INTRODUCTION
Gait initiation refers to the transient period between the quiet standing posture and steady state walking[1-5]. It is a functional task that is classically used in the literature to investigate how the central nervous system (CNS) controls balance during a whole-body movement involving change in the base of support dimensions and center of mass progression. Gait initiation is known to be a highly challenging task for the balance control system. Gait initiation indeed requires the integration of multiple sensory information arising from somatosensory, vestibular and visual systems, along with the coordination of multiple skeletal muscles distributed over the whole body. As such, it may potentially expose populations with sensory or motor deficits or disorders to the risk of fall[6-8]. Falls represent the second cause of mortality in the world, and one third of subjects over 65 years and 50% of those over 80 years living at home fall at least once a year[9]. Although being a very important issue gait analysis however received relatively little attention by orthopedic surgeons. This is particularly troublesome for understanding the pathogenesis of fractures, such as hip or wrist fractures, by those treating these highly frequent traumatic issues.
Understanding how the CNS in able-bodied subjects controls balance during such a challenging task is a pre-requisite to identifying motor disorders in populations with specific impairments of the postural system or fear of falling, such as the elderly or patients with neurological/orthopedics conditions. It may also provide the clinicians with objective measures to assess the efficiency of rehabilitation programs and to better target interventions according to individual impairments. Hence, beside a basic interest per se, it is therefore important to identify the different balance control mechanisms available to participants that ensure stabilization during whole-body progression. It is also important to define adequate biomechanical measures of stability to evaluate the efficiency of these mechanisms. Recent studies in the biomechanical field bring novel insights on these two aspects and open new research avenues, some of which will be mentioned in the present paper.
The present review thus proposes a state-of-the-art on: (1) the balance control mechanisms in play during gait initiation in able bodied subjects and in the case of some frail populations; and (2) the biomechanical parameters used in the literature to quantify postural stability during gait initiation. Before considering these aspects, let us first recall why balance is challenged during gait initiation.
BALANCE IS DISTURBED DURING GAIT INITIATION
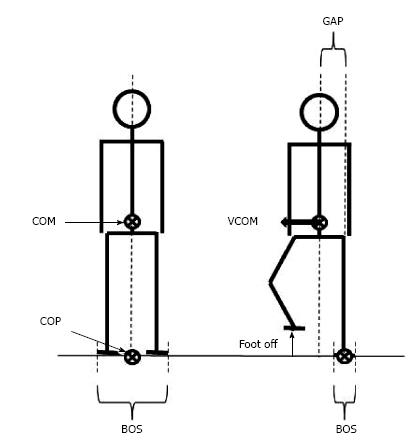
During quiet standing, stability requires that the vertical projection of the center of mass falls within the base of support[10,11] (Figure 1). The center of mass corresponds to the point where the mass of the whole body is concentrated. It is the point of application of the gravity force vector. In the standing posture, the base of support refers to the area that includes every point of contact that the foot (or the feet) make(s) with the supporting surface. When one lifts the foot from the ground to step in the desired direction, balance is potentially challenged along the mediolateral direction because the base of support width in this direction is then drastically reduced. If the center of mass is not repositioned above the new base of support, a mediolateral gap between the center of mass and the center of pressure will be created. The center of pressure corresponds to the barycenter of the ground reaction forces. As a consequence of the mediolateral gap between the center of pressure and center of mass, the whole body will fall towards the swing leg side during the unipodal (or “execution”) phase of gait initiation. The amplitude of this mediolateral fall can be estimated from the center of mass displacement and velocity at the time of swing foot contact, i.e., the greater these two quantities, the larger the mediolateral fall[12-15].
Figure 1 Representation of selected basic notions for balance analysis in biomechanics.
Note that in the quiet standing posture (left figure), the vertical projection of the COM onto the ground falls on the COP. When the subject lifts the foot to step forward (right figure), the base of support size is drastically reduced. A gap between the COP and the COM may then occur, thus causing a disequilibrium towards the stance leg. COM: Center of mass; COP: Center of pressure; BOS: Base of support; VCOM: COM velocity.
Although a recent modelling study reported that an attenuation of this mediolateral fall may theoretically occur during the execution phase of gait initiation via an action on the stance leg stiffness[15] (cf. paragraph “stabilizing features of gait initiation”), this fall towards the swing leg side seems mainly to be braked by the swing foot landing. Swing foot landing indeed acts to provide an immediate enlargement of the base of support size so that the center of pressure may then be shifted laterally beyond the center of mass and thus create a counterbalancing torque oriented toward the stance leg side. Now, this lateral fall may be too significant to be braked by swing foot contact, e.g., if the hip musculature of the swing leg becomes too weak to ensure this braking. This may be the case with aging or neurological/orthopedic conditions. In these cases, the center of mass may then be shifted laterally beyond the base of support with potential risk of the body falling onto the ground if appropriate actions are not undertaken.
It is noteworthy that such lateral falls are common in older adults and are associated with a 6-fold greater risk of hip fracture, compared with falls in other directions, i.e., anterior and posterior falls (e.g., Ref[16-19]). Deficits in the capacity to overcome the mediolateral perturbation to balance due to gravity force is thus thought to be of major importance in the aetiology of falls in frail populations[20,21].
Beside mediolateral instability, it is well known that the collision of the swing foot with the ground generates a large peak vertical ground reaction force. The amplitude of this peak may reach approximately twice body weight during barefoot walking at maximal speed (approximately 2 m/s). This peak, and probably most important, the slope of the vertical ground reaction force rise following the swing foot contact, may potentially cause discomfort or pain to body joints with task repetition (e.g., Ref[22,23]). This perturbing effect is due to the transmission of the vibration from the swing foot to the whole body via bones and soft tissues. When walking with shoes at a normal speed (approximately 1.3 m/s) onto an unobstructed track, the amplitude of this vibration can easily be supported by any subject with either pathology or frailty. This may not be the case if participants have to clear a large obstacle (e.g., Ref[15,24]), which may then increase the fall duration of the center of mass and therefore the vertical peak impact force and the associated slope. This perturbing effect may also be exacerbated if participants initiate gait barefoot and on a hard surface, if they descend large stairs, or if they suffer from knee joint pain or knee hypomobility, e.g., due to the use of an orthosis or to pathology.
Knee joint mobility (flexion) post swing foot contact is known to play an important role in damping these vertical ground reaction forces (e.g., Ref[25,26]). Mechanisms other than swing leg knee flexion are also developed in anticipation of swing foot contact. These mechanisms act in combination to attenuate these disturbing forces and thus avoid body collapse on the ground. As such, they also contribute to maintaining stability. These stabilizing mechanisms are described in the paragraph below.
To summarize, balance is disturbed during gait initiation because the act of lifting the swing foot from the ground induces a gap between the center of mass and the center of pressure. This gap is responsible for generating body disequilibrium and a fall towards the swing leg side. In addition, the collision of the swing foot with the ground generates a peak vertical ground reaction force which is transmitted from swing foot to the whole body via bones and soft tissues. These perturbations may be responsible of falls if not properly counterbalanced.
STABILIZING MECHANISMS INTO PLAY DURING GAIT INITIATION
Once the different sources of disturbance are identified, the question arises as to what the nature of the different mechanisms allowing able-bodied subjects to progress safely (i.e., without falling) in the desired direction is.
Anticipatory postural adjustments
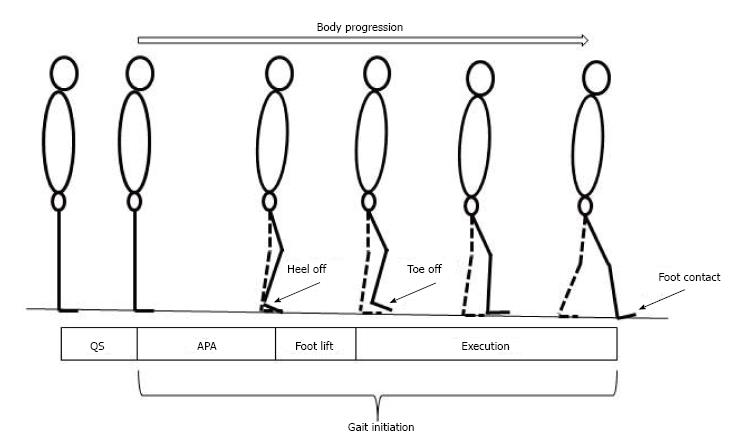
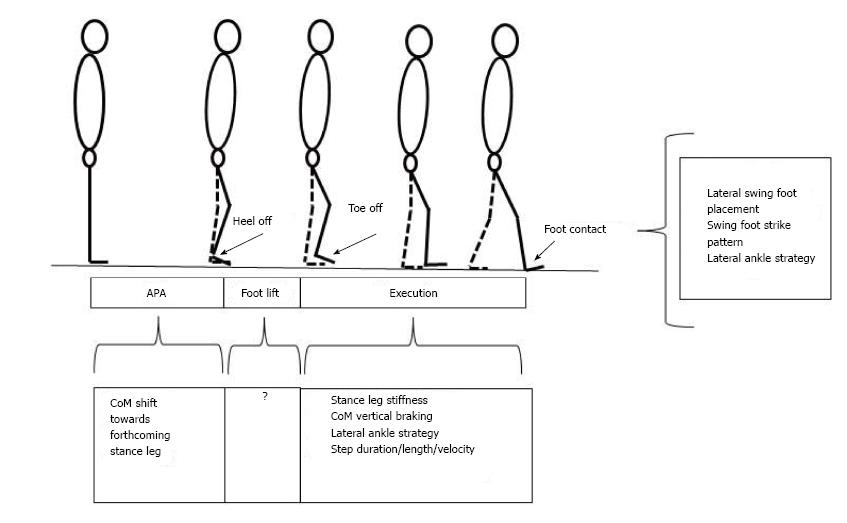
Gait initiation is classically divided in three successive phases: A postural phase which precedes the swing heel off (this phase corresponds to the so-called anticipatory postural adjustments, APAs, described in this paragraph), followed by the foot lift phase that ends at the time of swing toe clearance (the mass of the body is transferred to the stance leg during this phase), and an execution phase that ends at the time of swing foot contact with the supporting surface (Figures 2 and 3).
Figure 2 Stick representation of the different phases and temporal events of gait initiation.
APA: Anticipatory postural adjustments.
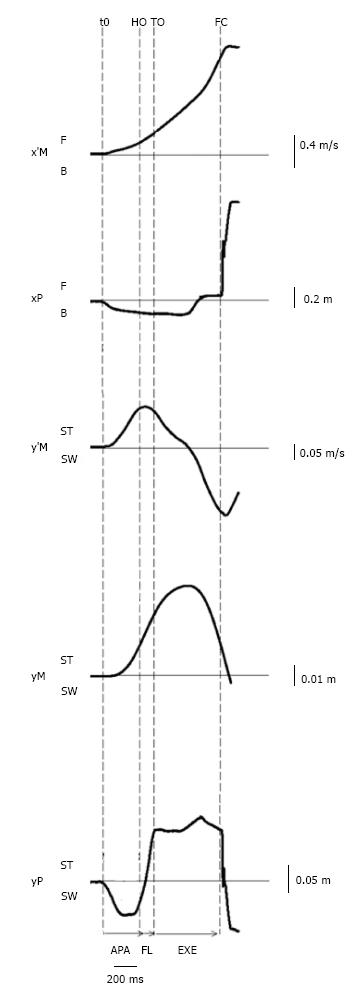
Figure 3 Example of biomechanical traces obtained for one representative subject initiating gait at a maximal velocity (one trial).
Anteroposterior direction x’M: center of mass (COM) velocity; xP: Center of pressure (COP) displacement; F: Forward; B: Backward. Mediolateral direction y’M: Mediolateral COM velocity; yM: Mediolateral COM displacement; yP: Mediolateral COP displacement; ST: Stance limb; SW: Swing limb. Vertical dashed lines: t0 onset variation of biomechanical traces; HO: Swing heel off; FO: Swing foot off; FC: Swing foot contact. Horizontal arrows: APA: Anticipatory postural adjustments; FL: Foot lift; EXE: Execution phase.
It is now well established that dynamical and electromyographical phenomena are developed during APAs. Their functional role depends on the axis considered. APAs along the anteroposterior axis are predictive of motor performance[2,27] while APAs along the mediolateral axis are predictive of postural stability[14,15,28-31].
Along the anteroposterior axis, APAs include a backward center of pressure shift which promotes the initial forward propulsive forces (prior to toe off) required to reach the intended motor performance, in terms of step length and progression velocity[2,27]. The anticipatory backward center of pressure shift is due to bilateral inhibition of the ankle plantar-flexors activity followed by activation of ankle dorsi-flexors[32,33].
Along the mediolateral axis, APAs include center of pressure shift toward the swing leg which promotes center of mass shift in the opposite direction, i.e., towards the stance leg[15,24,31] (Figure 3). These mediolateral APAs thus reduce the gap between the center of mass and the center of pressure at the foot off time. This gap reduction attenuates the mediolateral fall of the center of mass toward the swing leg during the execution phase due to gravity[12,15,28,29] (cf. paragraph above).
The anticipatory mediolateral center of pressure shift has been classically attributed to the loading of the swing leg associated with the activation of swing leg hip adductors[1,11]. Recent studies further reported that, the stance knee and hip are slightly flexed during APAs[31,34], which acts to unload the ipsilateral leg and therefore complement the action of swing hip abductors. EMG analysis revealed that the flexion of the stance knee is favored by bilateral soleus silencing and a greater ipsilateral tibialis anterior activity with respect to contralateral activity, while stance hip flexion was associated with activation of the stance rectus femoris. It is to note that, due to biomechanical constraints, initiating gait from a wider stance decreases the effectiveness of hip abductor activity and increases the reliance on stance knee flexion and vice versa[31].
As a direct consequence of this muscle synergy, when mediolateral balance control is examined in patients suffering from motor problems during gait initiation, hip abduction, stance hip and knee flexion should be considered. Knee flexion control in the frontal plane during APAs could be inadequate in patients suffering from gait problems such as cerebral palsy, Parkinson’s disease[35-39], stroke, amputees[40]. For instance, freezing of gait in Parkinsonian patients is associated with knee trembling[41-45]. Jacobs et al[46] found that during gait initiation, knee trembling causes multiple APAs that are observable as a right-left leg loading-unloading cycles (cf. also[47]). Interestingly, the alternating unloading and loading of the legs was accompanied by similar alternating activation and deactivation of right-left tibialis anterior (Figure 2 in Jacobs et al[46]). Therefore, knee trembling in Parkinsonian patients may be preventing them from correctly displacing their center of mass towards the stance leg and thus not allowing them to initiate gait properly. Indeed, the smaller mediolateral center of pressure displacement during APAs and larger step width of the first step at gait initiation have been observed in Parkinson’s disease[37,38]. This could be in part associated to inappropriate knee flexion. Therefore, it has been proposed by Honeine et al[31] that correcting the knee flexion angle of the stance leg during APAs with a smart orthosis could be an effective solution to enhance gait initiation and possibly steady-state gait in such patients. Future studies should investigate this aspect.
To summarize, lifting the swing foot for stepping forward induces a potential lateral body imbalance. This imbalance is partly countered is advance before swing foot off, i.e., during APA. This APA includes center of pressure shift towards the swing leg which act to move the center of mass towards the stance leg. This lateral postural dynamics is due to motor synergy involving swing hip adduction, combined with stance knee and stance hip flexion. Deficits in this motor synergy with aging or pathology may increase the risk of imbalance.
Stance leg stiffness
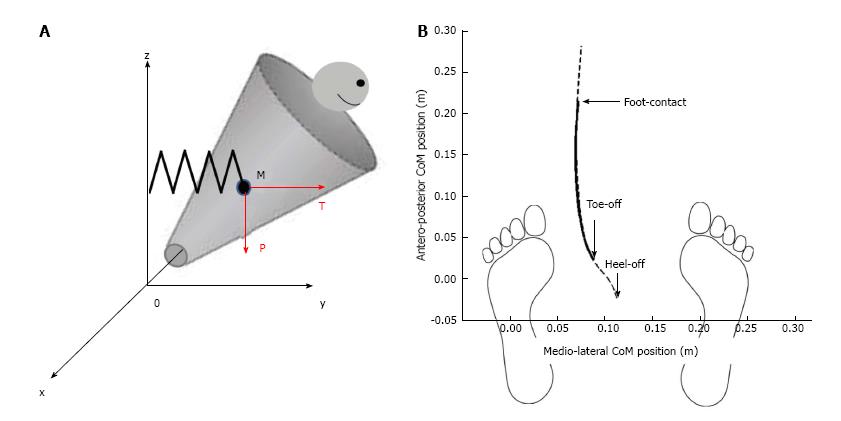
To investigate the link between mediolateral APAs and postural stability during the execution phase of gait initiation (from toe off to foot contact), a recent study[15] modeled the human body as a single conic inverted pendulum which rotates about a fixed point (Figure 4).
Figure 4 Mechanical model of the body during the execution phase of gait initiation.
A: The mechanical model is represented as conic inverted pendulum which pivots around fixed point 0. Body displacement presents five degrees of freedom. The center of mass M falls under the influence of the gravity force “P” and the elastic restoring force “T”; B: Anteroposterior vs mediolateral path of the center of gravity. Dash line represents experimental data during the whole trial and full line represents theoretical data during the execution phase of gait initiation. Note the excellent fitting between these two traces.
This model was based on work carried out in earlier studies[3,12,13,48]. During the execution phase, it was considered that the center of mass was falling laterally under the influence of two forces: The gravity force P = mg (where m is the mass of the solid, and g is the gravitational acceleration) and an elastic restoring force T that reflects active muscular control of movement[49,50], with T = k|yM| (where k is the stiffness of the stance leg muscles during the execution phase[11] and |yM| is the absolute value of the mediolateral center of mass shift, which was systematically oriented towards the swing leg (positive values) during the execution phase. The initial position and velocity of the cone corresponds to the position and velocity of the subject’s center of mass at toe off. The addition of a restoring force on the conic model was necessary in order to control the initial velocity at toe off. A visual analysis of Figure 2 illustrates the excellent fit between the experimental traces obtained during gait initiation and those obtained with the mechanical model. The best fit between experimental (dashed line) and theoretical (full line) data was obtained for stance leg stiffness in the frontal plane of about 1000 N/m. This corresponded to a restoring force of approximately T = 50 N, applied at the center of mass of the participant.
The results obtained in this latter study[15] suggested that changing the stance leg stiffness during the execution phase of gait initiation has the potential to influence the amplitude of the mediolateral fall of the center of mass. Stance leg stiffness can theoretically be modified by changing the co-activation level of agonistic/antagonistic pairs of muscles crossing the ankle, knee or hip joints. Whether the global mediolateral leg stiffness is equally sensitive to co-activation of the muscles groups crossing each of these joints remains to be investigated. Whether the CNS uses this theoretical leg stiffness tuning strategy in combination with mediolateral APAs in order to attenuate the fall of the center of mass during gait initiation, and whether the use of this strategy depends on the sensorimotor state of the postural system also remain unanswered.
Interestingly, an increase in leg stiffness is commonly found in many neurological patients such as patients with Parkinson’s disease, multiple sclerosis, stroke, etc. Based on this mechanical model, it can be speculated that part of the unstable state that is classically observed in these populations during gait initiation can be ascribed to an increase in stance leg stiffness. Besides, the use of medical devices such as leg orthoses, prostheses, plaster, etc. may also be expected to modify stance leg stiffness. On this aspect, Delafontaine et al[51] showed that wearing an orthosis over the ankle of the stance leg induced an increase in the mediolateral fall of the center of mass. In contrast, unpublished observations from our laboratory showed that wearing an orthosis over the knee of the stance leg did not induced any change in the mediolateral fall of the center of mass. These findings suggest that stance leg stiffness in the frontal plane may not be equally sensitive to ankle or knee stiffness. Future studies should investigate this aspect.
To summarize, stance leg stiffness during the execution phase of gait initiation may theoretically influence the mediolateral fall of the center of mass. It can thus be speculated that the increased leg stiffness in neurological patients such as patients with Parkinson’s disease, multiple sclerosis, stroke, etc., or in patients wearing a leg orthosis, may be responsible of part of their unstable state.
Foot placement and lateral ankle strategy
Although modulations of both the mediolateral APAs and the stance leg stiffness may influence the extent to which the center of mass falls toward the swing leg during step execution, it is known that these mechanisms do not fully stabilize the whole-body in the frontal plane during gait initiation[3,12,14,15]. Yet, mediolateral stability must necessarily be restored in order to ensure safe forward progression. It is acknowledged that the primary mechanism employed to restore stability in the frontal plane following the swing foot off is the foot placement[48,52,53]. As stated above, the action of repositioning the swing foot onto the ground allows to enlarge the base of support and opens the possibility of displacing laterally the center of pressure beyond the center of mass. In this way, it becomes possible to create a mediolateral gap between the center of mass and the center of pressure that will brake the lateral body fall and accelerate the center of mass in the direction of the stance leg (Figure 5).
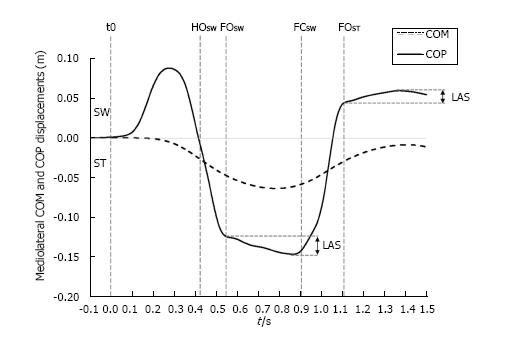
Figure 5 Typical time course of the center of mass and the center of pressure along the mediolateral direction during gait initiation.
The traces were obtained for one subject initiating gait at self-selected speed (one trial). SW and ST indicate swing limb and stance limb, respectively. t0, HOSW, FOSW, FCSW, FOST: Onset variation of biomechanical traces, swing heel-off, swing foot off, swing foot contact, stance foot off, respectively. LAS indicates “lateral ankle strategy”. The extent of the corrections achievable by this strategy may be appreciated by the difference between both the initial and the maximum lateral positions of the COP during the single support phase. COM: Center of mass; COP: Center of pressure.
Results from the literature reveal that the foot placement is actively regulated by the CNS to restore and control balance in the mediolateral direction[54-57]. Foot placement would be mainly adjusted by the activity of the hip abductors of the swing limb in response to the mechanical state of the body, in terms of center of mass position and velocity[58,59]. Interestingly, Caderby et al[60] have investigated the effect of the progression velocity on the mediolateral stability control during gait initiation. These authors noted that when participants initiated gait at high speed, the lateral fall of the center of mass toward the swing limb during step execution was increased compared with gait initiation performed at low and normal speeds. Nevertheless, it was observed that the participants were able to compensate this higher mediolateral instability in the high speed condition by enlarging the step width (i.e., the base of support) such that the mediolateral dynamic stability at the time of foot contact remained unchanged. These findings underlined that healthy young adults were able to finely tune the mediolateral foot placement such as to maintain an invariant mediolateral stability during gait initiation. Results obtained during steady state walking indicate that the accuracy of the foot placement may be altered in patients suffering from sensory and motor impairments. Specifically, it has been observed that above-knee amputees[58] and patients with stroke[61] exhibited a reduced ability to appropriately control foot placement, which may consequently contribute to a higher lateral instability.
It is worth noting that small errors in the foot placement may be corrected even after foot landing. Indeed, after the swing foot is positioned onto the ground, it remains possible to adjust the mediolateral position of the center of pressure located beneath this foot (Figure 5). This mechanism, called “lateral ankle strategy”, would be mainly controlled by the ankle inversor/eversor muscles of the supporting foot[58,62]. Although the extent of the corrections achievable by this mechanism is small, as it is limited by the width of the foot, it allows a fine-tuning of the torque induced by the mediolateral gap between the center of pressure and the center of mass that acts to brake the lateral fall of the body. During steady state walking, Hof et al[58] have shown that the range of corrections attainable by this mechanism was reduced in above-knee amputees for their prosthetic leg (1-2 cm) compared with their sound leg (1.7-4.4 cm) and compared with healthy subjects (0.7-3 cm). These findings suggest that this mechanism could also be altered in people suffering from sensorimotor problems.
Reinmann et al[63] advanced that foot placement and lateral ankle strategy may be two independent mechanisms that are likely coupled and temporally coordinated. What the relative importance of each mechanism is in balance maintenance and how they are coordinated in normal subjects and in patients with postural disorders are questions that remain to be elucidated.
To summarize, lateral swing foot placement and lateral ankle strategy are two independent mechanisms that are likely coupled and temporally coordinated. These mechanisms may complement the mediolateral APAs and stance leg stiffness regulation to stabilize the whole-body in the frontal plane[64-67].
Vertical center of mass braking
As stated above, during the execution phase of gait initiation, the backward center of pressure shift that is generated during APAs propels the center of mass away from the base of support[27,68]. The distance between the center of mass and the center of pressure allows gravity to generate a disequilibrium torque which accelerates the center of gravity in both the anterior and downward directions[11]. Consequently, the lowest center of mass position throughout gait initiation is measured at the instant of foot contact. Nonetheless, in healthy adults, the center of mass velocity reaches a maximum absolute value around mid-single stance and then is decreased (Figure 6). This center of mass vertical deceleration has been shown to result from the increase in triceps surae activity that occurs during the second half of the execution phase of gait initiation[69].
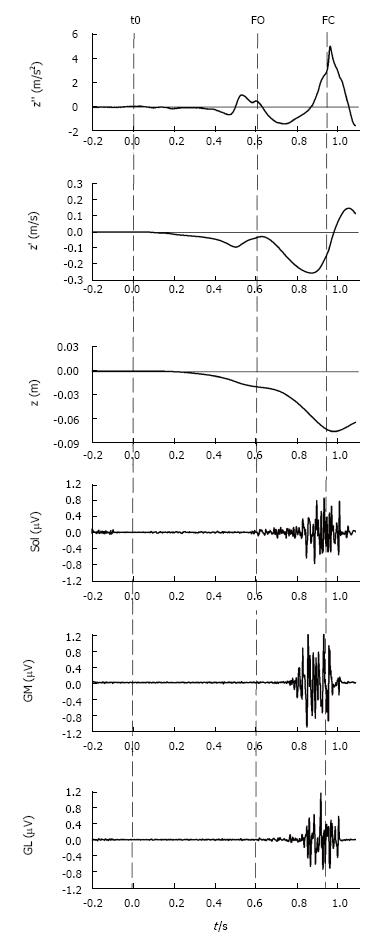
Figure 6 Vertical braking of center of mass during gait initiation.
This figure shows, from top to bottom, the timelines of the COM vertical acceleration (z’’), COM vertical velocity (z’), COM vertical position (z) as well as the electromyographical activity of stance leg triceps surae activity, i.e., soleus (Sol), gastrocnemius medialis (GM) and gastrocnemius lateralis (GL) of a single recording during gait initiation. The dashed lines indicate the instants of initiation (t0), foot off (FO) and foot contact (FC). As can be seen in the figure, step execution is accompanied by a downward (negative) COM acceleration. During mid-single stance, triceps surae activity counteracts gravity and brakes the vertical fall of COM. The braking action of COM is observable as a positive acceleration (top panel) which causes the vertical absolute velocity at foot contact to be lower than the peak absolute velocity measured in mid-single stance. COM: Center of mass.
By counteracting gravity, the triceps surae also plays a role in modulating the disequilibrium torque and setting velocity and duration of step execution as well as step length[70]. On the one hand, it has been argued that the braking action limits the amplitude of the impact of the swinging leg with the ground at foot contact[71,72]. On the other hand, Kuo[73] reasoned that the vertical force produced during late single-stance decreases the work needed to raise the center of gravity in the ensuing double stance phase. Consistent with this hypothesis, Bregman et al[74] showed that the spring assistive ankle foot-orthosis decreases the energetic cost of hemiplegic patients by 10% during double support.
The active braking of the center of mass during step execution is not observed before the age of 4[75]. This implies that active braking requires a process of neural learning[76-78]. In addition, progressive supranuclear palsy[72] and Parkinson patients[79,80] as well as elderly people[81] have all been found to have difficulties in decelerating the center of mass’ downward velocity. The dysfunction in the braking action of center of mass has been linked to lesion or dysfunction of the network linking the primary motor cortex and the mesencephalic locomotor region which is involved in the control of gait and balance[82]. Furthermore, induced ankle joint mobility on healthy individuals has also been shown to play a role in modulating the active braking of center of mass[51,83]. In those studies, the modifications in the proprioceptive inputs, due to strapping the ankle joint or by wearing a rigid ankle foot orthosis, are likely the cause of the deterioration in vertical braking. While proprioception has been shown to play a role in modulating the APAs phase of gait[84-86], more research is needed to understand how somatosensory inputs are integrated in order to generate the central commands responsible for the vertical braking action on center of mass.
To summarize, the center of mass’ downward velocity is actively braked during step execution thanks to the activation of the triceps surae of the stance leg. Difficulties to perform this active vertical braking, as observed in patient with progressive supranuclear palsy and with Parkinson disease as well as in elderly people, may induce postural instability.
Swing foot strike pattern
During locomotion, it is known that the collision of the swing foot with the ground can occur in three ways (e.g., Ref[22,23]): A rear foot strike, in which the heel lands first; a mid-foot strike, in which the heel and ball of the foot land simultaneously; and a fore foot strike, in which the ball of the foot lands before the heel comes down. During running, the strike patterns vary within subjects and whether participants are shod or barefoot. Kinematic and kinetic analyses showed that even on hard surfaces, barefoot runners who fore-foot strike generate smaller collision forces than shod rear-foot strikers[23]. This difference results primarily from a more plantar-flexed foot at landing and more compliance during impact, decreasing the effective mass of the body that collides with the ground. To date, in most studies on gait initiation, participants initiate gait unshod on an unobstructed track and over the hard surface of a force plate. In these classical conditions, participants spontaneously use a rear foot strike whatever the progression velocity. Recent study[15] however reported that when participants initiate gait at maximal velocity with the instruction to clear an obstacle, the percentage of fore-foot strike progressively increased with the obstacle distance, passing from 8% for obstacle at 10% of body height to 21% for obstacle at 30% of body height. Forefoot strike was also reported in young healthy adults during stairs descend[64]. The question of how the CNS in young healthy adults vs frail subjects with high risk of fall coordinates their foot strike pattern with the strategy of active braking of the vertical center of mass fall (cf. paragraph above), remains to be explored in both walking and running. Impairment in the coordination between these two strategies of vertical ground reaction force damping may potentially increase the risk of injuries (e.g., tibial stress fractures[65,66] and plantar fasciitis[67]) and/or of body collapse on the ground. This research avenue is currently under investigation in our laboratory.
To summarize, the swing foot strike pattern used by participants during locomotion (rear foot, mid-foot, or fore foot strike pattern) influences the damping of the ground impact force. Swing foot strike pattern may be combined with active vertical braking of the center of mass to attenuate the potentially damaging effects of the collision forces generated at the time of foot contact.
The different balance control mechanisms at play during gait initiation are summarized in the Figure 7.
Figure 7 Synthesis of balance control mechanisms into play during gait initiation.
The coordination between these different mechanisms remains to be elucidated.
MEASURING DYNAMIC STABILITY DURING GAIT INITIATION
As stated above, the condition for stability during quiet standing holds that the vertical projection of the center of mass falls within the base of support[10,11]. As stressed in the literature, this condition is sufficient during quiet standing when the velocity of the center of mass can be neglected. However, during dynamical tasks such as gait initiation, the velocity of the center of mass cannot be neglected and this quantity has to be taken into consideration in the condition for stability[87,88]. To illustrate this necessity, Hof et al[87] emphasized “that even if the center of mass is above the base of support, balance may be impossible if the center of mass velocity is directed outward. The reverse is also possible: Even if the center of mass is outside the base of support, but its velocity directed towards it, balance can be achieved”. The former situation is particularly relevant to the gait initiation process where the center of mass position falls normally within the base of support at the time of foot contact while its velocity is then directed outwards, i.e., towards the swing leg side.
Until recently, authors compared stability across stepping task conditions with measures of mediolateral center of mass shift, velocity and step width at the time of swing foot contact (e.g., Ref[12,14,29,89,90]). It was assumed that the lower these kinematical center of mass variables are and the larger the step width, the greater the stability. These variables were however considered separately, which made comparison of stability across conditions potentially difficult. Difficulty would indeed arise in a situation where the base of support size is increased, thus yielding a greater stability, and where the center of mass velocity or shift at swing foot contact is also increased, thus yielding a lower stability: It could not be clearly determine whether stability is improved or not.
Hof et al[87] proposed an extension of the classical condition for stability in static situations to dynamical situations where the position of the vertical projection of the center of mass plus its velocity times a factor (square root L/g) should fall within the base of support, l being leg length and g the acceleration of gravity. These authors suggested naming this vector quantity “extrapolated centre of mass position”, because the centre of mass trajectory is extrapolated in the direction of its velocity. According to these authors, the definition put forward the “margin of stability”, which was defined as the minimum distance from extrapolated centre of mass position to the boundaries of the base of support, as a measure of dynamical stability. The margin of stability can thus be considered as a “synthetic” variable since it simultaneously takes into account the position of the center of mass, its velocity and the base of support size. Since the publication of Hof et al[87], this quantity is increasingly used in the literature to quantify stability during dynamical tasks such as steady-state locomotion[21,91,92], gait initiation[15,24,60,93], leg flexion[20,94], sit-to-stand[95], etc.
To summarize, the condition for dynamic stability holds that the position of the vertical projection of a quantity termed the “extrapolated center of mass” should fall within the base of support. The distance between the boundaries of the base of support and the extrapolated center of mass (i.e., “the margin of stability”) is increasingly used in the literature to quantify stability during dynamical tasks.
STABILITY-RELATED CONTROLLED VARIABLES
The mediolateral margin of stability was fruitfully used in recent studies which investigated the adaptability of the stabilizing features of various stepping tasks (gait initiation, rapid leg flexion or abduction) to spatial or temporal constraints imposed on the postural system in young healthy adults and seniors. These constraints included temporal pressure[15,20,24,94], obstacle clearance[15,24], fear of falling[96-98], velocity instruction[60], and symmetrical or asymmetrical body loading[93,99]. In brief, these studies have repeatedly shown that participants were able to develop adaptive postural strategies in the forms of changes in the APAs’ spatio-temporal features, step width and/or swing foot strike pattern (cf. paragraph above) so as to maintain an invariant margin of stability value across the different conditions. A recent study using mechanical modeling of the human body during gait initiation over obstacles of varying heights and distances (cf. paragraph Stance leg stiffness) reinforced this idea of adaptive stabilizing features by showing that a negative value of the margin of stability at foot contact would occur (thus yielding an instable state) should these changes not be developed[15]. This invariance of the margin of stability under various postural constraints led authors to suggest that this quantity may function as a possible balance control parameter during gait initiation.
As a marked exception, unpublished data obtained in twenty seven healthy young adults showed that unilateral knee joint hypomobility experimentally induced by the wear of an orthosis over the stance or the swing leg induced an increased margin of stability compared to unconstrained gait initiation, thus yielding a more stable state. This result was due to an enlarged step width in the conditions with an orthosis. In addition, participants spontaneously initiated gait with a smaller step length and reduced progression velocity, which allowed them to maintain an invariant peak vertical ground reaction force and associated slope values. This statement was strengthened by the result that participants were in fact able to reach the same step length and progression velocity when instructed to do so. But, in this case, the slope (and to a lesser extent, the peak vertical ground reaction force) was then largely increased compared to the control condition. As an expected consequence, participants reported discomfort at the heel, knee and hip with repetition.
To summarize, these results showed that when a mechanical constraint is applied to the leg, the CNS uses a more protective strategy by giving priority to stability and joint comfort rather than to motor performance. It can thus be proposed that the CNS set reference values for stability and vertical disturbance before stepping. The CNS would then plan the stabilizing features and motor performance of gait initiation so as to reach these desired reference values. These references values may change according to the instructions given to participants and the sensorimotor state of the locomotor apparatus.