Published online Jul 18, 2012. doi: 10.5312/wjo.v3.i7.101
Revised: June 5, 2012
Accepted: July 10, 2012
Published online: July 18, 2012
Osteochondral lesions of the talus are common injuries in the athletic patient. They present a challenging clinical problem as cartilage has a poor potential for healing. Current surgical treatments consist of reparative (microfracture) or replacement (autologous osteochondral graft) strategies and demonstrate good clinical outcomes at the short and medium term follow-up. Radiological findings and second-look arthroscopy however, indicate possible poor cartilage repair with evidence of fibrous infill and fissuring of the regenerative tissue following microfracture. Longer-term follow-up echoes these findings as it demonstrates a decline in clinical outcome. The nature of the cartilage repair that occurs for an osteochondral graft to become integrated with the native surround tissue is also of concern. Studies have shown evidence of poor cartilage integration, with chondrocyte death at the periphery of the graft, possibly causing cyst formation due to synovial fluid ingress. Biological adjuncts, in the form of platelet-rich plasma (PRP) and bone marrow aspirate concentrate (BMAC), have been investigated with regard to their potential in improving cartilage repair in both in vitro and in vitro settings. The in vitro literature indicates that these biological adjuncts may increase chondrocyte proliferation as well as synthetic capability, while limiting the catabolic effects of an inflammatory joint environment. These findings have been extrapolated to in vitro animal models, with results showing that both PRP and BMAC improve cartilage repair. The basic science literature therefore establishes the proof of concept that biological adjuncts may improve cartilage repair when used in conjunction with reparative and replacement treatment strategies for osteochondral lesions of the talus.
- Citation: Smyth NA, Murawski CD, Haleem AM, Hannon CP, Savage-Elliott I, Kennedy JG. Establishing proof of concept: Platelet-rich plasma and bone marrow aspirate concentrate may improve cartilage repair following surgical treatment for osteochondral lesions of the talus. World J Orthop 2012; 3(7): 101-108
- URL: https://www.wjgnet.com/2218-5836/full/v3/i7/101.htm
- DOI: https://dx.doi.org/10.5312/wjo.v3.i7.101
The ankle is one of the most common sites of injury in athletes, with a sprain being the most frequent mechanism[1]. The incidence has been described to be as high as nearly 1 per 1000 athlete exposures, leading to 27 000 ankle sprains every day in the United States[2,3]. These injuries are known to lead to cartilage insult in up to 50% of patients[4] and therefore potentially resulting in an osteochondral lesion of the talus (OCL). The increasing recognition of the prevalence of these lesions has brought the etiology and treatment of OCLs to the forefront of sports medicine.
The first description of an ankle OCL was likely given by Monro[5], who removed a loose body from the ankle caused by traumatic injury. Since then, various etiologies have been described which may contribute to the formation of these lesions, including acute trauma, chronic microtrauma, endocrine or metabolic factors, genetic predisposition, joint displacement, osteoarthritis, and avascular necrosis[6-13]. Trauma, however, remains the most common instigating factor, with Flick and Gould[7] concluding that of 500 patients with OCLs, 90% of lateral dome and 70% of medial dome lesions could be attributed to a traumatic event.
Multiple treatment strategies have been defined for managing osteochondral lesions of the talus. These include conservative management, in the form of immobilization or protective weight bearing, and surgical treatment, consisting of either reparative or replacement therapies[14].
Articular hyaline cartilage is avascular and therefore has a poor propensity for healing, additionally when the osteochondral lesion does not extend beyond the subchondral plate, the body does not mount an inflammatory response to promote regeneration. With lesions that involve the subchondral bone, an inflammatory response stimulates marrow cells to produce repair tissue in an attempt to fill the defect[15]. This is the principle behind bone marrow stimulation techniques such as microdrilling and microfracture.
The replacement techniques for treating OCLs consist of substituting the lesion with viable tissue, such as an autologous osteochondral graft or an osteochondral allograft. Studies on both reparative and replacement treatment strategies, however, have shown concern with regard to poor post-operative cartilage repair[16-19]. Both surgical techniques have proven to demonstrate good short to medium term clinical results, however further long-term studies, second look arthroscopy, and radiological investigation have shown reasons for concern[16-20]. This review will describe and address the issue of poor cartilage healing following surgical treatment of osteochondral lesions of the talus, and the use the biological adjuncts to improve cartilage healing. The two most common surgical modalities currently in practice and those used by the senior surgeon are microfracture and autologous osteochondral transplantation, and will be the two strategies addressed in this article.
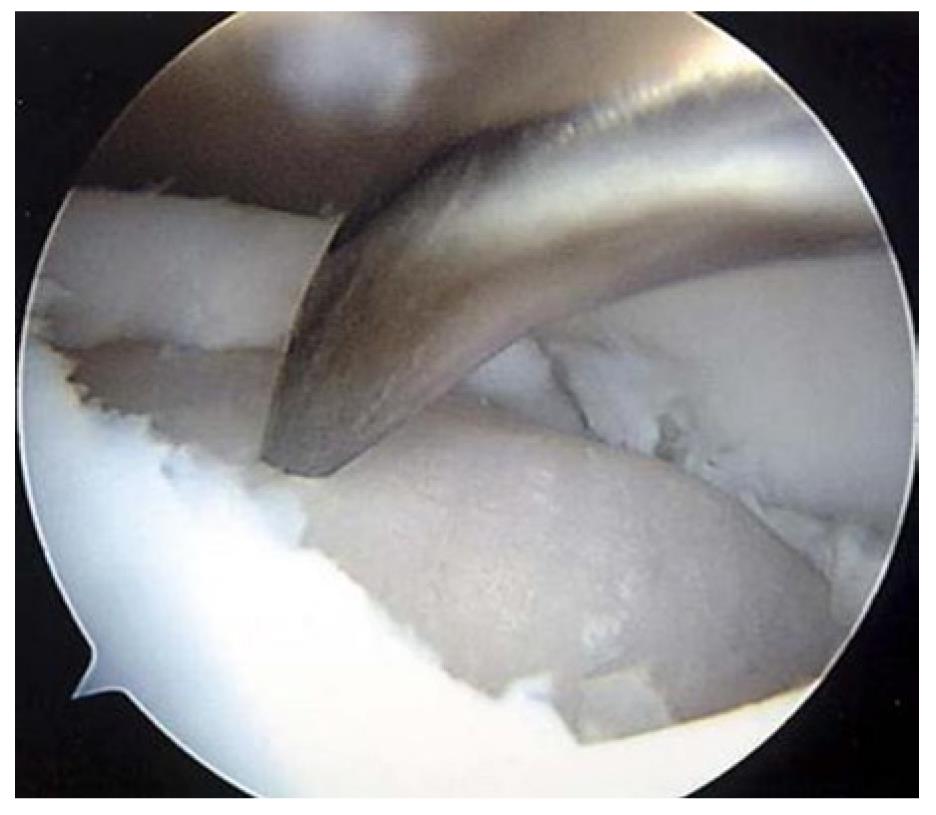
The microfracture procedure involves arthroscopically breaching the subchondral plate in order to stimulate an inflammatory response and migration of subchondral derived mesenchymal stem cells (Figure 1). The recruited cells differentiate into fibrocartilage in an attempt to fill the defect and protect the underlying subchondral bone from excessive loading over time[21].
Short to medium term clinical outcomes following microfracture have been good. Saxena and Eakin[4] reported their results in the athletic population (n = 26) at an average follow-up of 32 mo. 96% of patients reported good or excellent post-operative AOFAS scores, with the same percentage of the study group returning to their sporting activity. Chuckpaiwong et al[22] published results detailing the outcomes of 105 consecutive patients whom had the talar OCLs microfractured. Of those patients who had lesions smaller than 15 mm in diameter, there were no failures of treatment at a mean follow-up of 31.6 mo.
These results were further pooled and corroborated in a systematic review article by Zengerink et al[23]. The authors reviewed 18 studies reporting the outcomes of arthroscopic microfracture surgery and found the reported success rate to be 85%. While the satisfaction of the patient is paramount to determining whether a procedure is successful, the majority of these studies have a short to medium term follow-up.
Despite good clinical follow-up, microfracture relies on a biologic infill that is fundamentally flawed. The underlying issue prevalent with subchondral stimulation is that it stimulates a fibrocartilage repair, which is biomechanically inferior to hyaline cartilage (Figure 2). Additionally, upon further mechanical loading of the joint, fibrocartilage progressively degenerates with an increase in type I collagen[18,19,24-26].
In a study correlating clinical results with second-look arthroscopic findings of 20 ankles, Lee et al[16] found at 12 mo post-operatively, 90% of patients reported a good to excellent outcome with regard to their AOFAS score. However, on second-look arthroscopy, 35% of ankles were determined to show incomplete healing, only 30% of lesions were integrated with the native hyaline cartilage, and 80% had cracks and fissures. The authors found no correlation between clinical AOFAS scores and arthroscopic appearance of the lesion site. This may possibly be due to the short follow-up, and the patients may not experience a deterioration of their symptoms at 1 year post-operatively.
Becher et al[17] echoed these results when assessing the outcome of microfracture surgery using the Hannover Scoring System for clinical outcome and magnetic resonance imaging (MRI) in 45 cases. While the clinical outcomes were successful, with 4 ankles necessitating further surgery to address the chondral defect, MRI assessment indicated that 100% of the cases had cracks and fissuring of the regenerative tissue at a mean follow-up of 5.8 years.
There is an indication that the post-operative clinical outcome scores may deteriorate with time as the lesions fails to heal with adequate repair tissue. Ferkel et al[27], reporting on 50 patients with a mean follow-up of 71 mo, found that 36% of patients had fair to poor results, as measured by the modified Weber scale. Furthermore, 17 patients had been seen 5 years previously and evaluated using the same criteria. Of these 17 patients with a longer-term outcome, 35% demonstrated deterioration in their outcome scores over time. Hunt and Sherman[19], on reporting clinical outcomes as measured by the Martin Score at 66 mo follow-up (33 ankles), found fair or poor outcomes in 61% of patients. Presently, there is no consensus or evidence base as to the optimal size defect that should be treated with microfracture.
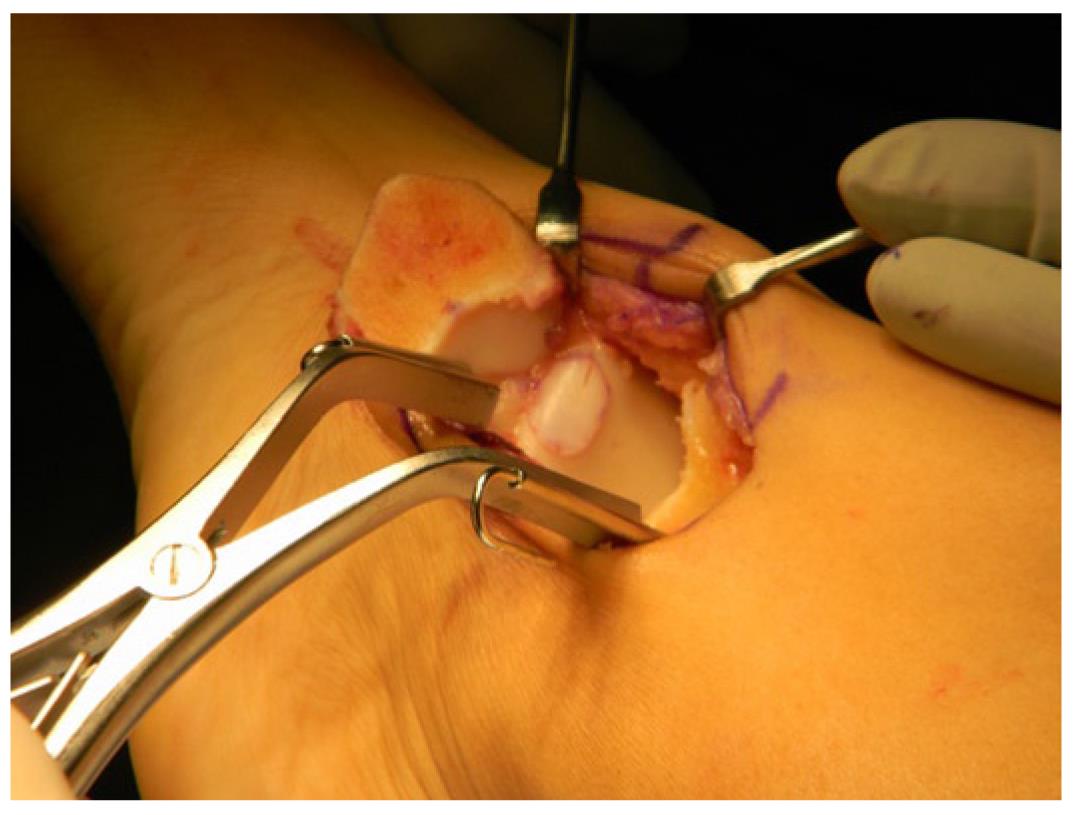
Autologous osteochondral grafts (OATS) involves replacing the damaged tissue on the talus, with a healthy osteochondral graft harvested from a non-weightbearing portion of the ipsilateral knee (Figure 3). This procedure has predominantly been advocated for treating large cystic lesions, or in patients who have failed previous subchondral stimulation[28-30].
Hangody et al[31] were the first to publish results following osteochondral autograft transplantation (mosaicplasty). In a study examining 34 cases with an average follow-up of 48 mo, the authors described good to excellent outcomes in 94% of patients, as measured by the Hannover scoring system.
In a retrospective study examining the outcomes of 50 patients with a cystic talar defect, Scranton et al[30] measured patient outcomes at a mean post-operative time point of 36 mo using the Karlsson-Peterson Ankle Score. 45 patients (90%) had a good to excellent outcome score, with a mean of 80.3.
These successful clinical post-operative outcomes were mirrored in one of the largest case series published by Kennedy and Murawski[32]. In a retrospective study reporting the outcomes of 72 patients with a mean follow-up of 28 mo, outcome was assessed using Foot and Ankle Outcome (FAOS) and Short Form-12 scores (SF-12). FAOS scores improved from 52.67 pre-operatively to 86.19 post-operatively. Similarly, the SF-12 scores improved from 59.4 pre-operatively to 88.63 post-operatively. One patient required a revision surgery for a decompression of a cyst that developed below the graft site through a standard retrograde sinus tarsi approach. Despite these encouraging results, there are some concerns that this procedure has inherent problems that may only manifest at a later time point.
An autologous osteochondral transplant allows degenerative tissue to be replaced with a viable hyaline cartilage graft. However, there remain issues with graft healing, particularly at the interface between the graft and native tissue[33]. In animal models it has been shown that there is poor integration at the cartilaginous border of the graft and surrounding talar cartilage[34]. Additionally, in the process of harvesting the graft from the ipsilateral knee and press fitting it into the cored out lesions site, up to 25% of cell death may occur at the periphery[35].
In a case series published by Valderrabano et al[20], reporting the outcomes of 21 patients treated with the osteochondral graft procedure with a mean follow-up of 72 mo, the authors described not only the clinical outcomes using the AOFAS ankle score, but also their MRI and SPECT-CT findings. While the patients reported a satisfaction rate of good to excellent in 92% of cases, and mean AOFAS score improved from 45.9 pre-operatively to 80.2 points post-operatively, radiological findings were less encouraging. On MRI, there was evidence of recurrent cyst formation in 75% of patients. SPECT-CT showed that some level of cyst formation in all cases.
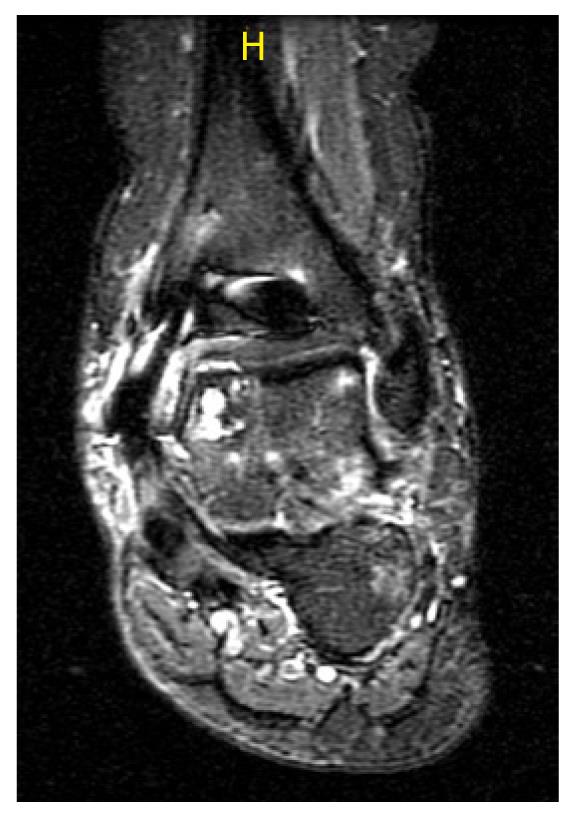
A poorly healed interface may allow synovial fluid ingress around the osteochondral plug and cause cyst formation (Figure 4). When the subchondral bone is under stress, such as from increased hydrostatic pressure from synovial fluid, it leads to upregulation of interleukin-1 and interleukin-6[36]. The upregulation of catabolic factors causes increased osteoclastic activity and ultimately bone resorption. This may cause cyst formation, therefore undermining the graft, and leading to failure of the procedure.
In order to detect poor cartilage healing, adequate imaging must be ordered that is able to visualize the problem. Standard weightbearing radiographs on the ankle are still used in the initial post-operative assessment. However, it is known that up to 50% of osteochondral lesions may not be visualized on X-ray[29]. Helical computed tomography is favored by many surgeons as an initial assessment of OCLs. It is useful in assessing bony detail and determining specific size, shape and extent of subchondral cystic formation[37]. The visualization of cartilage though, is not possible with CT imaging.
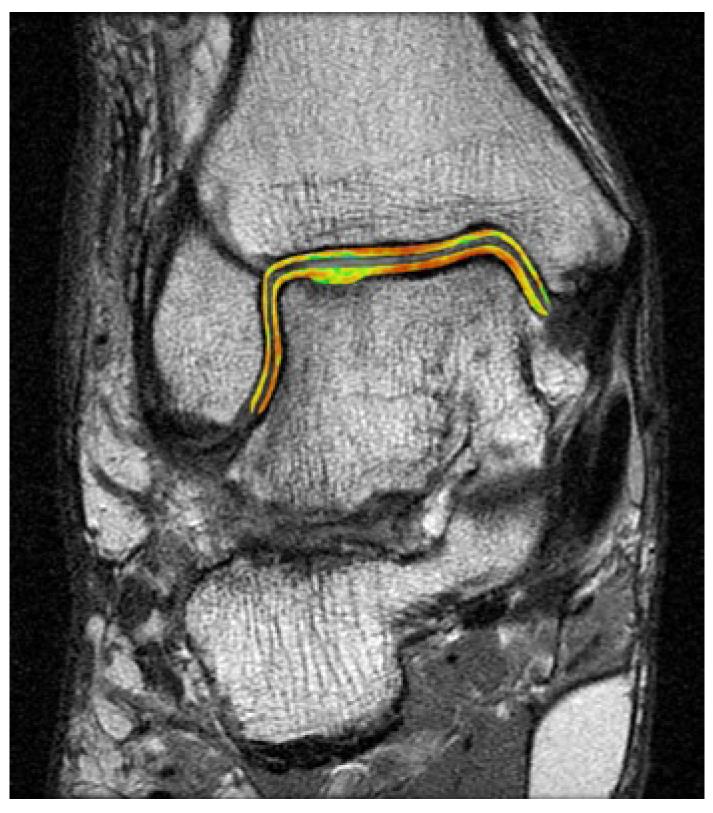
Soft tissue pathology, which is the object of concern when determining if proper regeneration of cartilage has occurred, is best assessed using magnetic resonance imaging[38]. MRI is useful is detecting the degree of cartilage repair and if any other soft tissue insult has occurred. Additionally, it has been shown that the radiological images correlate well arthroscopic findings[39]. The authors prefer using the recently developed quantitative MRI technique of T2 mapping which provides quantitative and qualitative information about cartilage repair (Figure 2)[40].
Improving the biological environment is crucial in order to stimulate the regeneration of cartilage-like tissue and prevent long-term deterioration of outcome following cartilage repair and replacement surgeries. Growth factors and mesenchymal stem cells have long been of interest to the orthopaedic community as a potential adjunct to both microfracture and osteochondral graft transplantation. Historically, individual growth factors have been study in isolation in their recombinant form[41-45]. However, given the vast assortment of growth factors and their interaction within the joint environment, it is doubtful that any single growth factor will lead to comprehensive cartilage regeneration[38]. Therefore, biological adjuncts in the form of platelet-rich plasma (PRP) and bone marrow aspirate concentrate (BMAC) are currently being investigated for their chondrogenic and anti-inflammatory effects and may improve poor cartilage healing as a post-operatively[46-61].
PRP is defined as a sample of plasma with a twofold or more increase in platelet concentration above baseline level or greater than 1.1 × 106 platelets/μL[62]. Platelets’ physiological role in healing has led to the concept that PRP may improve cartilage restoration. Additionally, the multitude of growth factors (Table 1) stored within the platelets’ alpha granules are believed to improve the biological environment within which cartilage may heal[63]. Multiple in vitro and in vivo studies are present in the literature delineating the potential of PRP to improve chondrogenesis in ankle cartilage repair[46-61].
| Growth factor | Activity |
| TGF-β1 | Stimulates MSCs and chondrocytes inhibit catabolic activity of IL-1 |
| FGF | Stimulate bone growth |
| Decrease aggrecanase activity | |
| EGF | Cellular proliferation epithelial cell differentiation |
| PDGF | Stimulation of fibroblasts and collagen synthesis stimulation of osteoblasts |
| VEGF | Promotes angiogenesis and vasculogenesis |
In a study culturing porcine chondrocytes in 10% PRP, the authors reported a 115% (P < 0.001) increase in proteoglycansynthesis compared to the control (fetal bovine serum). Furthermore, PRP augmented collagen production by 163% (P < 0.001)[41]. The proliferative effect of PRP is not limited to chondrocytes alone. Human mesenchymal stem cells (MSC), which may be recruited through subchondral stimulation (e.g., microfracture) or by the addition of BMAC, have also been shown to be positively affected by PRP. Subchondral progenitor MSCs are stimulated to migrate in the presence of PRP[47]. This is particularly relevant to improving the outcomes of the arthroscopic microfracture technique as this is the reasoning behind the use of this procedure. In addition, human MSCs, when cultured in 10% PRP, demonstrate increased levels of DNA compared to an FBS control[48]. Kruger et al[47] demonstrated that the addition of PRP caused MSCs to undergo chondrogenic differentiation and increase type II collagen matrix deposition. MSCs are also difficult to recruit in significant amounts as their concentration in peripheral blood and bone marrow is relatively low, representing only 0.001% to 0.01% of mononuclear cells in bone marrow aspirate[49,50]. Their proliferation though has been shown to increase when cultured with PRP[51-53]. Increasing the synthetic capacity and proliferation of chondrocytes and mesenchymal stem cells potentially improves the cartilage infill of both an OCL that has undergone microfracture and the interface between an osteochondral graft and native tissue.
Osteochondral lesions cannot be managed in isolation if the surgeon hopes to avoid a poor outcome. The development of an OCL indicates the presence of an intra-articular inflammatory environment. While surgical intervention in the form of subchondral stimulation or an osteochondral graft treats the focal defect, the presence of catabolic cytokines may cause further cartilage degeneration and inhibit the production of regenerative tissue. Haemarthrosis following trauma or surgery, causes iron-catalyzed oxygen metabolites to induce macrophage activation and matrix metalloproteinase (MMP)-2 and MMP-9 production by synoviocytes[54]. Additionally, neutrophils chemotactically drawn to the intra-articular space produce interleukin 1 beta (IL-1β) and tumor necrosis factor (TNF)-α which further increase matrix metalloproteinase, ADAMTS, and elastases by both synovial cells and chondrocytes[55]. This catabolic cascade serves to alter the composition of synovial fluid and promote degradation of cartilage extracellular matrix, ultimately causing detriment to any surgical procedure.
PRP is known to counter the catabolic mediators in order to reduce the inflammatory damage to the joint. PRP has been shown to increase the production of hyaluronic acid and hepatocyte growth factorby synoviocytes excised from arthritic patients[56]. The increase in hepatocyte growth factor is particularly relevant as there is evidence that it blocks the activity of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)[57]. Furthermore, in human chondrocytes cultured in IL-1β to simulate an osteoarthritic environment, PRP decreased IL-1β mediated inhibition of COL2A1 and ACAN gene expression. Additionally, itdecreased the IL-1β induced increase of ADAMTS4 and PTGS2 gene expression[58]. These findings have been further confirmed by additional study culturing chondrocytes in IL-1β and TNF-α with collagen matrix enhanced PRP, showing increased chondrogenesis, collagen type II deposition and inhibition of IL-1β and TNF-α[59]. The combination of both the anabolic effect and the inhibition of inflammatory catabolism may contribute to improving cartilage repair and decreasing the risks of a poor outcome following surgery for OCLs. These studies have since been translated to an in vivo model.
In a rabbit model, PRP treated polylactic-co-glycolic acid (PLGA) scaffolds improved osteochondral lesion healing compared to OCLs treated with a PLGA scaffold alone. The authors noted that while the controls showed fibrous healing with deep fissures, the PRP treated group showed hyaline-like infill which was well integrated with the surrounding tissue[60]. Milano et al[61] reported on the results of using PRP and PRP combined with fibrin as an adjunct to microfracture surgery for osteochondral defects in a sheep model, comparing their treatment groups to microfracture surgery alone. The study showed evidence that the PRP treated group showed improved cartilage repair that was both histologically differentiated and mechanically competent.
BMAC is obtained through density gradient centrifugation of bone marrow typically aspirated from the iliac crest. Similar to PRP, BMAC contains platelets, and therefore growth factors, but in lesser concentrations[64]. The principle reason for using BMAC as a biological adjunct to osteochondral lesion surgeries of the talus is to introduce MSCs to the site[64]. Wilke et al[65] demonstrated, in an equine model, that the introduction of MSCs to a full thickness cartilage defect improves cartilage repair. Furthermore, the authors noted that the repair tissue contained primarily type II collagen and was therefore more hyaline-like. The principle that MSCs improve cartilage healing has been further corroborated in the literature[66,67].
The role of BMAC in improving the outcomes of OCL surgery has also been investigated in an in vivo setting. In an equine model, BMAC augmented microfracture was compared to microfracture alone for treatment of a full thickness chondral defect, 15 mm in diameter. At the 8 mo post-operative time point, the authors reported a vast improvement in both ICRS macroscopic and histological scores, 9.4 ± 1.2 compared with 4.4 ± 1.2 and 11.1 ± 1.6 compared with 6.4 ± 1.2 respectively. Moreover, there was improved collagen orientation and collagen type II content in the BMAC treated group[68]. Saw et al[69] showed similar results in a study extrapolating the use of BMAC to a goat model. In a comparison of treatment between microfracture alone, microfracture plus hyaluronan, and microfracture plus hyaluronan and BMAC, there was a statistically significant difference in repair tissue with the last group showing the most favorable results. At the 24 wk following surgery, the BMAC treated group demonstrated almost complete coverage of the defect with evidence of hyaline cartilage repair. In comparison, the group that received microfracture in isolation, showed only partial healing of the lesion with predominantly scar tissue.
Osteochondral lesions are currently treated predominantly by either attempting to repair the lesion with arthroscopic subchondral microfracture or replacement of the non-viable tissue with an autologous osteochondral graft. The short to medium-term clinical results of these surgeries are positive, however longer-term clinical outcomes, as well as radiographic and arthroscopic findings, indicate that surgeons must improve the quality of regenerative tissue in order to avoid long-term post-operative deterioration of outcome.
PRP and BMAC, with their array of bioactive factors have been shown to improve cartilage regeneration in both in vitro and in vivo models. They amalgamate two of the three factors of the tissue engineering trifecta, bringing stem cells and growth factors to the site of injury. These biological adjuncts are simple and easy to generate and are not known to cause any adverse clinical event. Additional research is required to analyze the long-term outcomes of employing biological adjuncts in a clinical setting using carefully designed randomized levelI clinical trials. As we seek to improve the outcomes of surgical treatments for osteochondrallesions, the body of evidence surrounding PRP and BMAC will grow to encompass long-term clinical outcome studies. Researchers are encouraged to continue investigating these biological adjuncts using rigorous scientific methodology.
Peer reviewers: Seung-Hoon Baek, MD, PhD, Assistant Professor, Department of Orthopedic Surgery, Daegu Catholic University Medical Center, 3056-6 Dae-myung-4-dong, Nam-gu, Daegu 705-718, South Korea; Dr., Christiaan JA van Bergen, Department of Orthopaedic Surgery, Academic Medical Center, Meibergdreef 9, Amsterdam 1105 AZ, The Netherlands
S- Editor Yang XC L- Editor A E- Editor Yang XC
| 1. | Fong DT, Hong Y, Chan LK, Yung PS, Chan KM. A systematic review on ankle injury and ankle sprain in sports. Sports Med. 2007;37:73-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 768] [Cited by in F6Publishing: 708] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 2. | Collins CL, Micheli LJ, Yard EE, Comstock RD. Injuries sustained by high school rugby players in the United States, 2005-2006. Arch Pediatr Adolesc Med. 2008;162:49-54. [PubMed] [Cited in This Article: ] |
| 3. | Baumhauer JF, Alosa DM, Renström AF, Trevino S, Beynnon B. A prospective study of ankle injury risk factors. Am J Sports Med. 1995;23:564-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 275] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Saxena A, Eakin C. Articular talar injuries in athletes: results of microfracture and autogenous bone graft. Am J Sports Med. 2007;35:1680-1687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 208] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 5. | Munro A; Part of the cartilage of the joint separated and ossified. In: Medical Essays and Observations. 2nd ed. Edinburgh: Ruddimans; 1737: 305. . [Cited in This Article: ] |
| 6. | Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 2004;86-A:1336. [PubMed] [Cited in This Article: ] |
| 7. | Flick AB, Gould N. Osteochondritis dissecans of the talus (transchondral fractures of the talus): review of the literature and new surgical approach for medial dome lesions. Foot Ankle. 1985;5:165-185. [PubMed] [Cited in This Article: ] |
| 8. | Guettler JH, Demetropoulos CK, Yang KH, Jurist KA. Osteochondral defects in the human knee: influence of defect size on cartilage rim stress and load redistribution to surrounding cartilage. Am J Sports Med. 2004;32:1451-1458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 178] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Konig F. UberfreieKorper in den Gelenken (On the presence of loose bodies in joints). Deutsche Zeitschr f Chi. 1888;27:90-109. [Cited in This Article: ] |
| 10. | Mubarak SJ, Carroll NC. Familial osteochondritis dissecans of the knee. Clin Orthop Relat Res. 1979;140:131-136. [PubMed] [Cited in This Article: ] |
| 11. | Mullet H, Kennedy JG, Quinlan W. Subchondral talar cyst following open reduction and internal fixation of an ankle fracture. Foot Ankle Surgery. 1999;5:147-149. [DOI] [Cited in This Article: ] |
| 12. | PICK MP. Familial osteochondritis dissecans. J Bone Joint Surg Br. 1955;37-B:142-145. [PubMed] [Cited in This Article: ] |
| 13. | Thordarson DB. Talar body fractures. Orthop Clin North Am. 2001;32:65-77, viii. [PubMed] [Cited in This Article: ] |
| 14. | O'Loughlin PF, Heyworth BE, Kennedy JG. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am J Sports Med. 2010;38:392-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 15. | O'Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80:1795-1812. [PubMed] [Cited in This Article: ] |
| 16. | Lee KB, Bai LB, Yoon TR, Jung ST, Seon JK. Second-look arthroscopic findings and clinical outcomes after microfracture for osteochondral lesions of the talus. Am J Sports Med. 2009;37 Suppl 1:63S-70S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 17. | Becher C, Driessen A, Hess T, Longo UG, Maffulli N, Thermann H. Microfracture for chondral defects of the talus: maintenance of early results at midterm follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18:656-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Kumai T, Takakura Y, Higashiyama I, Tamai S. Arthroscopic drilling for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 1999;81:1229-1235. [PubMed] [Cited in This Article: ] |
| 19. | Hunt SA, Sherman O. Arthroscopic treatment of osteochondral lesions of the talus with correlation of outcome scoring systems. Arthroscopy. 2003;19:360-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Valderrabano V, Leumann A, Rasch H, Egelhof T, Hintermann B, Pagenstert G. Knee-to-ankle mosaicplasty for the treatment of osteochondral lesions of the ankle joint. Am J Sports Med. 2009;37 Suppl 1:105S-111S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 198] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 21. | Murawski CD, Foo LF, Kennedy JG. A review of arthroscopic bone marrow stimulation techniques of the talus: the good, the bad, and the causes for concern. Cartilage. 2010;1:137-144. [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy. 2008;24:106-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 315] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 23. | Zengerink M, Struijs PA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18:238-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 441] [Cited by in F6Publishing: 372] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 24. | Furukawa T, Eyre DR, Koide S, Glimcher MJ. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J Bone Joint Surg Am. 1980;62:79-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 25. | Hjertquist SO, Lemperg R. Histological, autoradiographic and microchemical studies of spontaneously healing osteochondral articular defects in adult rabbits. Calcif Tissue Res. 1971;8:54-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Mitchell N, Shepard N. The resurfacing of adult rabbit articular cartilage by multiple perforations through the subchondral bone. J Bone Joint Surg Am. 1976;58:230-233. [PubMed] [Cited in This Article: ] |
| 27. | Ferkel RD, Zanotti RM, Komenda GA, Sgaglione NA, Cheng MS, Applegate GR, Dopirak RM. Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med. 2008;36:1750-1762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in F6Publishing: 276] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 28. | Hepple S, Winson IG, Glew D. Osteochondral lesions of the talus: a revised classification. Foot Ankle Int. 1999;20:789-793. [Cited in This Article: ] |
| 29. | Loomer R, Fisher C, Lloyd-Smith R, Sisler J, Cooney T. Osteochondral lesions of the talus. Am J Sports Med. 1993;21:13-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 275] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 30. | Scranton PE, Frey CC, Feder KS. Outcome of osteochondral autograft transplantation for type-V cystic osteochondral lesions of the talus. J Bone Joint Surg Br. 2006;88:614-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 31. | Hangody L, Kish G, Módis L, Szerb I, Gáspár L, Diószegi Z, Kendik Z. Mosaicplasty for the treatment of osteochondritis dissecans of the talus: two to seven year results in 36 patients. Foot Ankle Int. 2001;22:552-558. [PubMed] [Cited in This Article: ] |
| 32. | Kennedy JG, Murawski CD. The treatment of osteochondral lesions of the talus with autologous osteochondral transplantation and bone marrow aspirate concentrate: Surgical technique. Cartilage. 2011;2:327-336. [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 33. | Huang FS, Simonian PT, Norman AG, Clark JM. Effects of small incongruities in a sheep model of osteochondral autografting. Am J Sports Med. 2004;32:1842-1848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Siebert CH, Miltner O, Schneider U, Wahner T, Koch S, Niedhart C. [Healing of osteochondral transplants--animal experiment studies using a sheep model]. Z Orthop Ihre Grenzgeb. 2001;139:382-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Tibesku CO, Szuwart T, Kleffner TO, Schlegel PM, Jahn UR, Van Aken H, Fuchs S. Hyaline cartilage degenerates after autologous osteochondral transplantation. J Orthop Res. 2004;22:1210-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Cavazzana-Calvo M, Hacein-Bey S, de Saint-Basile G, Le Deist F, Fischer A. [Gene therapy of severe combined immunodeficiencies]. Transfus Clin Biol. 2000;7:259-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Verhagen RA, Maas M, Dijkgraaf MG, Tol JL, Krips R, van Dijk CN. Prospective study on diagnostic strategies in osteochondral lesions of the talus. Is MRI superior to helical CT? J Bone Joint Surg Br. 2005;87:41-46. [PubMed] [Cited in This Article: ] |
| 38. | Dipaola JD, Nelson DW, Colville MR. Characterizing osteochondral lesions by magnetic resonance imaging. Arthroscopy. 1991;7:101-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 253] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 39. | Ferkel RD, Flannigan BD, Elkins BS. Magnetic resonance imaging of the foot and ankle: correlation of normal anatomy with pathologic conditions. Foot Ankle. 1991;11:289-305. [PubMed] [Cited in This Article: ] |
| 40. | Potter HG, Black BR, Chong le R. New techniques in articular cartilage imaging. Clin Sports Med. 2009;28:77-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Chubinskaya S, Segalite D, Pikovsky D, Hakimiyan AA, Rueger DC. Effects induced by BMPS in cultures of human articular chondrocytes: comparative studies. Growth Factors. 2008;26:275-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Diao H, Wang J, Shen C, Xia S, Guo T, Dong L, Zhang C, Chen J, Zhao J, Zhang J. Improved cartilage regeneration utilizing mesenchymal stem cells in TGF-beta1 gene-activated scaffolds. Tissue Eng Part A. 2009;15:2687-2698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Fan H, Tao H, Wu Y, Hu Y, Yan Y, Luo Z. TGF-β3 immobilized PLGA-gelatin/chondroitin sulfate/hyaluronic acid hybrid scaffold for cartilage regeneration. J Biomed Mater Res A. 2010;95:982-992. [PubMed] [Cited in This Article: ] |
| 44. | Chubinskaya S, Hurtig M, Rueger DC. OP-1/BMP-7 in cartilage repair. Int Orthop. 2007;31:773-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 45. | Ellman MB, An HS, Muddasani P, Im HJ. Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Gene. 2008;420:82-89. [PubMed] [Cited in This Article: ] |
| 46. | Akeda K, An HS, Okuma M, Attawia M, Miyamoto K, Thonar EJ, Lenz ME, Sah RL, Masuda K. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14:1272-1280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 251] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 47. | Krüger JP, Hondke S, Endres M, Pruss A, Siclari A, Kaps C. Human platelet-rich plasma stimulates migration and chondrogenic differentiation of human subchondral progenitor cells. J Orthop Res. 2012;30:845-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 48. | Cho HS, Song IH, Park SY, Sung MC, Ahn MW, Song KE. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med. 2011;31:212-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 49. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15372] [Cited by in F6Publishing: 14770] [Article Influence: 590.8] [Reference Citation Analysis (0)] |
| 50. | Martin DR, Cox NR, Hathcock TL, Niemeyer GP, Baker HJ. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp Hematol. 2002;30:879-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 187] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 51. | Mishra A, Tummala P, King A, Lee B, Kraus M, Tse V, Jacobs CR. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods. 2009;15:431-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 265] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 52. | Vogel JP, Szalay K, Geiger F, Kramer M, Richter W, Kasten P. Platelet-rich plasma improves expansion of human mesenchymal stem cells and retains differentiation capacity and in vivo bone formation in calcium phosphate ceramics. Platelets. 2006;17:462-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 53. | Kasten P, Vogel J, Beyen I, Weiss S, Niemeyer P, Leo A, Lüginbuhl R. Effect of platelet-rich plasma on the in vitro proliferation and osteogenic differentiation of human mesenchymal stem cells on distinct calcium phosphate scaffolds: the specific surface area makes a difference. J Biomater Appl. 2008;23:169-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985-997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 689] [Cited by in F6Publishing: 774] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 55. | Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, Griggs DW. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 289] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 56. | Anitua E, Sánchez M, Nurden AT, Zalduendo MM, de la Fuente M, Azofra J, Andía I. Platelet-released growth factors enhance the secretion of hyaluronic acid and induce hepatocyte growth factor production by synovial fibroblasts from arthritic patients. Rheumatology (Oxford). 2007;46:1769-1772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 207] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 57. | Bendinelli P, Matteucci E, Dogliotti G, Corsi MM, Banfi G, Maroni P, Desiderio MA. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-κB inhibition via HGF. J Cell Physiol. 2010;225:757-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 295] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 58. | van Buul GM, Koevoet WL, Kops N, Bos PK, Verhaar JA, Weinans H, Bernsen MR, van Osch GJ. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med. 2011;39:2362-2370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 257] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 59. | Wu CC, Chen WH, Zao B, Lai PL, Lin TC, Lo HY, Shieh YH, Wu CH, Deng WP. Regenerative potentials of platelet-rich plasma enhanced by collagen in retrieving pro-inflammatory cytokine-inhibited chondrogenesis. Biomaterials. 2011;32:5847-5854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 60. | Sun Y, Feng Y, Zhang CQ, Chen SB, Cheng XG. The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int Orthop. 2010;34:589-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 61. | Milano G, Sanna Passino E, Deriu L, Careddu G, Manunta L, Manunta A, Saccomanno MF, Fabbriciani C. The effect of platelet rich plasma combined with microfractures on the treatment of chondral defects: an experimental study in a sheep model. Osteoarthritis Cartilage. 2010;18:971-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 62. | Miller Y, Bachowski G, Benjamin R, Eklund DK, Hibbard AJ, Lightfoot T. Practice guideline for blood transfusion: A compilation from recent peer-reviewed literature. 2nd ed. Washington, DC: American Red Cross; 2007; 56. [Cited in This Article: ] |
| 63. | Smyth NA, Fansa AM, Murawski CD, Kennedy JG. Platelet-rich plasma as a biological adjunct for the surgical treatment of osteochondral lesions of the talus. Techniques in Foot and Ankle Surgery. 2012;11:18-25. [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 64. | Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469:2706-2715. [PubMed] [Cited in This Article: ] |
| 65. | Wilke MM, Nydam DV, Nixon AJ. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthop Res. 2007;25:913-925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 66. | Fortier LA, Nixon AJ, Williams J, Cable CS. Isolation and chondrocytic differentiation of equine bone marrow-derived mesenchymal stem cells. Am J Vet Res. 1998;59:1182-1187. [PubMed] [Cited in This Article: ] |
| 67. | Chen FH, Tuan RS. Mesenchymal stem cells in arthritic disease. Arthritis Res Ther. 2008;10:223-232. [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 68. | Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, Stokol T, Cheetham J, Nixon AJ. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92:1927-1937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 298] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 69. | Saw KY, Hussin P, Loke SC, Azam M, Chen HC, Tay YG, Low S, Wallin KL, Ragavanaidu K. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic Acid: an experimental study in a goat model. Arthroscopy. 2009;25:1391-1400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |