser. Physical Therapy Methods in the Movement System Diseases. İstanbul: Nobel Tıp Kitapevi 2002; 73-81. [Cited in This Article: ] |
| 53. | Schindl A, Heinze G, Schindl M, Pernerstorfer-Schön H, Schindl L. Systemic effects of low-intensity laser irradiation on skin microcirculation in patients with diabetic microangiopathy. Microvasc Res. 2002;64:240-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 54. | Kaviani A, Djavid GE, Ataie-Fashtami L, Fateh M, Ghodsi M, Salami M, Zand N, Kashef N, Larijani B. A randomized clinical trial on the effect of low-level laser therapy on chronic diabetic foot wound healing: a preliminary report. Photomed Laser Surg. 2011;29:109-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 55. | Akgun K. Magnetic field therapy. Physical Therapy Methods in the Movement System Diseases. İstanbul: Nobel Tıp Kitapevi 2002; 65-73. [Cited in This Article: ] |
| 56. | Weintraub MI, Wolfe GI, Barohn RA, Cole SP, Parry GJ, Hayat G, Cohen JA, Page JC, Bromberg MB, Schwartz SL. Static magnetic field therapy for symptomatic diabetic neuropathy: a randomized, double-blind, placebo-controlled trial. Arch Phys Med Rehabil. 2003;84:736-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 57. | Eryavuz M. Direct current (Galvanic current). Physical Therapy Methods in the Movement System Diseases. İstanbul: Nobel Tıp Kitapevi 2002; 19-27. [Cited in This Article: ] |
| 58. | Abbott CA, Garrow AP, Carrington AL, Morris J, Van Ross ER, Boulton AJ. Foot ulcer risk is lower in South-Asian and african-Caribbean compared with European diabetic patients in the U.K.: the North-West diabetes foot care study. Diabetes Care. 2005;28:1869-1875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 59. | Flahr D. The effect of nonweight-bearing exercise and protocol adherence on diabetic foot ulcer healing: a pilot study. Ostomy Wound Manage. 2010;56:40-50. [PubMed] [Cited in This Article: ] |
| 60. | Goldsmith JR, Lidtke RH, Shott S. The effects of range-of-motion therapy on the plantar pressures of patients with diabetes mellitus. J Am Podiatr Med Assoc. 2002;92:483-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | Chantelau E, Kushner T, Spraul M. How effective is cushioned therapeutic footwear in protecting diabetic feet? A clinical study. Diabet Med. 1990;7:355-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 75] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Mantey I, Foster AV, Spencer S, Edmonds ME. Why do foot ulcers recur in diabetic patients? Diabet Med. 1999;16:245-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |