Published online Jun 10, 2017. doi: 10.5306/wjco.v8.i3.214
Peer-review started: February 10, 2017
First decision: March 27, 2017
Revised: May 4, 2017
Accepted: May 18, 2017
Article in press: May 20, 2017
Published online: June 10, 2017
High-resolution pelvic magnetic resonance imaging (MRI) is the primary method for staging rectal cancer. MRI is highly accurate in the primary staging of rectal cancer; however, it has not proven to be effective in re-staging, especially in complete response evaluation after neoadjuvant therapy. Neoadjuvant chemoradiotherapy produces many changes in rectal tumors and on adjacent area, as a result, local tumor extent may not be accurately determined. However, adding diffusion-weighted sequences to the standard approach can improve diagnostic accuracy. In this pictorial review, an overview of the situation of MRI in the staging and re-staging of rectal cancer is exhibited as a pictorial assay. An experience- and literature-based discussion of limitations and difficulties in interpretation are also presented.
Core tip: Accurate staging and circumferential resection margin evaluation significantly impacts determining optimal treatment scheme. Preoperative magnetic resonance imaging (MRI) is highly accurate; however, it has yet to be proved as effective in re-staging. The adding of diffusion-weighted sequences to standard T2-weighted MRI can positively affect its diagnostic accuracy.
- Citation: Engin G, Sharifov R. Magnetic resonance imaging for diagnosis and neoadjuvant treatment evaluation in locally advanced rectal cancer: A pictorial review. World J Clin Oncol 2017; 8(3): 214-229
- URL: https://www.wjgnet.com/2218-4333/full/v8/i3/214.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i3.214
Multimodal treatment of rectal cancer, with the combination of preoperative (neoadjuvant) chemoradiotherapy (CRT) followed by surgery increases local control in locally advanced cancers and has become the standard approach to such rectal cancers[1-5].
High-resolution pelvic magnetic resonance imaging (MRI) is the primary method for evaluation in rectal cancer[6-10]. When applied according to the optimal protocols, high-resolution MRI accurately determining patients regarding neoadjuvant CRT requirement[11]. Moreover, assessing treatment response in tumors using MRI also predicts probable survival outcomes, and could be used in the future to further adjust treatment according to the patients’ response[12]. In recurrent rectal cancer, MRI enables the depiction of the extent of tumor growth, and can establish the resectability of disease[13,14].
MRI has not met expectations in re-staging, especially in complete response evaluation after neoadjuvant CRT because of post-therapeutic fibrosis and inflammation[15-19]. However, adding functional MR sequences such as dynamic contrast-enhanced and diffusion-weighted sequences to the standard approach can improve diagnostic accuracy of MRI[20-23].
In this pictorial review, we present a synopsis of the current standing of MRI in the staging and re-staging of rectal cancer. We also present an experience- and literature-based discussion of limitations and difficulties in interpretation.
Rectal MRI should be performed with pelvic phased-array coils. Rectal MRI using this technique provides overall assessment of the rectal wall layers with high-spatial-resolution and benefits from a large field of view[15,24].
Routine rectal filling using endoluminal contrast agents such as ultrasonography gel is discouraged[24] because this can distend of the rectum and compress the mesorectal fat, which may result in overestimation of fascial involvement and interfere with assessment of mesorectal nodes[25].
Bowel preparation is generally not necessary before the examination, but spasmolytics can be used when excessive fecal matter is visible on the planning images[15,24]. For this purpose, a dose of 40 mg butylscopolamine is used intramuscularly unless contraindicated, immediately prior to placing the patient on the MRI table.
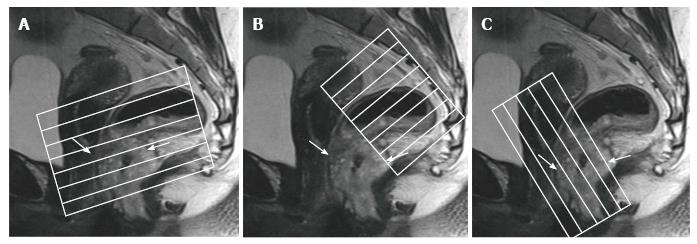
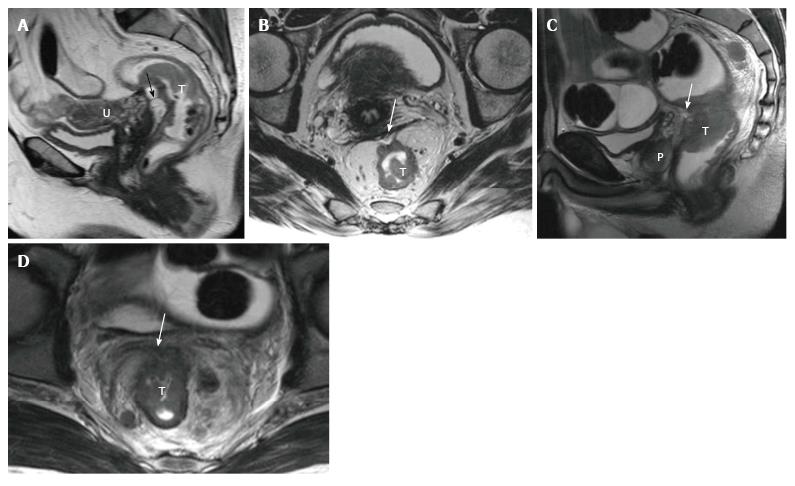
Standard MR rectal protocols must at least include 2D T2-weighted sequences in sagittal, axial, and oblique coronal planes with 1-3 mm slice thicknesses. Sagittal sequences are used to identify the longitudinal tumor axis such that axial and coronal planes may be angled as perpendicular and parallel to the tumor axis as possible, respectively. Coronal planes must be angled in line with the anal canal for low tumors in order to evaluate the relation to the anal complex and pelvic floor muscles[15,24,26] (Figure 1). Axial images are useful for evaluation of the tumor and its relationship with the intestinal wall, mesorectal fascia (MRF), and the adjacent pelvic tissue. Sagittal images are useful for the assessment of the tumor height and length and its relationship with peritoneum and other adjacent tissue.
In addition to T2-weighted sequences, diffusion-weighted imaging (DWI) sequences are recommended for inclusion in restaging protocols. DWI provides no additional benefit in primary staging; however, evidence is accumulating suggesting that it increases the diagnostic capability of MRI in the assessment of therapy response (yT-stage) after CRT[24]). DWI also helps T2-weighted fast-spinecho (FSE) sequences to distinguish patients having good vs poor response[20-23]. However, there is not adequate proof for supporting the usage of DWI for primary T-staging and lymph node assessment[27].
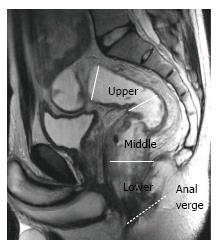
The rectum is approximately 15 cm in length from the anal verge, which is the lowest part of the anal canal. The rectum has traditionally been divided into three segments according to the distance from the anal verge: Upper (> 10 cm), middle (5-10 cm), and lower (< 5 cm)[27,28] (Figure 2).
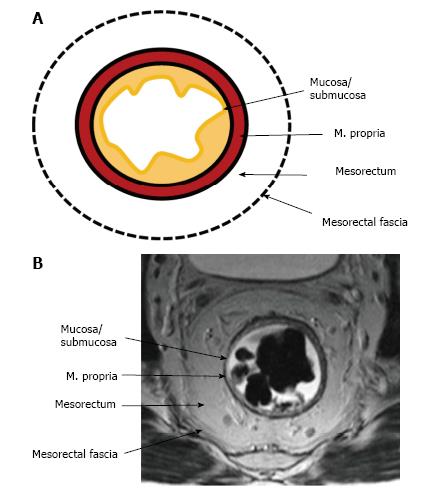
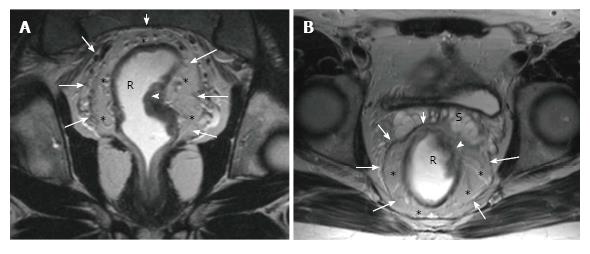
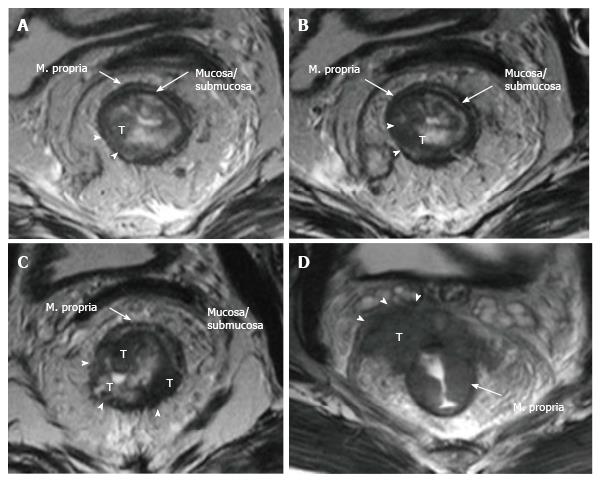
The upper and middle rectal walls consist of three separate layers that can be distinguished in MRI. T2-weighted MRI sequences are the best for visualizing rectal wall anatomy. The internal hyperintense layer represents the mucosa and submucosa (no distinction is possible between in two layers); the medial hypointense layer and external hyperintense area represent the muscularis propria and the mesorectum, respectively[15,29] (Figure 3).
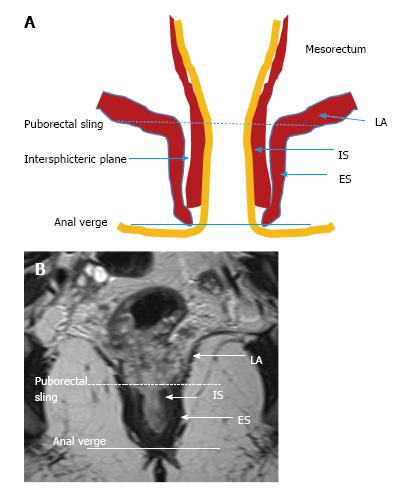
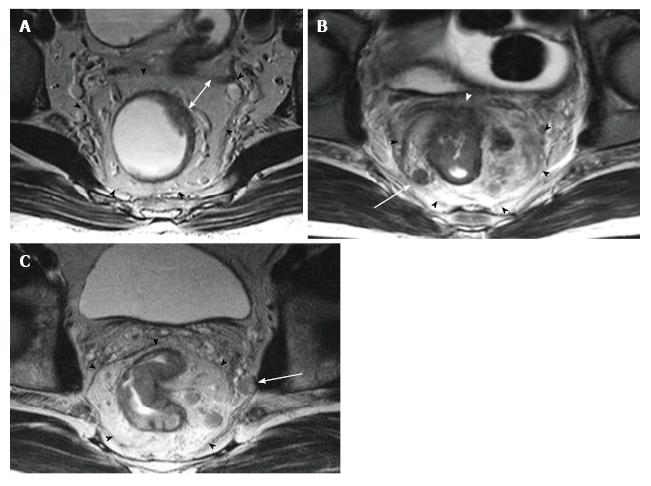
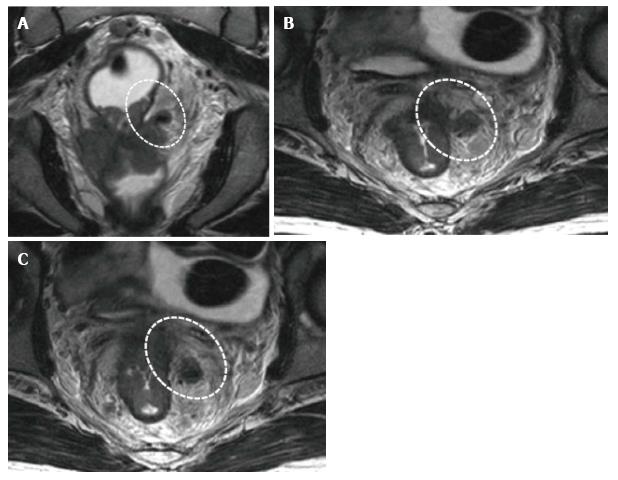
The puborectal sling constitutes the upper limit of the anal canal. The inner muscular wall of the anal canal comprises the internal sphincter, which is the direct continuation of the circular layer of the muscularis propria of the rectum. The outer muscular wall of the anal canal is cranially composed of the puborectal muscle and caudally of the external sphincter[15,26] (Figure 4).
The puborectal sling constitutes the upper limit of the anal canal. The internal sphincter (the internal muscular wall) of the anal canal is consisted of the direct continuity of the muscularis propria circular layer of the rectum. The external muscular wall of the anal canal is formed by the puborectal muscle in cranially and the external sphincter in caudally[15,26] (Figure 4).
The peritoneal reflection covers the anterior wall of the upper rectum; the risk of peritoneal perforation in upper rectal tumors is high[27]. The peritoneal reflection can be easily displayed on sagittal and axial high-resolution T2-weighted images. In sagittal images, it can be depicted whereon upper pole of the seminal vesicles in men and at the uterocervical angle in women[15]. The evaluation of the peritoneal invasion is very important in staging, because rectal tumor is staged as T4a in the presence of peritoneal invasion (Figure 5).
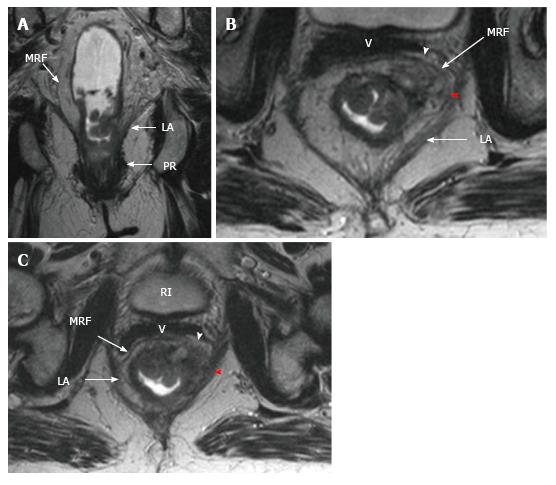
The middle rectum, which lies below the peritoneal reflection, is completely surrounded by mesorectal fatty tissue which is called the mesorectum. Mesorectum is encircled by the MRF which is constitutes the circumferential resection margin (CRM)[26-29]. The MRF can be seen as a thin, low-signal intensity envelop which surrounds the rectum and mesorectum (Figure 6). MRF tapers downward at the lower rectal level[26]. The MRF is easily seen in posterolateral views, although it is difficult to distinguish it from Denonvilliers’ fascia in the anterior wall[30].
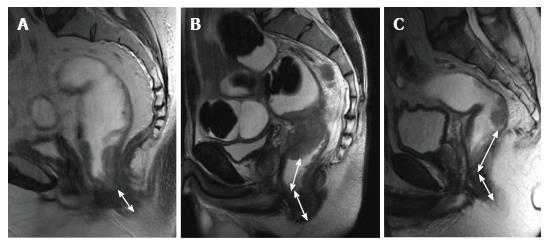
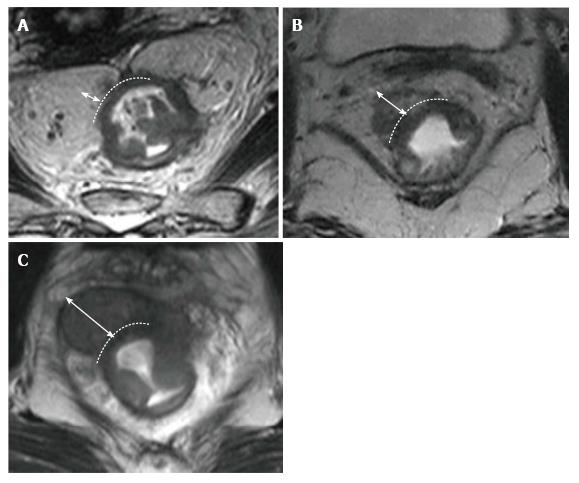
Tumor height and length should be routinely reported because outcomes and surgical management are affected by the location of the tumor[24].
The distance and length are measured on a line drawn on the sagittal MR images. For tumor localization, the distance of the lowest portion of the tumor from the anal verge is measured. Rectal tumors are classified as high, middle or low when their most caudal border is > 10 cm, 5-10 cm, or < 5 cm from the anal verge, respectively[15] (Figure 7).
On T2-weighted imaging, the muscularis propria is seen as a hypointense line between the hyperintense mesorectal fat and the inner submucosa and mucosa, which show intermediate to mild hyperintensity. The signal intensity of a rectal tumor on T2-weighted images is typically intermediate between the signal intensity of the muscularis propria and mucosa (Figure 8).
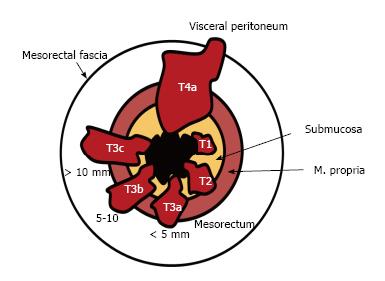
T1 tumors are confined to the submucosa; T2 tumors extend into, but not beyond, the muscularis propria. The differentiation of T1 tumors from T2 tumors on MRI is usually not reliable without an endorectal coil or endorectal ultrasound, and tumors should generally be staged as T1/T2[15]. A tumor is staged as T3 when it extends beyond the muscularis propria and strands the mesorectal fat. Disruption of the muscularis propria because of penetrating vessels should not be overstaged as T3 (Figures 8 and 9).
The extramural depth of invasion refers to extension of tumor beyond the muscularis propria[31]. The American Joint Committee on Cancer suggested an optional stratification of T3 tumors based on the extramural depth of invasion: Less than 5 mm, T3a; 5-10 mm, T3b; and more than 10 mm, T3c[32]. An extramural depth of invasion of less than 5 mm presents a significantly higher survival rate, and these early T3 tumors may be adequately managed with surgery alone and have a prognosis comparable to that of tumors characterized as T1/T2[33]. T4 tumors extend onto the surface of the visceral peritoneum or an adjacent structure (Table 1, Figures 8 and 10).
| Stage | MRI findings |
| T stage for middle and high tumors1 | |
| T1 | Tumor signal intensity is confined to the submucosal layer |
| T2 | Tumor signal intensity extends into the muscle layer, with loss of the interface between the submucosa and circular muscle layer |
| T3 | Tumor signal intensity extends through the muscle layer into the perirectal fat, with obliteration of the interface between muscle and perirectal fat |
| T3a | Tumor < 5 mm into the perirectal fat |
| T3b | Tumor 5-10 mm into the perirectal fat |
| T3c | Tumor > 10 mm into the perirectal fat |
| T4a | Tumor signal intensity extends to surface of visceral peritoneum |
| T4b | Tumor signal intensity extends into an adjacent structure or viscus |
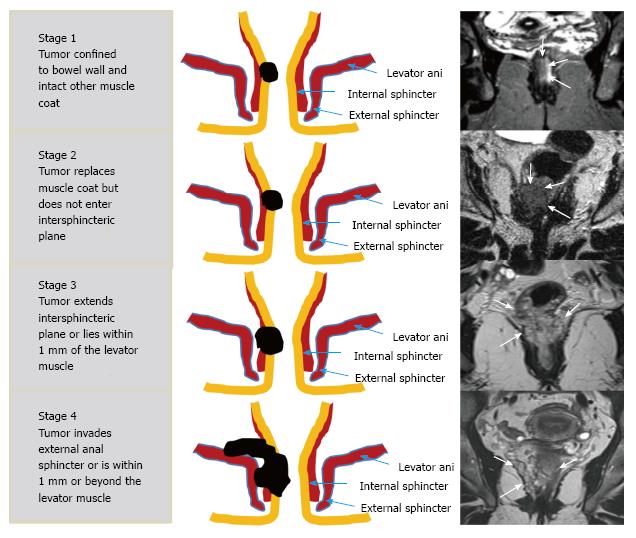
| T stage for low tumors2 | |
| T1 | Tumor signal intensity confined to bowel wall, outer muscle coat intact |
| T2 | Tumor signal intensity replaces muscle coat but does not enter intersphincteric plane |
| T3 | Tumor signal intensity extends intersphincteric plane or lies within 1 mm of levator muscle |
| T4 | Tumor signal intensity extends external anal sphincter or is within1 mm or beyond levator muscle with/without adjacent organ invasion |
| N stage | |
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in 1-3 regional lymph nodes |
| N2 | Metastasis in > 3 regional lymph nodes |
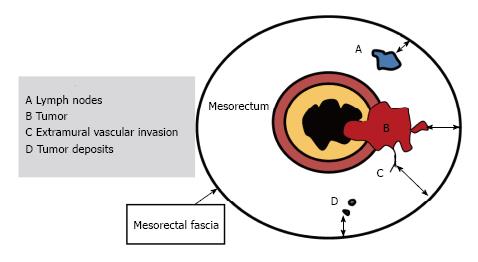
For T3 tumors, the shortest distance between the most penetrating parts of the tumor and the MRF should be measured[34,35]. The distance to the MRF is a critical local prognostic factor for rectal cancer[36,37]. A tumor-MRF distance of more than 1 mm is a reliable predictor for negative margins after TME[38]. In the presence of satellite nodules such as tumor deposits, lymph nodes or extramural vascular invasion (EMVI), the shortest distance between the nodules and the MRF should also be reported[15] (Figures 11 and 12).
EMVI is associated with local and distant recurrence and poor survival[39]. It is defined as the presence of malignant cells within blood vessels located beyond the muscularis propria in the mesorectal fat. EMVI is suggested when vessels close to the tumor are obviously irregular or expanded by tumoral signal intensity[39] (Figure 13).
The assessment of EMVI is a routine component of MR evaluation for primary staging; however, for restaging, there is no agreement as to whether evaluation of EMVI remains beneficial[24].
A specific T staging system is used to identify tumors and its circumferential resection margin (CRM)[40] (Table 1, Figures 14 and 15).
Staging of nodes is very important for planning preoperative treatment[41]. In the TNM system, disease involving only the regional nodes, including the mesorectal and internal iliac nodes, accounts for the N stage (Table 1); involvement of other nodes is regarded as metastasis[38].
Mesorectal nodes are often the first and the most commonly involved group of nodes. Nodal metastases are usually within the proximal 5 cm of the tumor[41].
Extramesorectal nodes (iliac, superior rectal or inferior mesenteric nodes) are generally involved in locally advanced cancers[42]. Low rectal tumors can also spread superficial inguinal nodes and imply poor prognosis[43].
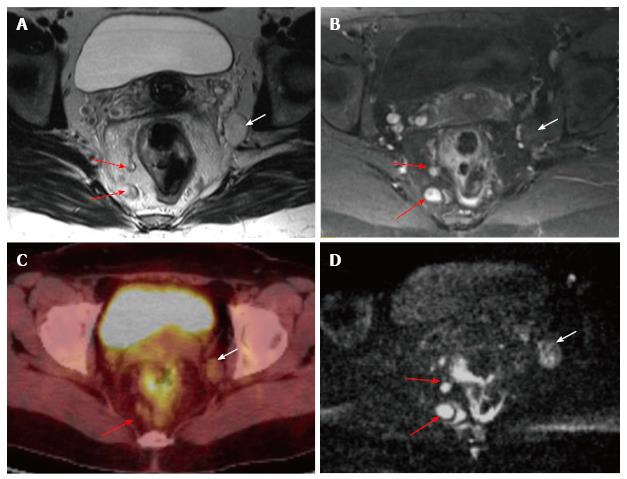
Node size is the usual criterion in nodal staging using MRI. Lymph nodes are usually considered pathologic when their short axis is longer than 0.5 cm; however, no optimal cut-off threshold exists for involved nodes[24]. The inclusion of morphologic features such as round shape, irregular contour, and nonhomogeneous signal intensity to a size cutoff increases the accuracy of MR[44]. Although DW MRI is not accurate enough for characterizing nodes, it may be useful for locating them[45] (Figure 16).
Neoadjuvant CRT provides downstaging and downsizing along with improvement in less extensive surgery, decreased local recurrence, and general survival[12,46]. Tumor restaging involves correlating the posttreatment images with the pretreatment images with respect to all the elements assessed in the initial staging, and necessitates image acquisition with almost the same protocol and on the same planes.
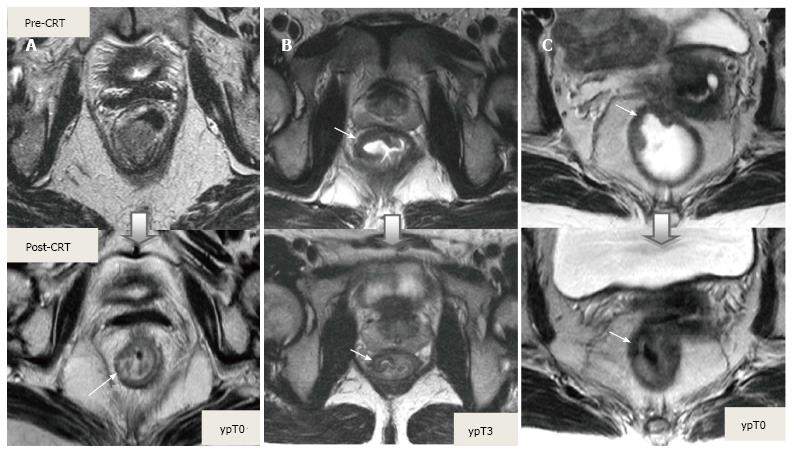
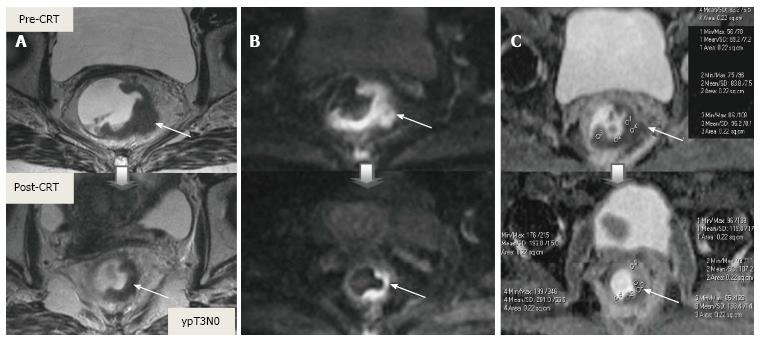
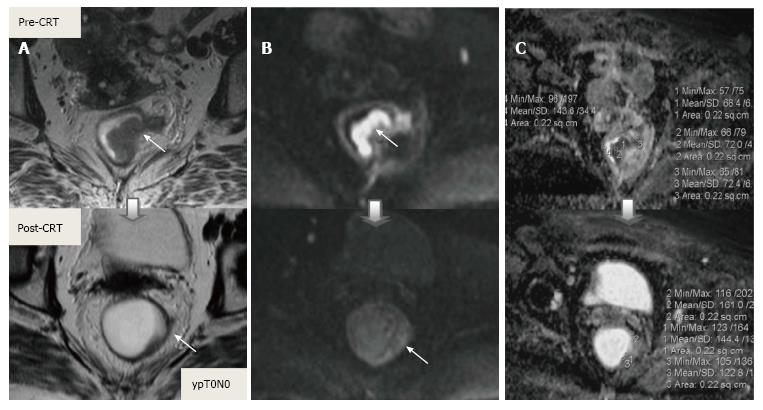
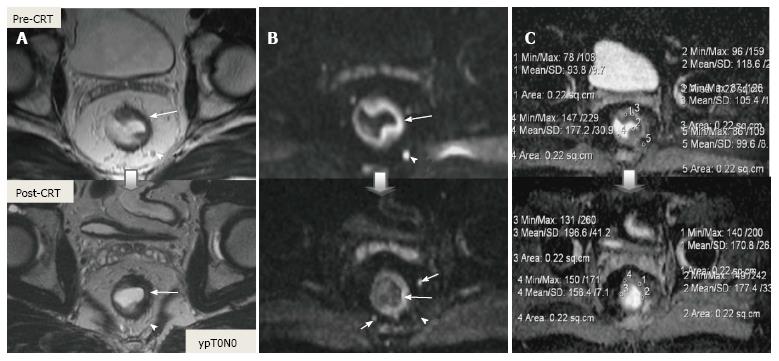
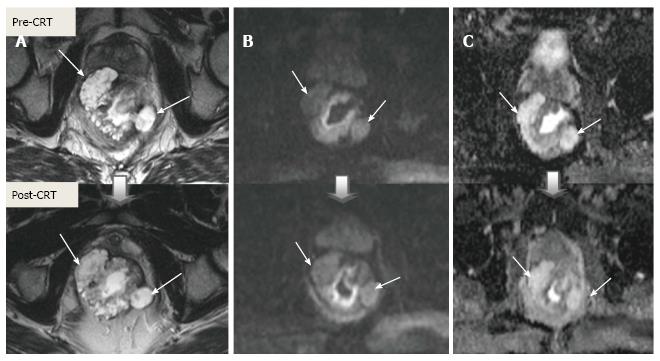
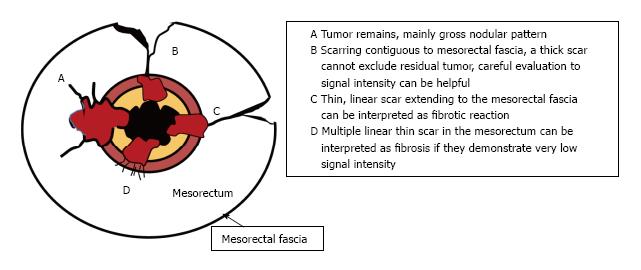
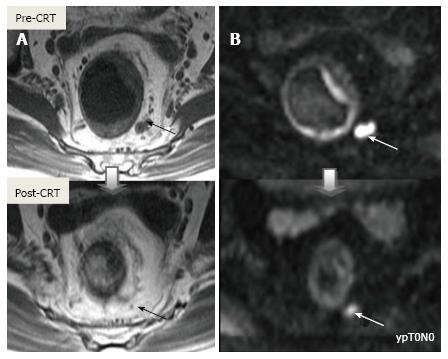
Post-CRT restaging using conventional MR sequences is less accurate than primary staging, especially when confirming complete response (yT0), mostly because it is difficult to distinguish fibrosis, edema and normal mucosa from small foci of residual tumor[46-48]. As such, a normal, two-layered rectal wall after CRT is indicative of complete response, whereas residual fibrosis indicates either residual tumor or complete response (Figure 17). In practice, areas of fibrosis have very low signal intensity on post-CRT T2-weighted MRI, in contrast, areas of residual tumor have intermediate signal-intensity[46]. Careful review of high-resolution images and DWI can enable distinction of small residual tumor within fibrosis (Figure 18).
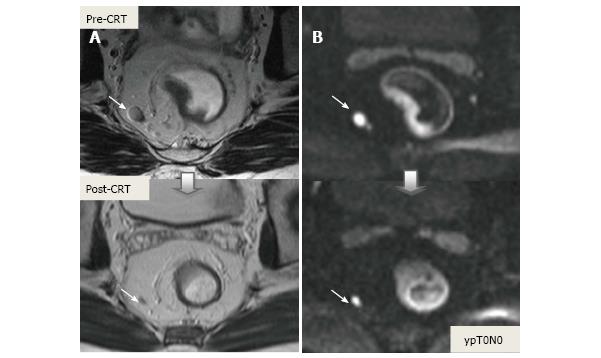
In addition to morphologic findings, DWI can provide functional information that can be correlated with changes at the cellular level in response to treatment. After CRT, the decrease in cellularity and development of fibrosis or necrosis in responders results in an increase in diffusion, which decreases diffusion signal intensity in diffusion-weighted images and increases ADC values and ADC signal intensity in ADC images[20,23] (Figures 18 and 19). Although DWI can differentiate viable tumor from fibrosis and good and bad response, it does not allow for predicting complete response[19] (Figure 20). Moreover, the response of mucinous tumors to CRT cannot be assessed using DWI because they exhibit ADC hyperintensity even before treatment (Figure 21).
CRM is considered uninvolved if a tumor free margin is seen at least 1 mm from MRF after CRT. This finding has strong negative predictive value (98%) of MR imaging for CRM involvement, whereas it has low positive predictive value[49]. In some rectal tumor, however, CRT results in a markedly reduction tumor volume, but also in retraction of pre-existing contacts with MRF. It is difficult to determine whether this area contains tumor cells or completely devoid of tumor cells[50] (Figures 22 and 23).
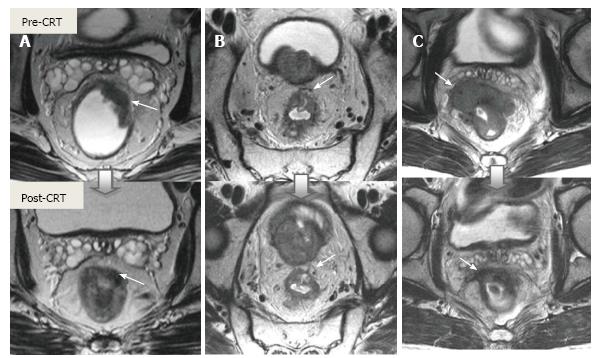
After CRT, nodal size (short axis diameter) is more reliable for nodal re-staging. It is difficult to differentiate a metastatic lymph node from a healthy lymph node with irradiation changes using morphologic criteria or DWI; therefore, lymph node restaging often results in overstaging[27,50] (Figures 24 and 25).
The accuracy of MRI for restaging is generally lower than the accuracy of MRI for initial staging, mainly owing to overstaging of nodal disease, failure to differentiate tumoral infiltration or residual tumor from desmoplastic reaction or radiation fibrosis[50]. According to recent meta-analysis results, MRI accuracy was variable for restaging rectal cancer after neoadjuvant treatment; however, significantly better results were achieved when DWI was used or with experienced observers. The authors also reported that MRI could be used for evaluating CRM staging, but nodal staging remained a challenge[51].
Using high-resolution MRI, standardizing image acquisition techniques and interpretation of images, comparative evaluation of pre- and post-CRT MR images, adding DWI to the standard approach, and importantly, experience and awareness of the limitations can improve diagnostic accuracy of MRI for re-staging.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kim HS, Koda K, Li XX S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | National Institute for Health and Clinical Excellence. NICE guidelines [CG131]. Colorectal cancer: the diagnosis and management of colorectal cancer. [accessed 2017; Jan 2] Available from: https://www.nice.org.uk/Guidance/CG131. [Cited in This Article: ] |
| 2. | van de Velde CJ, Boelens PG, Borras JM, Coebergh JW, Cervantes A, Blomqvist L, Beets-Tan RG, van den Broek CB, Brown G, Van Cutsem E. EURECCA colorectal: multidisciplinary management: European consensus conference colon & amp; rectum. Eur J Cancer. 2014;50:1.e1-1.e34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 298] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 3. | Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20:207-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 451] [Cited by in F6Publishing: 460] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 4. | Monson JR, Weiser MR, Buie WD, Chang GJ, Rafferty JF, Buie WD, Rafferty J. Practice parameters for the management of rectal cancer (revised). Dis Colon Rectum. 2013;56:535-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 278] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 5. | Millard T, Kunk PR, Ramsdale E, Rahma OE. Current debate in the oncologic management of rectal cancer. World J Gastrointest Oncol. 2016;8:715-724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123. [PubMed] [Cited in This Article: ] |
| 7. | Roh MS, Colangelo LH, O’Connell MJ, Yothers G, Deutsch M, Allegra CJ, Kahlenberg MS, Baez-Diaz L, Ursiny CS, Petrelli NJ. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124-5130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 627] [Cited by in F6Publishing: 665] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 8. | Network , N . C.C. NCCN clinical practice guidelines in oncology: rectal cancer, version 2.2017. J Natl Compr Cancer Netw. 2016;. [Cited in This Article: ] |
| 9. | Poston GJ, Tait D, O’Connell S, Bennett A, Berendse S. Diagnosis and management of colorectal cancer: summary of NICE guidance. BMJ. 2011;343:d6751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 727] [Cited by in F6Publishing: 696] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 11. | Lahaye MJ, Engelen SM, Nelemans PJ, Beets GL, van de Velde CJ, van Engelshoven JM, Beets-Tan RG. Imaging for predicting the risk factors--the circumferential resection margin and nodal disease--of local recurrence in rectal cancer: a meta-analysis. Semin Ultrasound CT MR. 2005;26:259-268. [PubMed] [Cited in This Article: ] |
| 12. | Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, Quirke P, Sebag-Montefiore D, Moran B, Heald R. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29:3753-3760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 431] [Cited by in F6Publishing: 446] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 13. | Georgiou PA, Tekkis PP, Constantinides VA, Patel U, Goldin RD, Darzi AW, John Nicholls R, Brown G. Diagnostic accuracy and value of magnetic resonance imaging (MRI) in planning exenterative pelvic surgery for advanced colorectal cancer. Eur J Cancer. 2013;49:72-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Chew MH, Brown WE, Masya L, Harrison JD, Myers E, Solomon MJ. Clinical, MRI, and PET-CT criteria used by surgeons to determine suitability for pelvic exenteration surgery for recurrent rectal cancers: a Delphi study. Dis Colon Rectum. 2013;56:717-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Jhaveri KS, Hosseini-Nik H. MRI of Rectal Cancer: An Overview and Update on Recent Advances. AJR Am J Roentgenol. 2015;205:W42-W55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Chen CC, Lee RC, Lin JK, Wang LW, Yang SH. How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy? Dis Colon Rectum. 2005;48:722-728. [PubMed] [Cited in This Article: ] |
| 17. | Kuo LJ, Chern MC, Tsou MH, Liu MC, Jian JJ, Chen CM, Chung YL, Fang WT. Interpretation of magnetic resonance imaging for locally advanced rectal carcinoma after preoperative chemoradiation therapy. Dis Colon Rectum. 2005;48:23-28. [PubMed] [Cited in This Article: ] |
| 18. | Patel UB, Brown G, Rutten H, West N, Sebag-Montefiore D, Glynne-Jones R, Rullier E, Peeters M, Van Cutsem E, Ricci S. Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer. Ann Surg Oncol. 2012;19:2842-2852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 159] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 19. | Curvo-Semedo L, Lambregts DM, Maas M, Thywissen T, Mehsen RT, Lammering G, Beets GL, Caseiro-Alves F, Beets-Tan RG. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy--conventional MR volumetry versus diffusion-weighted MR imaging. Radiology. 2011;260:734-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 216] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 20. | Kim SH, Lee JY, Lee JM, Han JK, Choi BI. Apparent diffusion coefficient for evaluating tumour response to neoadjuvant chemoradiation therapy for locally advanced rectal cancer. Eur Radiol. 2011;21:987-995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Sun YS, Zhang XP, Tang L, Ji JF, Gu J, Cai Y, Zhang XY. Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion-weighted MR imaging for early detection of tumor histopathologic downstaging. Radiology. 2010;254:170-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 22. | Kim SH, Lee JM, Hong SH, Kim GH, Lee JY, Han JK, Choi BI. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology. 2009;253:116-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 277] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 23. | Engin G, Sharifov R, Güral Z, Sağam EK, Sağlam S, Balik E, Asoğu O, Yamaner S, Güllüoğu M, Kapran Y. Can diffusion-weighted MRI determine complete responders after neoadjuvant chemoradiation for locally advanced rectal cancer? Diagn Interv Radiol. 2012;18:574-581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Beets-Tan RG, Lambregts DM, Maas M, Bipat S, Barbaro B, Caseiro-Alves F, Curvo-Semedo L, Fenlon HM, Gollub MJ, Gourtsoyianni S. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2013;23:2522-2531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 206] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 25. | Slater A, Halligan S, Taylor SA, Marshall M. Distance between the rectal wall and mesorectal fascia measured by MRI: Effect of rectal distension and implications for preoperative prediction of a tumour-free circumferential resection margin. Clin Radiol. 2006;61:65-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Salerno G, Daniels IR, Brown G. Magnetic resonance imaging of the low rectum: defining the radiological anatomy. Colorectal Dis. 2006;8 Suppl 3:10-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Nougaret S, Reinhold C, Mikhael HW, Rouanet P, Bibeau F, Brown G. The use of MR imaging in treatment planning for patients with rectal carcinoma: have you checked the “DISTANCE”? Radiology. 2013;268:330-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 28. | Gowdra Halappa V, Corona Villalobos CP, Bonekamp S, Gearhart SL, Efron J, Herman J, Kamel IR. Rectal imaging: part 1, High-resolution MRI of carcinoma of the rectum at 3 T. AJR Am J Roentgenol. 2012;199:W35-W42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Torkzad MR, Påhlman L, Glimelius B. Magnetic resonance imaging (MRI) in rectal cancer: a comprehensive review. Insights Imaging. 2010;1:245-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Lindsey I, Warren B, Mortensen N. Optimal total mesorectal excision for rectal cancer is by dissection in front of Denonvilliers’ fascia (Br J Surg 2004; 91: 121-123). Br J Surg. 2004;91:897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Cho SH, Kim SH, Bae JH, Jang YJ, Kim HJ, Lee D, Park JS. Prognostic stratification by extramural depth of tumor invasion of primary rectal cancer based on the Radiological Society of North America proposal. AJR Am J Roentgenol. 2014;202:1238-1244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Edge SB, Byrd DR, Compton CC. AJCC cancer staging handbook: from the AJCC cancer staging manual, 7th ed. New York, NY: Springer 2010; 718. [Cited in This Article: ] |
| 33. | Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, Sebag-Montefiore DJ, Tekkis P, Brown G. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011;253:711-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 421] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 34. | Rao SX, Zeng MS, Xu JM, Qin XY, Chen CZ, Li RC, Hou YY. Assessment of T staging and mesorectal fascia status using high-resolution MRI in rectal cancer with rectal distention. World J Gastroenterol. 2007;13:4141-4146. [PubMed] [Cited in This Article: ] |
| 35. | Brown G, Kirkham A, Williams GT, Bourne M, Radcliffe AG, Sayman J, Newell R, Sinnatamby C, Heald RJ. High-resolution MRI of the anatomy important in total mesorectal excision of the rectum. AJR Am J Roentgenol. 2004;182:431-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 36. | Cawthorn SJ, Parums DV, Gibbs NM, A’Hern RP, Caffarey SM, Broughton CI, Marks CG. Extent of mesorectal spread and involvement of lateral resection margin as prognostic factors after surgery for rectal cancer. Lancet. 1990;335:1055-1059. [PubMed] [Cited in This Article: ] |
| 37. | Wibe A, Rendedal PR, Svensson E, Norstein J, Eide TJ, Myrvold HE, Søreide O. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg. 2002;89:327-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 482] [Cited by in F6Publishing: 508] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 38. | Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, St Rose S, Sebag-Montefiore DJ, Tekkis P, Brown G. One millimetre is the safe cut-off for magnetic resonance imaging prediction of surgical margin status in rectal cancer. Br J Surg. 2011;98:872-879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 39. | Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 297] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 40. | Taylor FG, Swift RI, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR Am J Roentgenol. 2008;191:1827-1835. [PubMed] [Cited in This Article: ] |
| 41. | Koh DM, Brown G, Temple L, Blake H, Raja A, Toomey P, Bett N, Farhat S, Norman AR, Daniels I. Distribution of mesorectal lymph nodes in rectal cancer: in vivo MR imaging compared with histopathological examination. Initial observations. Eur Radiol. 2005;15:1650-1657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Engelen SM, Beets-Tan RG, Lahaye MJ, Kessels AG, Beets GL. Location of involved mesorectal and extramesorectal lymph nodes in patients with primary rectal cancer: preoperative assessment with MR imaging. Eur J Surg Oncol. 2008;34:776-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Luna-Pérez P, Corral P, Labastida S, Rodríguez-Coria D, Delgado S. Inguinal lymph node metastases from rectal adenocarcinoma. J Surg Oncol. 1999;70:177-180. [PubMed] [Cited in This Article: ] |
| 44. | Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, Williams GT. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003;227:371-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 616] [Cited by in F6Publishing: 571] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 45. | Mir N, Sohaib SA, Collins D, Koh DM. Fusion of high b-value diffusion-weighted and T2-weighted MR images improves identification of lymph nodes in the pelvis. J Med Imaging Radiat Oncol. 2010;54:358-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 46. | Blazic IM, Campbell NM, Gollub MJ. MRI for evaluation of treatment response in rectal cancer. Br J Radiol. 2016;89:20150964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Patel UB, Blomqvist LK, Taylor F, George C, Guthrie A, Bees N, Brown G. MRI after treatment of locally advanced rectal cancer: how to report tumor response--the MERCURY experience. AJR Am J Roentgenol. 2012;199:W486-W495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 48. | De Nardi P, Carvello M. How reliable is current imaging in restaging rectal cancer after neoadjuvant therapy? World J Gastroenterol. 2013;19:5964-5972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 27] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Salerno G, Daniels IR, Moran BJ, Wotherspoon A, Brown G. Clarifying margins in the multidisciplinary management of rectal cancer: the MERCURY experience. Clin Radiol. 2006;61:916-923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Kim DJ, Kim JH, Lim JS, Yu JS, Chung JJ, Kim MJ, Kim KW. Restaging of Rectal Cancer with MR Imaging after Concurrent Chemotherapy and Radiation Therapy. Radiographics. 2010;30:503-516. [PubMed] [Cited in This Article: ] |
| 51. | van der Paardt MP, Zagers MB, Beets-Tan RG, Stoker J, Bipat S. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology. 2013;269:101-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 258] [Article Influence: 23.5] [Reference Citation Analysis (0)] |