Peer-review started: August 30, 2018
First decision: October 22, 2018
Revised: November 10, 2018
Accepted: November 29, 2018
Article in press: November 29, 2018
Published online: December 21, 2018
The immune system plays a pivotal role in defending our body from invading pathogens and in surveillance against cancer. While most cells that acquire mutations are detected and destroyed by immunocytes, a small number of transformed cells succeed in evading immune destruction by inhibiting immune checkpoint regulatory pathways, leading to suppression of anti-cancer immune responses. Under normal conditions, immune checkpoint receptors maintain self-tolerance, prevent immunopathology, and regulate overall immune homeostasis. However, their skewed activation by cancer cells may lead to the suppression of nascent anti-tumor immunity and the promotion of tumor growth. Discovering the role of immune checkpoints in cancer and understanding their mode of operation has led to the development of novel strategies for cancer immunotherapy, which are based on the intervention or blockade of immune checkpoint-regulated pathways. Clinical studies have demonstrated that immune checkpoint co-inhibitory receptor-blocking antibodies can revert tumor-induced immunosuppression and augment overall anti-tumor immunity. These antibodies induced durable clinical responses and unprecedented therapeutic benefits in multiple types of malignancies. Although immune checkpoint inhibitors have revolutionized cancer therapy, the clinical benefits of these drugs have been limited to subsets of cancer patients and treatments frequently associated with a unique spectrum of toxicities, termed immune-related adverse events. Future discoveries of novel immune checkpoint receptors, identification of new prognostic and predictive biomarkers, and improvement of combination therapies are likely to boost the success rate of cancer immunotherapy and increase the survival rates of patients with different types of cancers.
Core tip: The major functions of immune checkpoint receptors are to maintain self-tolerance, prevent immunopathology, and regulate overall immune homeostasis. However, skewed activation of these receptors by cancer cells may lead to suppression of nascent anti-tumor immunity and promote tumor cell growth. Clinical studies have demonstrated that blocking inhibitory immune checkpoint receptors induced durable clinical responses and unprecedented therapeutic benefits in multiple types of malignancies. The present editorial addresses some of the major immune checkpoint receptor targets in cancer immunotherapy, discusses some of the side effects and limitations in their utilization, and highlights some of the future challenges in the field.
- Citation: Isakov N. Cancer immunotherapy by targeting immune checkpoint receptors. World J Immunol 2018; 8(1): 1-11
- URL: https://www.wjgnet.com/2219-2824/full/v8/i1/1.htm
- DOI: https://dx.doi.org/10.5411/wji.v8.i1.1
T lymphocytes include several functionally distinct cell populations that are essential for combating virally-infected and neoplastic cells. A large fraction of the T cells in healthy individuals are naive resting cells that require antigen priming to acquire effector functions. The priming process involves the engagement of multiple T cell surface receptors that activate signaling pathways, leading to T cell proliferation, differentiation and acquisition of effector functions. A signal from the T cell antigen receptor (TCR) is sine qua non for the activation process, as it provides the very early activation step and ensures that only T cell clones specific to the appropriate antigen are expanded. The TCR interacts with a peptide antigen-loaded major histocompatibility complex (MHC) molecule on the surface of antigen presenting cells (APCs). T cell activation and responsiveness are carefully regulated by a number of accessory co-stimulatory and co-inhibitory cell surface proteins, termed immune checkpoint receptors, that can be triggered by cognate ligands on the surface of APC or target cells. The balance between signals provided by the TCR and the immune checkpoint receptors determines the cell’s ability to respond and differentiate into a fully active immunocyte.
The immune checkpoint receptors are implicated in immune response initiation and the regulation of their intensity and duration. Two of the best studied T cell-specific co-inhibitory receptors are the cytotoxic T lymphocyte associated antigen-4 (CTLA-4) and programed cell death-1 (PD-1). The main function of these co-inhibitory receptors is to maintain self-tolerance and prevent autoimmunity. Furthermore, they participate in the termination of T cell-mediated immune responses and limit collateral tissue injury during anti-microbial immune responses.
Recent studies demonstrated that different types of cancers utilize immune checkpoints to their own benefit. By activating co-inhibitory receptors, these tumors can suppress T cell-mediated functions and promote their own escape from immune destruction. The appreciation of the regulatory role and the mode of operation of immune checkpoints in cancer diseases have led to the development of new strategies for cancer immunotherapy based on immune checkpoint blockade. Multiple clinical studies have demonstrated that immune checkpoint blocking antibodies can be highly efficient in inducing durable clinical responses in different types of malignancies.
Nevertheless, the clinical benefits of these antibodies have been limited to subsets of cancer patients and treatments that are frequently associated with a unique spectrum of toxicities, termed immune-related adverse events.
CTLA-4 (CD152) is the prototypic immune checkpoint receptor and the most studied in the context of cancer immunotherapy. It is a disulfide-linked homodimeric transmembrane glycoprotein expressed exclusively on T cells, and it participates in the repression of T cell proliferation, cell cycle progression and cytokine production[1,2]. The CTLA-4 protein is highly homologous to the co-stimulatory molecule CD28, and both receptors utilize the same ligands, CD80 (B7.1) and CD86 (B7.2), which are expressed on the surface of APCs[3-5].
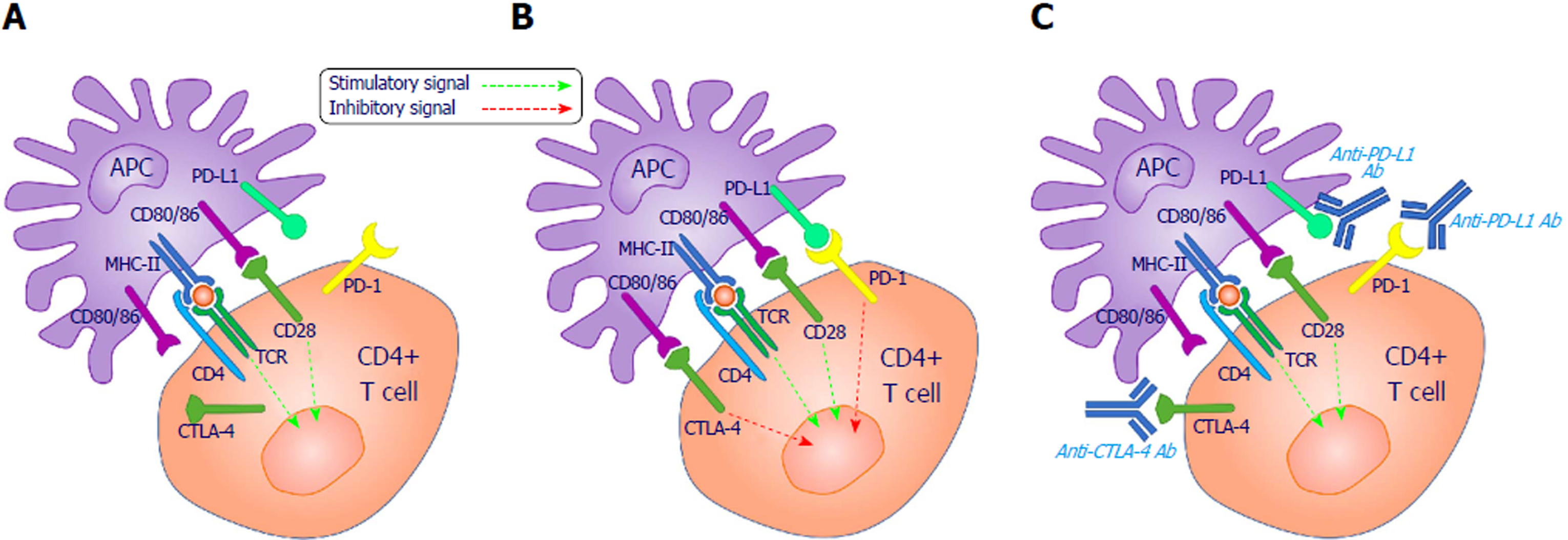
CD28 co-stimulation is necessary for maximal T cell activation. It promotes T cell expansion via interleukin-2 (IL-2)-dependent and independent mechanisms[6], but requires a primary signal (signal 1) in the T cell, which is generated by TCR engagement with MHC-bound cognate antigens on APCs (Figure 1).
In contrast to CD28, which is constitutively expressed on both resting and activated T cells, CTL4 is only marginally expressed on the outer surface of resting T cells and localizes predominantly in intracellular stores. In response to antigen stimulation, CTLA-4 is temporarily transported to the outer cell membrane of the memory and regulatory T cells[7]. At this stage, CTLA-4 can out-compete CD28 for ligand binding and induce T cell suppression via several independent mechanisms[8-10]. One mechanism relates to the fact that CTLA-4 has a higher binding affinity for both ligands. Its transient expression on the surface of T cells prevents CD28 from interacting with CD80/CD86 and delivering co-stimulatory signals[11]. Another mechanism reflects the ability of CTLA-4 to directly deliver inhibitory signals to effector T cells through its cytoplasmic tail, which associates with signaling proteins[12]. Furthermore, CTLA-4 can suppress T cell responses by upregulating the activity of Treg cells. The engagement of CTLA-4 on Tregs delivers activating signals that enhance their suppressive activity and inhibit antitumor immunity[8,13].
The role of CTLA-4 in maintaining T-cell activation under control is well demonstrated in studies of CTLA-4-deficient mice generated by homologous recombination. These mice suffer from a CD4+ T cell-mediated lymphoproliferative disease that is driven by excessive CD28 signaling[14-16]. CTLA-4-deficient mice also exhibit impaired differentiation of Treg cells, resulting in massive lymphoproliferation, splenomegaly and lymphadenopathy, including multiorgan lymphocyte infiltration and tissue destruction[17]. These results substantiate the inhibitory role of CTLA-4 in the regulation of both Th and Treg development and function and in immune homeostasis.
Studies on the biological role of CTLA-4 and its involvement in the suppression of T cell functions laid the groundwork for the development of new strategies for cancer immunotherapy, which are based on CTLA-4 blockade[13].
The most studied CTLA-4 blocking antibody that was used in clinical trials is Ipilimumab (Yervoy®, Bristol-Myers Squibb), which showed significant survival benefit for patients with advanced metastatic melanoma. In early 2011, Ipilimumab was the first anti-immune checkpoint antibody to be approved by the United States Food and Drug Administration (commonly known as the FDA) for the treatment of melanoma[18,19]. Ipilimumab enhances antitumor immunity by blocking the negative effects of CTLA-4 and augmenting effector cytotoxic T cell functions.
Clinical trials with a second anti-CTLA-4 antibody, tremelimumab (ticilimumab, CP-675,20, Pfizer and AstraZeneca), demonstrated its effectiveness in patients with melanoma[20], refractory metastatic colorectal cancer[21], and hepatocellular carcinoma[22].
Additional clinical trials using a variety of CTLA-4 blocking drugs, either alone or in combination therapy, are being conducted on patients with a wide range of tumors. Combination of anti-CTLA-4 antibodies with immunotherapy, chemotherapy, or radiotherapy was found to improve long-term survival of cancer patients suffering from different types of malignancies[23-26].
The PD-1 immune checkpoint receptor is expressed on activated T cells and helps to preserve self-tolerance and the prevention of autoimmunity. It was initially cloned in 1992 in a search for molecules involved in the negative selection of thymocytes that undergo programed cell death[27]. The role of PD-1 as an immune checkpoint became clear in 2000 upon identification of its physiological ligand, programed cell death ligand-1 (PD-L1), which is expressed on APCs and on some tumor cells[28]. These tumors can co-opt the PD-1/PD-L1 pathway by upregulating PD-L1 expression as a mechanism of immune resistance[29]. Preclinical studies in animal models demonstrated that targeting the PD-1/PD-L1 pathway can serve as another promising immunotherapeutic strategy for augmenting endogenous T cell antitumor immunity[30].
In addition to its fundamental role in maintaining self-tolerance and preventing autoimmunity, the PD-1 (CD279) immune checkpoint receptor is also critical for terminating immune responses. Its absence or down-regulation may cause tolerance breakdown or induction of autoimmune responses. PD-1 is expressed on activated T lymphocytes, and upon engagement with its ligand, it delivers signals that counteract the TCR-induced signals and inhibit IL2 production and T cell proliferation.
PD-1-mediated intervention with TCR-coupled signals involves the inhibition of ZAP70 binding to CD3, which down-regulates ZAP70 phosphorylation and catalytic activity. This occurs concomitantly with a reduction in the phosphorylation and activity of protein kinase C-theta (PKCθ), which attenuates the NF-κB and AP-1 transcription factors[31]. As a result, PD-1 signals can promote apoptosis of antigen-specific effector T-cells and induce opposite effects on Tregs, leading to their exemption from programmed cell death[32,33]. One possible explanation for these contrasting effects might rely on the spatially-regulated distinct functions of PKCθ in these two cell types[34-36]. Thus, PKCθ is recruited to the center of the immunological synapse of activated effector T-cells[34,35], but is sequestered away from the immunological synapse of Tregs, instead concentrating on the opposite pole[36]. Therefore, it is highly probable that PKCθ activation in the temporally-organized distinct subcellular structures will result in PKCθ-mediated phosphorylation of different substrate proteins that regulate distinct biological processes.
Although signaling through PD-1 helps maintain homeostasis in the immune system, PD-1-induced signals in cancer patients may facilitate tumor progression by suppressing effector T cell functions, particularly the induction of effective anti-tumor immunity[37].
The realization that PD-1 blockade might help boost the immune system in cancer patients has led to the development of an array of PD-1 targeting drugs that have already demonstrated remarkable clinical success in experimental models and in cancer patients.
In December 2014, the first monoclonal anti-PD-1 antibody to gain FDA approval was nivolumab (Opdivo®, Bristol-Myers Squibb), which was used in a clinical trial (CA209037) in metastatic melanoma patients that had deteriorated after CTLA-4-blocking antibody treatment. The overall objective response rate of the PD-L1+ melanoma patients was approximately 40%. In 2015, nivolumab received FDA approval for the treatment of patients with metastatic squamous non-small cell lung cancer (commonly known as NSCLC) and metastatic renal cell carcinoma, whereby the treatment extended the overall survival rate of cancer patients in both study groups.
In recent years, nivolumab received additional FDA approval for the treatment of a wide range of cancers.
A combination therapy of nivolumab and ipilimumab, which simultaneously target PD-1 and CTLA-4, was also used in clinical studies and found to improve the outcome of patients with melanoma[38-40] and other types of solid tumors[41-43].
In August 2016, pembrolizumab (Keytruda®, Merck Sharp and Dohme Corp.) was the second anti-PD-1 antibody that received FDA approval for the treatment of metastatic head and neck squamous cell carcinoma patients. Several clinical studies are being conducted with pembrolizumab in patients with head and neck cancer (NCT02454179, NCT02707588), in addition to multiple clinical studies that evaluate the efficacy of newly designed PD-1-targeting drugs in a wide range of cancers.
PD-L1 [CD274; also known as B7 homolog 1(B7-H1)] is a 40 kDa type I transmembrane protein that serves as a ligand for PD-1[44]. Under normal physiological conditions, PD-L1 plays a vital role in the regulation of Treg functions and suppression of immunity during pregnancy and autoimmune diseases[45,46].
Upregulation of PD-L1 on APCs delivers inhibitory signals to PD-1+ T cells, which keep immune responses at bay by terminating them once antigens have been eliminated.
However, some tumors can disrupt this equilibrium and manipulate the PD-1/PD-L1 checkpoint pathways by expressing PD-L1 and delivering signals that turn off effector T cells[47].
Observations that support this mechanism were made in several studies where high PD-L1 expression on the tumor cells correlated with reduced immune responses, increased tumor aggressiveness and relatively poor survival[48,49].
Checkpoint inhibitors that target PD-L1 can therefore prevent its interaction with PD-1, restore T-cell activation, and amplify antitumor immunity[47,50].
The first FDA-approved anti-PD-L1 antibody atezolizumab (Tecentriq®, Genentech) was authorized in 2016 for the treatment of metastatic non-small cell lung cancer and advanced and metastatic urothelial carcinoma[50,51]. The clinical results indicated that tumors with a high frequency of PD-L1+ tumor infiltrating immune cells demonstrated particularly high response rates.
Two additional anti-PD-L1 antibodies, avelumab (Bavencio®, Merck KGaA, Pfizer and Eli Lilly) and durvalumab (Imfinzi®, AstraZeneca), received FDA approval in 2017 for the treatment of Merkel-cell carcinoma and advanced bladder cancer, respectively. Additional PD-L1 blocking antibodies are currently being evaluated for the treatment of a wide range of cancers in multiple clinical trials[52-54].
Cancer immunotherapies directed against the CTLA-4- and PD-1/PD-L1 co-inhibitory receptors exhibited undisputed efficacy in selected types of cancer diseases, but many patients were nonresponsive to these therapies, and several tumor types are refractory to these therapies. To increase the repertoire of drug targets in different cancers, scientists are searching for additional immune checkpoint receptors and testing their usefulness as drug targets in cancer immunotherapy.
The T cell immunoglobulin and ITIM domain (TIGIT) protein is a novel inhibitory receptor discovered in a genomic search for genes expressed in T cells. Their protein domain structure represents a potential inhibitory receptor[55]. The expression mechanism of TIGIT on the outer surface of T cells is somewhat similar to that of CTLA-4, as it is upregulated by T cell activation and is transient in nature.
Other inhibitory receptors are constitutively expressed on “exhausted” T cells in patients with chronic cancer[56], including lymphocyte activation gene 3 (LAG3; CD223) and T cell immunoglobulin and mucin-containing molecule-3 (TIM-3; CD366).
The newly identified inhibitory receptors are being analyzed for their effectiveness as blocking antibody targets, while other surface proteins, such as CD137, CD27, ICOS, and GITR activating receptors, are being evaluated for their effectiveness as agonistic targets that amplify anti-cancer immune responses[57].
TIGIT (also known as WUCAM, Vstm3, VSIG9) is part of the CD28 family-like receptors that are expressed on T cells and various other hematopoietic cells[55,58,59]. TIGIT agonists include CD155 (poliovirus receptor-PVR) and CD122 (PVRL2, nectin-2), which are expressed by immune and non-immune cells, as well as tumor cells[55]. TIGIT is a co-inhibitory receptor with a role in tolerance induction and autoimmunity. In general, TIGIT-/- mice were found to be more sensitive to immunization with myelin oligodendrocyte glycoprotein peptide than wild-type mice, and they develop more severe experimental autoimmune encephalomyelitis (EAE). In contrast, TIGIT transgenic mice are less sensitive to immunization with myelin oligodendrocyte glycoprotein and develop reduced symptoms of EAE[58,60]. TIGIT-/- mice develop severe autoimmune responses in a model of collagen-induced arthritis and graft-versus-host disease, similar to the effects induced by anti-TIGIT blocking antibodies[58], indicating that TIGIT functions as an immunoreceptor that downregulates T cell-mediated immunity.
The mechanism of action of TIGIT and the membrane receptor CD226 is reminiscent of that of the CTLA-4/CD28 receptor pair, with TIGIT fulfilling the role of the co-inhibitory receptor that counterbalances co-stimulation mediated by CD226. Furthermore, TIGIT/CD226 expression kinetics are similar to those of CTLA-4/CD28, with the co-inhibitory receptor being expressed only following T cell activation, in contrast to the co-stimulatory receptor, which is constitutively expressed on resting T cells[55,58,59].
TIGIT expression on peripheral blood lymphocytes and in lymphoid organs of tumor bearing mice is relatively poor, but it is highly expressed on tumor infiltrating lymphocytes, including Tregs, in both mouse and humans[61,62].
The observations that TIGIT may impose negative effects on anti-tumor responses were made in studies in which tumor growth in TIGIT-deficient mice were retarded compared to tumor growth in wild type mice[61], and that co-blockade of TIGIT and PD-1 had an additive positive effect on lymphoid cell functions in melanoma patients[63]. Further studies revealed that the progression of multiple myeloma in mice and humans was associated with high TIGIT expression in CD8+ T cells that exhibited reduced functions, while targeting of TIGIT with monoclonal antibodies increased the effector function of CD8+ T cells and prolonged the survival of multiple myeloma patients[64].
In another set of studies, TIGIT, but not CTLA-4 or PD-1, was found to be associated with natural killer (NK) cell exhaustion in mice and humans with colon cancer[65]. Blockade of TIGIT prevented NK cell exhaustion and promoted NK cell-dependent and T cell-mediated anti-tumor immunity[65].
The knowledge accumulated thus far on the immune-inhibitory role of TIGIT in tumor bearing mice and human cancer patients suggests that future designed TIGIT-directed blocking strategies could lead to the development of highly effective and improved anti-cancer therapies.
A phase I clinical trial using anti-TIGIT monoclonal antibodies (MTIG7192A) in patients with advanced or metastatic tumors is in progress (NCT02794571).
Another phase I clinical trial (NCT03119428) in patients with locally advanced or metastatic solid tumors investigates the safety and pharmacokinetics of the OMP-313M32 antibody, which blocks TIGIT binding to PVR.
TIM-3 [also known as Hepatitis A virus cellular receptor 2 (HAVCR2)] belongs to the TIM family of cell surface receptor proteins, which consists of eight members (TIM-1-8) in mice and three members (TIM-1, TIM-3, and TIM-4) in humans[66]. The individual TIM proteins differ in molecular structure and expression patterns, as well as in their regulatory functions and impact on T-cell responses[66]. TIM-3 is expressed on T cells and additional hematopoietic cell types[67-69] and utilize the C-type lectin galectin-9 as its ligand.
Ligation of the TIM-3 receptor in vitro initiates signals that suppress T cell responses, and in vivo administration of anti-TIM-3 antibodies increase the severity of clinical symptoms in a mouse model of EAE[70]. Because EAE is a T helper 1 (Th1)-dependent autoimmune disease, it has been suggested that TIM-3 plays a role in the induction of autoimmune diseases by regulating macrophage functions[70].
Additional studies demonstrated that both TIM-3-deficient mice and wild-type mice treated with a TIM-3-Ig fusion protein exhibited defects in the induction of antigen-specific tolerance[71,72]. These studies and others indicated that TIM-3 is an immune checkpoint receptor that functions to specifically limit the duration and magnitude of T cell-mediated immune responses and contributes to the overall maintenance of immune tolerance[71,72].
TIM-3 is expressed on a large fraction of T cells in cancer patients where it is predominantly upregulated in tumor-infiltrating lymphocytes[73-75]. In addition, TIM-3 expression was found on various types of cancer cells where increased expression was frequently associated with disease progression and shorter survival[73-75].
Preclinical studies in cancer patients demonstrated that TIM-3+ T cells exhibited the most suppressed phenotype and were among the most severely exhausted lymphocytes[76,77].
In addition, a large fraction of TIM-3+ T cells in cancer patients co-expressed PD-1, and the dual receptor-expressing T cells exhibited greater immune function impairments compared to T cells that expressed PD-1 alone[76-78].
These investigations and additional studies support the hypothesis that TIM-3- and PD-1-coupled signaling pathways cooperate to induce severe T cell anergy or active T cell suppression in cancer diseases.
In contrast to CTLA-4 and PD-1, TIM-3 does not utilize immunoreceptor tyrosine-based inhibition motifs (commonly known as ITIM) or immunoreceptor tyrosine-based switch motifs (also known as ITSM) to transduce its signals. It is therefore suggested that TIM-3 is unlikely to be functionally redundant with other ITIM/ITSM-containing checkpoint receptors and that targeting of TIM-3 might have additive or synergistic effects with those induced by anti-CTLA-4 or PD-1 antibodies.
In agreement with this hypothesis, targeting the TIM-3 pathway was found to have modest antitumor effects in various experimental models and preclinical cancer studies[79,80]. However, the combination of anti-TIM-3 and anti-PD-1 monoclonal antibodies had a synergistic effect in suppressing tumor growth[73,76,81,82].
Multiple clinical trials (NCT03489343; NCT02817633; NCT03099109; NCT03066648) using a variety of anti-TIM-3 monoclonal antibodies (such as LY3321367, MBG453 and TSR-022) alone or in combination with anti-PD-1 or anti-PD-L1 antibodies are currently in progress in different types of leukemia and solid tumors.
LAG3 is a CD4-related 70 kDa type I transmembrane glycoprotein that is expressed on activated CD4+ and CD8+ T lymphocytes[83]. It binds MHC-II with a high affinity compared to CD4, suggesting that it might function as a CD4 competitor[84] and negatively regulate TCR-induced signals leading to T cell activation[85,86].
A negative regulatory role of LAG3 was demonstrated in human peripheral blood cells, when anti-LAG-3 blocking antibody combined with superantigen stimulation led to the increased proliferation of CD4+ and CD8+ T cells[87]. LAG3 was found to synergize with PD-1 in downregulating T cell functions and promoting immune evasion by cancer cells[88]. Furthermore, dual anti-LAG3/anti-PD-1 antibody treatment cured most mice of established fibrosarcoma tumors that were otherwise resistant to single antibody treatment[88].
Several LAG3-targeted therapies are currently at various stages of preclinical and clinical development. Combination therapies of anti-LAG-3 and anti-PD-1 antibodies showed promising results in melanoma patients who had relapsed or were refractory to anti-PD-1/PD-L1 antibody therapy.
Multiple clinical trials (NCT02658981; NCT03489369; NCT02061761; NCT03250832) using a variety of LAG3 blocking reagents [such as TSR-033, Sym022, and BMS-986016 (relatlimab) monoclonal antibodies or a soluble LAG-3Ig fusion protein (IMP321)] alone or in combination with a variety of other drugs are currently in progress in different types of hematological malignancies and solid tumors.
Although immune checkpoint targeting has shown a great deal of promise in treating a wide range of cancers, drug-induced side-effects termed “immune-related adverse events’’ (irAEs) have been observed in numerous patients due to non-tumor-specific activation of T cells by the immune checkpoint blocking antibodies.
Side effects of immune checkpoint therapy range from mild to severe and may include fatigue, cough, fever, diarrhea, nausea, loss of appetite, skin rash, itching and nerve inflammation. In addition, the irAEs may affect multiple organs and systems, including the gastrointestinal tract, liver, kidney, central nervous system, endocrine glands, and the pulmonary, cardiovascular and hematological systems.
The frequency and severity of irAEs usually correlate with antibody dosage. The median time of onset of irAEs is about ten weeks after the start of treatment, although it varies with respect to the affected tissues and between individual patients and may occur even after cessation of immune checkpoint therapy.
While the occurrence of irAEs normally indicates that the immune checkpoint blockade has activated the patient’s immune system, its correlation with improved antitumor immunity remains controversial, and the general assumption is that irAEs are not required for obtaining an effective anti-tumor response.
Although the majority of irAEs are completely reversible, immunosuppression using glucocorticoids or other drugs is recommended in cases of severe adverse events. This temporary immunosuppression was found to have no influence on the overall survival rate[89].
The discovery of additional biomarkers and the development of new tools to predict the patient’s risk of developing irAEs will facilitate the application of the full therapeutic potential of immune checkpoint-targeting drugs.
Immune checkpoints consist of inhibitory and stimulatory pathways that are essential for maintaining self-tolerance and to assist with the regulation of immune responses.
Co-inhibitory immune checkpoint receptors are often activated in cancer cells and enable tumor progression by dampening antitumor immune responses.
Antibody-mediated cancer immunotherapy based on the blockade of the signaling axis between co-inhibitory receptors and their ligands has shown remarkable clinical success in patients with different types of cancers.
The most effective reagents thus far are antibodies directed against CTLA-4, PD-1 and PD-L1 receptors, while other antibodies that react with receptors, such as TIGIT, TIM-3 and LAG3, are under evaluation.
While immune checkpoint blockade therapy has revolutionized oncology care, only a subset of treated cancer patients shows durable responses.
One reason relates to the inherent limitations of the antibody molecules that have poor tissue and tumor penetrance and harmful Fc-effector functions that deplete immune cells. These limitations can be partially overcome by using genetically-modified smaller sized antibodies (nanobodies; monomeric variable fragments of Camelid heavy-chain antibodies) or antibodies that lack their Fc portion. Another option is using smaller, high affinity non-antibody molecules directed against selected immune checkpoint receptors that function as soluble agonists. Such molecules can be designed to have a higher affinity to immune checkpoint receptors compared to physiological ligands, to have superior tumor penetration due to their smaller size, and to have no effect on Fc-mediated T cell depletion[90].
At present, immune checkpoint therapy is aided only by a limited number of biomarkers that can accurately predict optimal therapy for patients with different types of cancers. However, intense investigations are ongoing to identify novel biomarkers for immune checkpoint therapy that will increase treatment specificity and selectivity, minimize the risk of toxicity, and serve as non-redundant targets that can maximize the potential of combination therapies.
Manuscript source: Invited manuscript
Specialty type: Immunology
Country of origin: Israel
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ferrante A S- Editor: Cui LJ L- Editor: Filipodia E- Editor: Bian YN
| 1. | Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533-2540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 703] [Cited by in F6Publishing: 686] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 2. | Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP. CTLA-4-Mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813-5820. [PubMed] [Cited in This Article: ] |
| 3. | Ono M, Yaguchi H, Ohkura N, Kitabayashi I, Nagamura Y, Nomura T, Miyachi Y, Tsukada T, Sakaguchi S. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446:685-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 490] [Cited by in F6Publishing: 517] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 4. | Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 864] [Cited by in F6Publishing: 882] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 5. | Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065-1068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 923] [Cited by in F6Publishing: 994] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 6. | Appleman LJ, Berezovskaya A, Grass I, Boussiotis VA. CD28 costimulation mediates T cell expansion via IL-2-independent and IL-2-dependent regulation of cell cycle progression. J Immunol. 2000;164:144-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Wang XB, Zheng CY, Giscombe R, Lefvert AK. Regulation of surface and intracellular expression of CTLA-4 on human peripheral T cells. Scand J Immunol. 2001;54:453-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB. Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood. 2012;119:5155-5163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 9. | Carreno BM, Bennett F, Chau TA, Ling V, Luxenberg D, Jussif J, Baroja ML, Madrenas J. CTLA-4 (CD152) can inhibit T cell activation by two different mechanisms depending on its level of cell surface expression. J Immunol. 2000;165:1352-1356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 191] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229:12-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 552] [Cited by in F6Publishing: 612] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 11. | van der Merwe PA, Bodian DL, Daenke S, Linsley P, Davis SJ. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185:393-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 421] [Cited by in F6Publishing: 412] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Baroja ML, Vijayakrishnan L, Bettelli E, Darlington PJ, Chau TA, Ling V, Collins M, Carreno BM, Madrenas J, Kuchroo VK. Inhibition of CTLA-4 function by the regulatory subunit of serine/threonine phosphatase 2A. J Immunol. 2002;168:5070-5078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717-1725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 644] [Cited by in F6Publishing: 682] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 14. | Chambers CA, Sullivan TJ, Allison JP. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity. 1997;7:885-895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 318] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 15. | Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2111] [Cited by in F6Publishing: 2078] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 16. | Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2109] [Cited by in F6Publishing: 2110] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 17. | Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11:852-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 520] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 18. | Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17:6958-6962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 371] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 19. | Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3331] [Cited by in F6Publishing: 3269] [Article Influence: 251.5] [Reference Citation Analysis (0)] |
| 20. | Camacho LH, Antonia S, Sosman J, Kirkwood JM, Gajewski TF, Redman B, Pavlov D, Bulanhagui C, Bozon VA, Gomez-Navarro J. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27:1075-1081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 21. | Chung KY, Gore I, Fong L, Venook A, Beck SB, Dorazio P, Criscitiello PJ, Healey DI, Huang B, Gomez-Navarro J. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2010;28:3485-3490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 219] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 22. | Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 634] [Cited by in F6Publishing: 686] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 23. | Sckisel GD, Mirsoian A, Bouchlaka MN, Tietze JK, Chen M, Blazar BR, Murphy WJ. Late administration of murine CTLA-4 blockade prolongs CD8-mediated anti-tumor effects following stimulatory cancer immunotherapy. Cancer Immunol Immunother. 2015;64:1541-1552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Sacco PC, Maione P, Guida C, Gridelli C. The Combination of New Immunotherapy and Radiotherapy: A N ew Potential Treatment for Locally Advanced Non-Small Cell Lung Cancer. Curr Clin Pharmacol. 2017;12:4-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Hu ZI, Ho AY, McArthur HL. Combined Radiation Therapy and Immune Checkpoint Blockade Therapy for Breast Cancer. Int J Radiat Oncol Biol Phys. 2017;99:153-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Lesterhuis WJ, Salmons J, Nowak AK, Rozali EN, Khong A, Dick IM, Harken JA, Robinson BW, Lake RA. Synergistic effect of CTLA-4 blockade and cancer chemotherapy in the induction of anti-tumor immunity. PLoS One. 2013;8:e61895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 27. | Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1899] [Cited by in F6Publishing: 1974] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 28. | Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027-1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3572] [Cited by in F6Publishing: 3733] [Article Influence: 155.5] [Reference Citation Analysis (0)] |
| 29. | Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2838] [Cited by in F6Publishing: 3288] [Article Influence: 149.5] [Reference Citation Analysis (0)] |
| 30. | Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3785] [Cited by in F6Publishing: 3801] [Article Influence: 237.6] [Reference Citation Analysis (0)] |
| 31. | Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, Qiu Y, Jussif JM, Carter LL, Wood CR. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 497] [Cited by in F6Publishing: 563] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 32. | Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1419] [Cited by in F6Publishing: 1622] [Article Influence: 115.9] [Reference Citation Analysis (0)] |
| 33. | Fife BT, Pauken KE. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann NY Acad Sci. 2011;1217:45-59. [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 240] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 34. | Kong KF, Yokosuka T, Canonigo-Balancio AJ, Isakov N, Saito T, Altman A. A motif in the V3 domain of the kinase PKC-θ determines its localization in the immunological synapse and functions in T cells via association with CD28. Nat Immunol. 2011;12:1105-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 35. | Isakov N, Altman A. Protein kinase C(theta) in T cell activation. Annu Rev Immunol. 2002;20:761-794. [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 284] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 36. | Zanin-Zhorov A, Ding Y, Kumari S, Attur M, Hippen KL, Brown M, Blazar BR, Abramson SB, Lafaille JJ, Dustin ML. Protein kinase C-theta mediates negative feedback on regulatory T cell function. Science. 2010;328:372-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 234] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 37. | Syn NL, Teng MWL, Mok TSK, Soo RA. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18:e731-e741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 470] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 38. | Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, Linette GP, Meyer N, Giguere JK, Agarwala SS. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558-1568. [DOI] [Cited in This Article: ] [Cited by in Crossref: 635] [Cited by in F6Publishing: 687] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 39. | Tsui E, Madu A, Belinsky I, Yannuzzi LA, Freund KB, Modi YS. Combination Ipilimumab and Nivolumab for Metastatic Melanoma Associated With Ciliochoroidal Effusion and Exudative Retinal Detachment. JAMA Ophthalmol. 2017;135:1455-1457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 40. | Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377:1345-1356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2362] [Cited by in F6Publishing: 2482] [Article Influence: 354.6] [Reference Citation Analysis (0)] |
| 41. | Lussier DM, Johnson JL, Hingorani P, Blattman JN. Combination immunotherapy with α-CTLA-4 and α-PD-L1 antibody blockade prevents immune escape and leads to complete control of metastatic osteosarcoma. J Immunother Cancer. 2015;3:21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 42. | Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol. 2018;36:773-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1013] [Cited by in F6Publishing: 1282] [Article Influence: 213.7] [Reference Citation Analysis (0)] |
| 43. | Hammers HJ, Plimack ER, Infante JR, Rini BI, McDermott DF, Lewis LD, Voss MH, Sharma P, Pal SK, Razak ARA. Safety and Efficacy of Nivolumab in Combination With Ipilimumab in Metastatic Renal Cell Carcinoma: The CheckMate 016 Study. J Clin Oncol. 2017;35:3851-3858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 324] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 44. | Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365-1369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1806] [Cited by in F6Publishing: 1867] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 45. | Zhang YH, Tian M, Tang MX, Liu ZZ, Liao AH. Recent Insight into the Role of the PD-1/PD-L1 Pathway in Feto-Maternal Tolerance and Pregnancy. Am J Reprod Immunol. 2015;74:201-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 46. | Jiang L, Tang C, Gong Y, Liu Y, Rao J, Chen S, Qu W, Wu D, Lei L, Chen L. PD-1/PD-L1 regulates Treg differentiation in pregnancy-induced hypertension. Braz J Med Biol Res. 2018;51:e7334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol. 2017;8:561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 860] [Cited by in F6Publishing: 1080] [Article Influence: 154.3] [Reference Citation Analysis (0)] |
| 48. | Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA. 2004;101:17174-17179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 600] [Cited by in F6Publishing: 624] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 49. | Su S, Dong ZY, Xie Z, Yan LX, Li YF, Su J, Liu SY, Yin K, Chen RL, Huang SM. Strong Programmed Death Ligand 1 Expression Predicts Poor Response and De Novo Resistance to EGFR Tyrosine Kinase Inhibitors Among NSCLC Patients With EGFR Mutation. J Thorac Oncol. 2018;13:1668-1675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 50. | Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1718] [Cited by in F6Publishing: 1823] [Article Influence: 202.6] [Reference Citation Analysis (0)] |
| 51. | Sidaway P. Urological cancer: Atezolizumab: an alternative to cisplatin? Nat Rev Clin Oncol. 2017;14:139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | McKay RR, Bossé D, Xie W, Wankowicz SAM, Flaifel A, Brandao R, Lalani AA, Martini DJ, Wei XX, Braun DA. The Clinical Activity of PD-1/PD-L1 Inhibitors in Metastatic Non-Clear Cell Renal Cell Carcinoma. Cancer Immunol Res. 2018;6:758-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 78] [Reference Citation Analysis (0)] |
| 53. | Brower V. Anti-PD-L1 inhibitor durvalumab in bladder cancer. Lancet Oncol. 2016;17:e275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Luo W, Wang Z, Tian P, Li W. Safety and tolerability of PD-1/PD-L1 inhibitors in the treatment of non-small cell lung cancer: a meta-analysis of randomized controlled trials. J Cancer Res Clin Oncol. 2018;144:1851-1859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 55. | Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 760] [Cited by in F6Publishing: 936] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 56. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8693] [Cited by in F6Publishing: 9310] [Article Influence: 775.8] [Reference Citation Analysis (0)] |
| 57. | Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1413] [Cited by in F6Publishing: 1526] [Article Influence: 95.4] [Reference Citation Analysis (0)] |
| 58. | Levin SD, Taft DW, Brandt CS, Bucher C, Howard ED, Chadwick EM, Johnston J, Hammond A, Bontadelli K, Ardourel D. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur J Immunol. 2011;41:902-915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 243] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 59. | Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 axis regulates human T cell function. J Immunol. 2012;188:3869-3875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 319] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 60. | Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, Sharpe AH, Kuchroo VK. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186:1338-1342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 384] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 61. | Kurtulus S, Sakuishi K, Ngiow SF, Joller N, Tan DJ, Teng MW, Smyth MJ, Kuchroo VK, Anderson AC. TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest. 2015;125:4053-4062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 392] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 62. | Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26:923-937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 685] [Cited by in F6Publishing: 751] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 63. | Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, Kirkwood JM, Chen TH, Maurer M, Korman AJ. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J Clin Invest. 2015;125:2046-2058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 541] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 64. | Guillerey C, Harjunpää H, Carrié N, Kassem S, Teo T, Miles K, Krumeich S, Weulersse M, Cuisinier M, Stannard K. TIGIT immune checkpoint blockade restores CD8+ T-cell immunity against multiple myeloma. Blood. 2018;132:1689-1694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 184] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 65. | Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, Wang Z, Wu Q, Peng H, Wei H. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. 2018;19:723-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 491] [Cited by in F6Publishing: 648] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 66. | Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235:172-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 468] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 67. | Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1124] [Cited by in F6Publishing: 1194] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 68. | Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141-1143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 493] [Cited by in F6Publishing: 526] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 69. | Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, Zheng XX, Strom TB, Hafler DA, Kuchroo VK. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol. 2009;39:2492-2501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 70. | Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245-1252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1334] [Cited by in F6Publishing: 1459] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 71. | Sabatos CA, Chakravarti S, Cha E, Schubart A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4:1102-1110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 466] [Cited by in F6Publishing: 491] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 72. | Sánchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 519] [Cited by in F6Publishing: 552] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 73. | Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175-2186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 890] [Cited by in F6Publishing: 974] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 74. | Yang ZZ, Grote DM, Ziesmer SC, Niki T, Hirashima M, Novak AJ, Witzig TE, Ansell SM. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J Clin Invest. 2012;122:1271-1282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 75. | Shayan G, Srivastava R, Li J, Schmitt N, Kane LP, Ferris RL. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology. 2016;6:e1261779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 202] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 76. | Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187-2194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1279] [Cited by in F6Publishing: 1459] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 77. | Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501-4510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 501] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 78. | Arai Y, Saito H, Ikeguchi M. Upregulation of TIM-3 and PD-1 on CD4+ and CD8+ T Cells Associated with Dysfunction of Cell-Mediated Immunity after Colorectal Cancer Operation. Yonago Acta Med. 2012;55:1-9. [PubMed] [Cited in This Article: ] |
| 79. | Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540-3551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 655] [Reference Citation Analysis (0)] |
| 80. | Kikushige Y, Shima T, Takayanagi S, Urata S, Miyamoto T, Iwasaki H, Takenaka K, Teshima T, Tanaka T, Inagaki Y. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell. 2010;7:708-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 335] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 81. | Sakuishi K, Jayaraman P, Behar SM, Anderson AC, Kuchroo VK. Emerging Tim-3 functions in antimicrobial and tumor immunity. Trends Immunol. 2011;32:345-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 82. | Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 870] [Cited by in F6Publishing: 1062] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 83. | Baixeras E, Huard B, Miossec C, Jitsukawa S, Martin M, Hercend T, Auffray C, Triebel F, Piatier-Tonneau D. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med. 1992;176:327-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 275] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 84. | Huard B, Prigent P, Tournier M, Bruniquel D, Triebel F. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur J Immunol. 1995;25:2718-2721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 253] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 85. | Huard B, Prigent P, Pagès F, Bruniquel D, Triebel F. T cell major histocompatibility complex class II molecules down-regulate CD4+ T cell clone responses following LAG-3 binding. Eur J Immunol. 1996;26:1180-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 100] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 86. | Hannier S, Tournier M, Bismuth G, Triebel F. CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. J Immunol. 1998;161:4058-4065. [PubMed] [Cited in This Article: ] |
| 87. | Maçon-Lemaître L, Triebel F. The negative regulatory function of the lymphocyte-activation gene-3 co-receptor (CD223) on human T cells. Immunology. 2005;115:170-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 88. | Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 949] [Cited by in F6Publishing: 1122] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 89. | Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, Carvajal RD, Dickson MA, D’Angelo SP, Woo KM. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33:3193-3198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 690] [Cited by in F6Publishing: 757] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 90. | Maute RL, Gordon SR, Mayer AT, McCracken MN, Natarajan A, Ring NG, Kimura R, Tsai JM, Manglik A, Kruse AC. Engineering high-affinity PD-1 variants for optimized immunotherapy and immuno-PET imaging. Proc Natl Acad Sci USA. 2015;112:E6506-E6514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 256] [Article Influence: 28.4] [Reference Citation Analysis (0)] |