Published online Aug 29, 2019. doi: 10.5409/wjcp.v8.i3.43
Peer-review started: February 20, 2019
First decision: April 16, 2019
Revised: May 30, 2019
Accepted: July 30, 2019
Article in press: July 30,2019
Published online: August 29, 2019
Microvillus inclusion disease (MVID) is a rare autosomal recessive cause of severe congenital diarrhea with significant morbidity and mortality. Definitive treatment involves bowel transplant. The diagnosis of this condition can be challenging and a few genetic panels are available for the identification of the most common mutations. We present the case of an infant with MVID due to a mutation not reported in the literature before.
We report the case of an infant transferred to our institution with severe diarrhea of unknown etiology, failure to thrive, and significant metabolic derangements. An extensive work-up including stool studies for common gastrointestinal pathogens, abdominal ultrasound, esophagogastroduodenoscopy with duodenal biopsy and flexible sigmoidoscopy failed to reveal a diagnosis. Multiple dietary and formula regimens were introduced but all resulted in voluminous diarrhea. She remained on total parenteral nutrition (TPN) for the duration of her hospital stay. Genetic testing was done and she was subsequently found to have a novel mutation in the MYO5B gene [homozygous mutation for MYO5B c.1462del, p. (Ile488Leufs*93)] giving us the diagnosis of MVID. She remains on TPN while awaiting bowel transplant at the time of the compilation of this case report.
We report a novel mutation involved in MVID and highlight the importance of considering this disease when faced with a newborn presenting with life threatening diarrhea. At the time of this publication, 232 allelic variations of this gene (MIM#606540) exist in National Center for Biotechnology Information’s database. Our patient’s mutation has not been reported in literature as a cause of MVID.
Core tip: Microvillus inclusion disease is a rare autosomal recessive cause of severe congenital diarrhea with significant morbidity and mortality. Infants most commonly present with failure to thrive, severe diarrhea, cholestasis, and electrolyte abnormalities. Diagnosis can be challenging and a few genetic panels are available for the identification of the most common mutations. We present the case of an infant transferred to our institution with severe diarrhea, failure to thrive, and significant metabolic derangements who was subsequently found to have a novel mutation in the MYO5B gene [homozygous mutation for MYO5B c.1462del, p. (Ile488Leufs*93)]. Infants with microvillus inclusion disease generally require life-long intravenous nutritional support or bowel transplant.
- Citation: Sadiq M, Choudry O, Kashyap AK, Velazquez DM. Congenital diarrhea in a newborn infant: A case report. World J Clin Pediatr 2019; 8(3): 43-48
- URL: https://www.wjgnet.com/2219-2808/full/v8/i3/43.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v8.i3.43
Microvillus inclusion disease (MVID) is a rare autosomal recessive cause of severe congenital diarrhea with significant morbidity and mortality[1,2]. Infants most commonly present with failure to thrive, severe diarrhea, cholestasis, malnutrition, and electrolyte abnormalities. Diagnosis can be challenging and a few genetic panels are available for the identification of the most common mutations. We present the case of an infant transferred to our institution with severe diarrhea of unknown etiology, failure to thrive, and significant metabolic derangements who was subsequently found to have a novel mutation in the MYO5B gene [homozygous mutation for MYO5B c.1462del, p. (Ile488Leufs*93)]. Infants with MVID generally require life-long IV nutritional support with close monitoring of any electrolyte imbalances that may require correction.
The MYO5B gene is responsible for the coding of a protein called myosin Vb[2]. MYO5B gene mutations that cause MVID result in a decrease or absence of myosin Vb function[2]. In the enterocytes of the small bowel, a lack of myosin Vb function leads to abnormal formation of microvilli[2]. Microvilli are small finger-like projections from the surface of enterocytes that absorb nutrients and fluid from food passing through the intestine. In MVID, affected enterocytes have small clumps of abnormal microvilli mixed with digestive proteins that form microvillus inclusions[3]. These dysfunctional enterocytes with abnormal microvilli are unable to absorb nutrients and fluids. This subsequently leads to recurrent severe diarrhea, malnutrition, and dehydration in individuals with MVID.
A 17-day-old, premature, female infant was transferred to our neonatal intensive care unit (NICU) for failure to thrive, severe metabolic derangements, and severe, intractable diarrhea. She was a 34 5/7 wk gestation infant of African American descent born via vaginal delivery to a 22-year-old gravida 3, para 1-0-1-1 woman. Prenatal labs were mostly unremarkable except for a Penta screen that was abnormal with a 1:81 risk of Trisomy 18. Amniotic fluid was noted to be meconium-stained with a duration of rupture of membranes of 5 h. There was no diagnosis of polyhydramnios, although the delivery team did notice a large amount of amniotic fluid. Apgar scores were 8 and 9 at 1 and 5 min, respectively. The birthweight was 2.445 kg (62.7% percentile), length was 47 cm (69.2%), and head circumference was 34 cm (93.1%). The infant was admitted to the NICU for prematurity and respiratory distress. Family history was significant only for a maternal uncle with a history of gastroschisis. There was no known consanguinity.
Upon admission to the outside NICU, the patient was intubated for surfactant administration and subsequently extubated to nasal continuous positive airway pressure. She was treated with ampicillin and gentamicin for 5 d from birth for a diagnosis of clinical sepsis. On day of life (DOL) 1, she was started on feeds of breastmilk via orogastric tube, which were fortified the following day. Overnight, she developed extensive watery diarrhea. On DOL 4 she was noted to be down 25% from birthweight and developed significant metabolic acidosis with a serum CO2 level of 6 mmol/L and acute kidney injury (creatinine 3.02 mg/dL). She had profound electrolyte derangements including hypernatremia of 157 mmol/L, hyperkalemia of 9.2 mmol/L, hyperchloremia of 128 mmol/L, hyperglycemia of 227 mg/dL. She had unconjugated hyperbilirubinemia (total bilirubin 15.2 mg/dL, direct bilirubin 1.2 mg/dL), was started on phototherapy. Patient had leukocytosis to 25,000/µL with immature-to-total (I:T) ratio of 0.10. She was then made nil per os (NPO) while electrolyte abnormalities were corrected with normal saline and sodium bicarbonate replacement. Stool studies were sent and the culture was found to be positive for campylobacter antigen. The patient was subsequently treated with 3 d of azithromycin. Mother’s breastmilk was sent for microbial studies and was found to be positive for coagulase negative staph and rare E. coli. Patient’s blood cultures from admission and DOL 4 resulted negative.
Over the following 2 wk, she was again started on various feeding regimens of donor expressed breast milk, conventional cow’s milk formula (Similac Sensitive), and casein-based extensively hydrolyzed formulas (Alimentum). Every feeding trial resulted in a relapse of profuse, watery, non-bloody stools and electrolyte derangements, requiring cessation of enteral feeds. On DOL 17, the patient was transferred to our institution for further work-up and management of refractory diarrhea with feeds associated with dehydration and failure to thrive. At the time of transfer, the patient was receiving TPN with SMOFlipid® (soybean oil, medium chain triglycerides, olive oil, and fish oil). Physical exam on admission revealed signs of moderate dehydration including sunken eyes, dry skin, and capillary refill about 4 s. The patient continued to have significant loose stools (approximately 8/d). Infectious disease and gastrointestinal consults were obtained. With the patient’s history of positive campylobacter stool antigen test and leukocytosis, the clinical picture was initially thought to be consistent with infectious diarrhea. Stool studies, a full sepsis work-up, and maternal HIV testing were performed and the results were negative. A gastrointestinal (GI) pathogen, PCR panel which tests for 21 of the most common bacterial, and viral pathogens implicated in infectious diarrhea were also negative. Stool was positive for abundant leukocytes and occult blood. Stool and blood cultures were repeated and found to be negative. Stool osmotic gap was 30 mOsm/kg, and the newborn screen results were positive for glucose-6-phosphate dehydrogenase deficiency, which were subsequently confirmed following further testing. Over the hospital course, she developed worsening unconjugated hyperbilirubinemia, thought to be secondary to TPN-induced cholestasis.
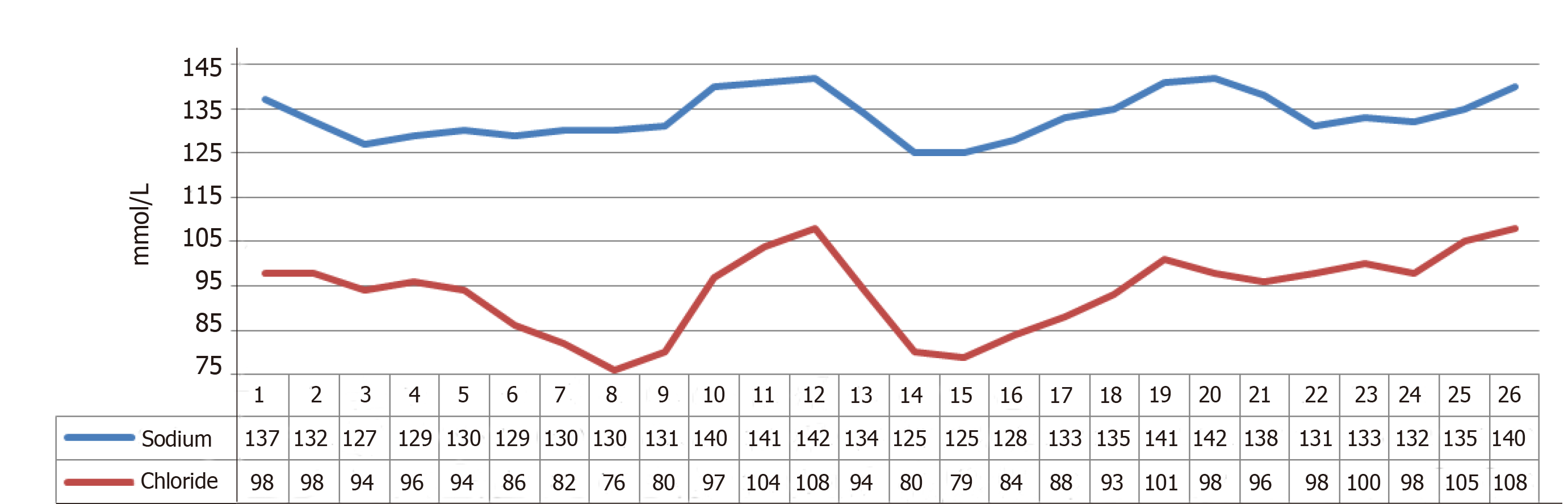
On DOL 19, the patient continued to have diarrhea despite strict NPO, raising suspicion of a secretory diarrhea. An extensive work-up was initiated including EGD and flexible sigmoidoscopy, which were visually normal and the results of a duodenal biopsy showed, “well preserved enterocytes with well-preserved brush borders with no evidence of brush border loss or MIVD.” The infant also continued to have electrolyte abnormalities, most significantly hyponatremia and hypochloremia. Stool sodium (Na) and chloride (Cl) were significantly increased while urine Na and Cl were decreased (Figure 1).
Once the infant’s electrolytes were stabilized and diarrhea improved, she was started once again on trophic feeds with Enfalyte, which were then advanced to amino acid-based formula (Elecare). Although initially tolerated, relapse of severe diarrhea occurred within 48 h. After this time, a congenital secretory diarrhea genetic panel that was sent weeks earlier returned positive for homozygous mutation for MYO5B c.1462del, p. (Ile488Leufs*93), which caused diarrhea with microvillus atrophy.
Based on the above work-up, she was diagnosed with MIVD. Treatment was supportive with IV nutritional support and correction of electrolyte imbalance. She was transferred to a bowel transplant center for evaluation and subsequently transferred back to our institution to be maintained on intravenous TPN until reaching a weight of 6.5 kg, at which point she will be placed on a waiting list for a bowel transplant.
The differential for diarrhea in a neonate is broad. The most common causes include food allergy, gastrointestinal infections, and antibiotic-associated diarrhea followed by congenital defects of ion transport[4]. Once the most common etiologies have been ruled out, the list of differential diagnoses must be shifted to the various enteropathies. These include immunodysregulation polyendocrinopathy enteropathy x-linked syndrome, autoimmune enteropathy, intestinal epithelial dysplasia (tufting enteropathy), brush border disaccharidase deficiencies, microvillous inclusion disease, and membrane channel/carrier protein defects such as congenital sodium and chloride channel defects[5]. Family history of chronic diarrhea, polyhydramnios, or dilated bowel loops on ultrasound should also raise the suspicion for congenital diarrhea[5].
Initial diagnostic steps include making the patient NPO and measuring the stool osmotic gap to differentiate between osmotic and secretory diarrhea. Stool osmotic gap is defined as [Osmolality - 2 x (stool Na + stool K)] and is normally between 50 and 100 mOsm/kg. Osmotic diarrhea is caused by impaired absorption of osmotically active solutes, which draws water into the intestinal lumen via osmosis as seen with lactase deficiency. Osmotic diarrhea improves with fasting and usually involves an osmotic gap > 100 mOsm/kg[5]. Conversely, secretory diarrhea is the result of either decreased absorption or increased secretion of electrolytes, does not improve with fasting, and usually involves an osmotic gap < 50 mOsm/kg[5]. This leads to active secretion of water into the intestinal lumen as seen in Congenital channelopathies.
In our patient, the stool osmotic gap was 30 mOsm/kg, consistent with secretory diarrhea. When suspecting secretory diarrheas, an evaluation of thyroid function may be beneficial as hyperthyroidism increases GI motility, decreasing transit time and the absorption of electrolytes. ESR and CRP may be used to screen for very-early onset inflammatory bowel disease, and liver function tests to evaluate for cholestatic liver disease and subsequent bile acid insufficiency. Endoscopy followed by genetic testing for the most commonly involved genes such a SLC26A3 (diarrhea, secretory chloride, congenital), SPINT2 and SLC9A3 (diarrhea, secretory sodium, congenital), and MYO5B (diarrhea with microvillus atrophy) can lead to the diagnosis[6].
MVID (OMIM 251850) is an autosomal recessive condition causing intractable secretory diarrhea. As in the case of our patient, early-onset MVID is known to cause symptoms within the first few days of life, while late-onset MVID is associated with onset of diarrhea around 2-3 mo of age[7]. Mutation in Myosin Vb (MYO5B) causes the classical form of MVID[7,8]. Myosin Vb is an actin motor protein that plays a key role in apical transport and polarization of enterocytes. Defect of this protein leads to failure of microvilli incorporation into apical membrane[6]. Classical histological features include shortening or loss of microvilli, microvillus inclusions and accumulation of tubulo-/vesicular structures in subapical secretory granules[7,8]. At the time of this publication, 232 allelic variations of this gene (MIM#606540) exist in National Center for Biotechnology Information’s database[1]. Our patient has a c.1462del that has not been reported in literature as a cause of MVID. Mutations in Syntaxin 3 have been reported as causes for atypical, milder form of MVID[9,10]. Syntaxin 3 is an apical SNARE receptor protein necessary for microvilli-lined vesicular docking. Histological features in the atypical variant are the same with the exception of location of secretory granules, which are located along the basolateral membrane and microvilli at the lateral surfaces[9,10]. Decreased expression of sodium hydrogen exchanger, sodium glucose co-transporter leads to impaired water absorption, while expression of cystic fibrosis transmembrane conductance regulator leads to chloride secretion and accounts for high losses of sodium and chloride[11,12].
Without functional microvilli and appropriate ion channels, enterocytes are unable to absorb ions and nutrients and are largely TPN-dependent with bowel transplant being the gold standard of treatment. MYO5b is found in epithelial cells of other organ systems; however, definitive causation of other disease processes remains under investigation. Some conditions thought to be associated with MVID include inguinal hernias, aganglionic megacolon, pneumocystic jiroveci pneumonia, autosomal dominant hypochondroplasia, hypophasphatemic rickets, bicuspidaortic valve, coarctation of aorta, liver dysfunction independent of TPN-associated cholestasis, multiple hepatic adenomas, Downs syndrome, renal dysplasia, and nephrocalcinosis[13-15]. Our patient developed nephrocalcinosis while she was at the transplant hospital for evaluation, which was attributed to dehydration which was managed with fluids being unfused at three-times the maintenance rate. Small bowel transplantation, despite its innate complications and risk of rejection, improves survival (100% in transplanted patients versus 40% in patients without transplant at a median follow-up of 3 years) and quality of life as all patients were able to be weaned off TPN[2].
Congenital diarrheas can pose a diagnostic challenge with high morbidity and mortality from electrolyte derangements and failure to thrive. Clinicians must keep a high index of suspicion for timely diagnosis and management. Prenatal diagnosis may be possible in suspected cases by genetic testing. Infants are at high risk of mortality if disease is not promptly diagnosed. Care for infants with MVID involves a multidisciplinary team approach consisting of gastroenterologists, nutritionists, transplant surgeons, social workers, and case managers. Pediatricians must be vigilant to screen for associated conditions. Parents and caretakers require extensive support, counseling and education in acceptance and management of this rare disorder.
MVID is a rare gastrointestinal condition that involves chronic, watery, life-threatening diarrhea caused by mutations in the MYO5B gene. Onset of symptoms is as early as the first few hours of life and patients usually require long term intravenous TPN. Consideration of this disease in the differential of any infant with unexplained, severe, intractable diarrhea is essential to ensure timely diagnostic investigations.
Manuscript source: Unsolicited manuscript
Specialty type: Pediatrics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Agrawal A, El-Radhi ASM, Nobile S, Pandey A S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Liu MY
| 1. | National Center for Biotechnology Information. ClinVar; c2012-2018 [Cited November 3, 2018]. Available from: https://www.ncbi.nlm.nih.gov/clinvar?term=606540. [Cited in This Article: ] |
| 2. | Halac U, Lacaille F, Joly F, Hugot JP, Talbotec C, Colomb V, Ruemmele FM, Goulet O. Microvillous inclusion disease: how to improve the prognosis of a severe congenital enterocyte disorder. J Pediatr Gastroenterol Nutr. 2011;52:460-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Ruemmele FM, Jan D, Lacaille F, Cézard JP, Canioni D, Phillips AD, Peuchmaur M, Aigrain Y, Brousse N, Schmitz J, Revillon Y, Goulet O. New perspectives for children with microvillous inclusion disease: early small bowel transplantation. Transplantation. 2004;77:1024-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Passariello A, Terrin G, Baldassarre ME, De Curtis M, Paludetto R, Berni Canani R. Diarrhea in neonatal intensive care unit. World J Gastroenterol. 2010;16:2664-2668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 41] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Terrin G, Tomaiuolo R, Passariello A, Elce A, Amato F, Di Costanzo M, Castaldo G, Canani RB. Congenital diarrheal disorders: an updated diagnostic approach. Int J Mol Sci. 2012;13:4168-4185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Blueprint genetics. Congenital Diarrhea Panel; c2018 [Cited November 3, 2018]. Available from: https://blueprintgenetics.com/. [Cited in This Article: ] |
| 7. | Vogel GF, Hess MW, Pfaller K, Huber LA, Janecke AR, Müller T. Towards understanding microvillus inclusion disease. Mol Cell Pediatr. 2016;3:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Moreau B, Nguyen VH. Sa2007 New Variant in MYO5B Gene for Microvillus Inclusion Disease. Gastroenterology. 2015;148:S-382. [DOI] [Cited in This Article: ] |
| 9. | Wiegerinck CL, Janecke AR, Schneeberger K, Vogel GF, van Haaften-Visser DY, Escher JC, Adam R, Thöni CE, Pfaller K, Jordan AJ, Weis CA, Nijman IJ, Monroe GR, van Hasselt PM, Cutz E, Klumperman J, Clevers H, Nieuwenhuis EE, Houwen RH, van Haaften G, Hess MW, Huber LA, Stapelbroek JM, Müller T, Middendorp S. Loss of syntaxin 3 causes variant microvillus inclusion disease. Gastroenterology. 2014;147:65-68.e10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 10. | Alsaleem BMR, Ahmed ABM, Fageeh MA. Microvillus Inclusion Disease Variant in an Infant with Intractable Diarrhea. Case Rep Gastroenterol. 2017;11:647-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Michail S, Collins JF, Xu H, Kaufman S, Vanderhoof J, Ghishan FK. Sodium-hydrogen exchanger isoform 3 (NHE-3) is not expressed in the jejunal mucosa of 2 patients with microvillus inclusion disease. Gastroenterology. 1998;114:A398-A399. [DOI] [Cited in This Article: ] |
| 12. | Engevik AC, Engevik MA, Meyer A, Shub M, Koepsell H, Ameen NA, Tyska M, Goldenring JR. Deficits in Apical Sodium and Water Transporters Along with Maintenance of CFTR Account for Diarrheal Pathology in MYO5B Ko Mice and Patients with MVID. Gastroenterology. 2018;154:S-179. [DOI] [Cited in This Article: ] |
| 13. | Guandalini S, Dhawan A, Branski D. Textbook of Pediatric Gastroenterology, Hepatology and Nutrition: A Comprehensive Guide to Practice. 1st edition. Available from: Springer International Publishing, 2016. [DOI] [Cited in This Article: ] |
| 14. | Burgis JC, Pratt CA, Higgins JP, Kerner JA. Multiple hepatic adenomas in a child with microvillus inclusion disease. Dig Dis Sci. 2013;58:2784-2788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Gathungu GN, Pashankar DS, Sarita-Reyes CD, Zambrano E, Reyes-Mugica M, Brueckner M, Mistry PK, Husain SZ. Microvillus inclusion disease associated with coarctation of the aorta and bicuspid aortic valve. J Clin Gastroenterol. 2008;42:400-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |