Published online Jun 24, 2021. doi: 10.5306/wjco.v12.i6.429
Peer-review started: October 22, 2020
First decision: December 3, 2020
Revised: December 30, 2021
Accepted: April 8, 2021
Article in press: April 8, 2021
Published online: June 24, 2021
Therapeutic manipulation of the immune system in cancer has been an extensive area of research in the field of oncoimmunology. Immunosuppression regulates antitumour immune responses. An immunosuppressive enzyme, indoleamine 2,3-dioxygenase (IDO) mediates tumour immune escape in various malignancies including breast cancer. IDO upregulation in breast cancer cells may lead to the recruitment of regulatory T (T-regs) cells into the tumour microenvironment, thus inhibiting local immune responses and promoting metastasis. Immunosuppression induced by myeloid derived suppressor cells activated in an IDO-dependent manner may enhance the possibility of immune evasion in breast cancer. IDO overexpression has independent prognostic significance in a subtype of breast cancer of emerging interest, basal-like breast carcinoma. IDO inhibitors as adjuvant therapeutic agents may have clinical implications in breast cancer. This review proposes future prospects of IDO not only as a therapeutic target but also as a valuable prognostic marker for breast cancer.
Core Tip: Indoleamine 2,3-dioxygenase might be utilized as a potential biomarker and immunotherapeutic target in breast cancer patients.
- Citation: Asghar K, Farooq A, Zulfiqar B, Loya A. Review of 10 years of research on breast cancer patients: Focus on indoleamine 2,3-dioxygenase. World J Clin Oncol 2021; 12(6): 429-436
- URL: https://www.wjgnet.com/2218-4333/full/v12/i6/429.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i6.429
Breast cancer is the most common cancer in women worldwide. A variety of genetic and non-genetic factors can be linked to breast cancer. Recent emerging epidemiologic, preclinical, and clinical data suggest the key role of the immune system in the aetiology of breast cancer[1]. The current understanding of the molecular and cellular mechanisms underlying cancer development suggests that immune cells functionally regulate epithelial cancer development and progression[2]. The innate and adaptive immune systems play a role in preventing relapse in breast cancer[1]. Lymphocytes, including T cells, T-regs, natural killer (NK) cells, and their cytokine release patterns are associated with both primary prevention and relapse of breast cancer[1,3]. Hence, breast cancer prognosis may be related to the functional status of the immune system.
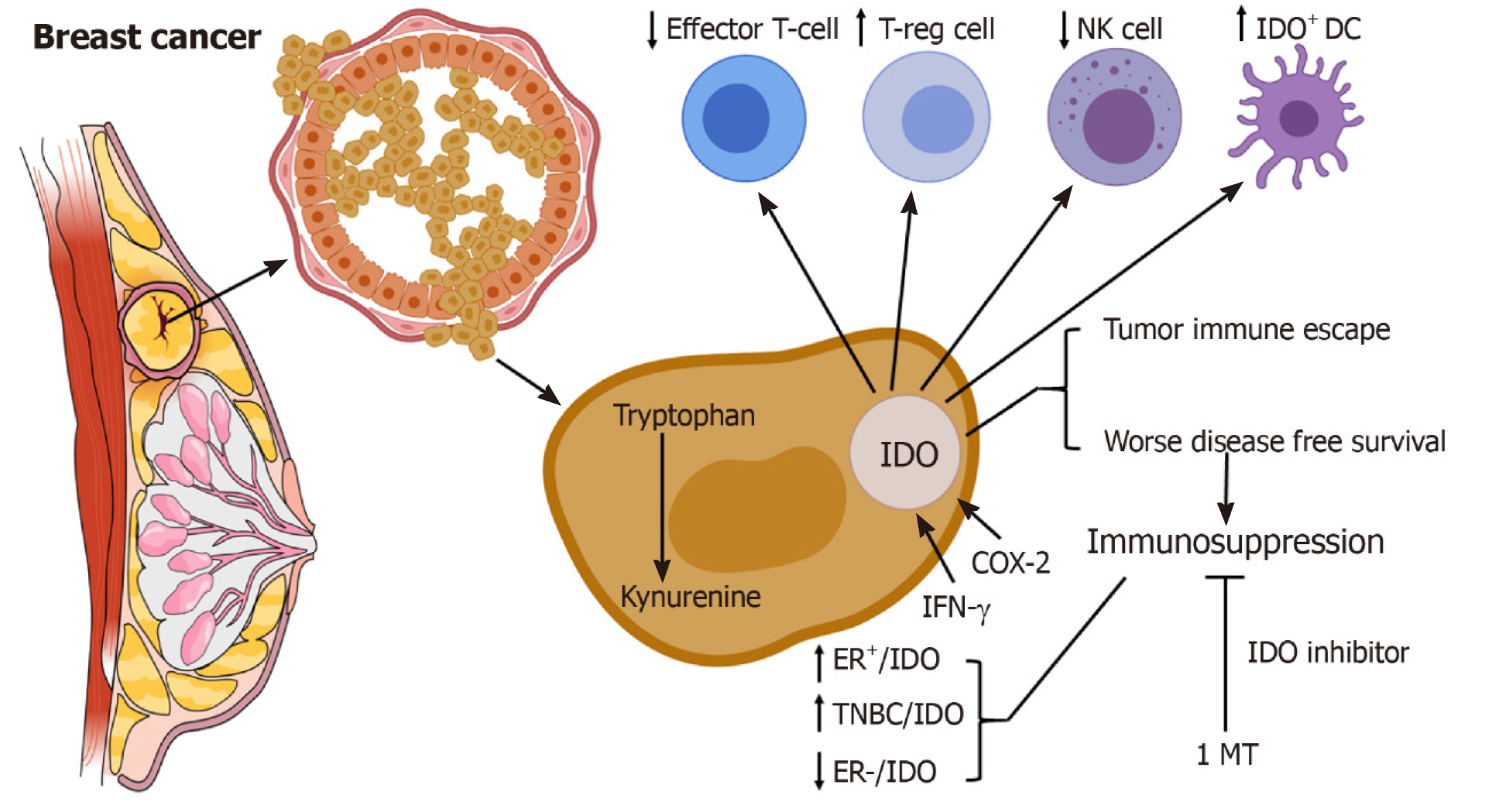
Breast cancer cells can evade immune responses, by various immunosuppressive mechanisms, such as the upregulation of indoleamine 2,3-dioxygenase (IDO/IDO-1)[4,5]. IDO is a heme-containing immunosuppressive enzyme, that degrades the essential amino acid L-tryptophan into kynurenine[6]. IDO is involved in the immune homeostasis and immune-related functions not only during pregnancy but also in chronic inflammatory diseases and tumour immune-escaping mechanisms[7-9]. IDO is chronically triggered in cancer patients[10]. Deprivation of tryptophan directly affects the cytotoxicity of T cells. In addition, the toxic metabolites produced from tryptophan degradation directly induce T cell apoptosis in vitro[9]. IDO may inhibit T cell immunity by inducing the differentiation and maturation of T-regs[11]. IDO overexpression induces immunosuppression and tolerance[8]. IDO-expressing cells are found at several sites of immune tolerance, including thymus, placenta, anterior chamber of the eye, mucosa of the gut and epididymis[12-14]. Human monocyte-derived macrophages and dendritic cells (DCs) express IDO[15,16]. IDO-expressing DCs are found in breast tumour tissue as well as draining lymph nodes of patients with breast cancers[17]. IDO expression may suppress immune responses by blocking NK cells (Figure 1)[18]. However, the immunosuppressive role of IDO in tumour immunology and its associations with other tolerogenic mechanisms have only recently begun to be elucidated. The molecular mechanisms underlying tumour immune escape are currently the topic of active research. In this review, we examine the potential role of IDO as a prognostic marker and therapeutic target in breast cancer patients.
It has been established that IDO in the tumour microenvironment has the capacity to inhibit antitumour immunity and promote metastasis, both hallmarks of cancer[19]. It is evident that IDO is consistently and robustly expressed in breast cancer[20]. IDO is suggested to play a pivotal role in the pathogenesis of breast cancer (Table 1). In 2011, Yu et al[21] observed that the upregulation of IDO in primary breast cancer might inhibit the local immune response by the infiltration of T-regs into the tumour microenvironment thereby promoting metastasis[21]. In 2013, Yu et al[22] further investigated IDO expression in myeloid-derived suppressive cells in breast cancer and observed that STAT3-dependent IDO expression induced the immunosuppressive effects of myeloid derived suppressor cells in breast cancer[22]. IDO has recently received more attention because of its involvement in regulating angiogenesis[23]. Wei et al[23] studied the effects of IDO on microvessel density and reported that high IDO expression is associated with microvessel density; causes a poor prognosis and subsequently promotes angiogenesis in breast cancer[23].
| Year | Ref. | Population | Description |
| 2011 | Yu et al[21] | Chinese (n = 26) | IDO upregulation inhibits local immune responses by infiltration of T-regs in the tumour microenvironment and promotes metastasis in breast cancer |
| 2011 | Lyon et al[27] | American (n = 33) | Increased tryptophan degradation may occur in women with early-stage breast cancer |
| 2012 | Jacquemier et al[31] | French (n = 1749) | Immunodetection of IDO-positive cells may be used for diagnosis of medullary breast cancer. IDO has a prognostic significance in basal like breast cancer |
| 2013 | Soliman et al[24] | American (n = 203) | IDO expression was higher in ER+ breast cancer than ER– breast cancer |
| 2013 | Yu et al[22] | Chinese (n = 85) | STAT3-dependent IDO expression induces immunosuppressive effects of MDSCs in breast cancer |
| 2014 | Bi et al[37] | Chinese (n = 110) | IDO and EGFR may serve as a potential biomarkers for breast cancer prognosis and treatment |
| 2014 | Isla Larrain et al[32] | Argentinian(n = 91) | IDO was expressed in a TNBC subgroup and was involved in the tumour immune escape |
| 2015 | Salvadori et al[33] | Brazilian (n = 20) | IDO inhibitor when combined with paclitaxel may be used as a new therapeutic strategy for breast cancer |
| 2017 | Kim et al[30] | South Korean (n = 200) | IDO might be an effective immunotherapeutic target in TNBC |
| 2017 | Dewi et al[25] | German (n = 15) | IDO-1 expression in ER– breast cancer may be associated with poor prognosis. IDO-1 maybe a promising therapeutic target for ER– breast cancer |
| 2017 | Carvajal-Hausdorf et al[36] | American (n = 362) | IDO-1 quantification has potential to differentiate a population that might get an advantage from IDO-1 blockade |
| 2017 | Li et al[38] | Chinese (n = 46) | Expression of IDO and IL-6 is associated with advanced breast cancer and poor response to neoadjuvant chemotherapy |
| 2018 | Ye et al[34] | Chinese (n = 963) | IDO and programmed cell death protein-1 pathways might be an effective therapeutic approach in breast cancer treatment |
| 2018 | Wei et al[23] | Chinese (n = 65) | IDO may induce angiogenesis in breast cancer, providing a molecular or gene therapy target for angiogenesis inhibition |
| 2018 | Li et al[39] | Chinese (n = 44) | Tumour-infiltrating T-regs, MDSCs and IDO expression may be used as a prognostic marker for the outcome of neoadjuvant chemotherapy |
| 2018 | Dill et al[29] | American (n = 281) | IDO expression in high-grade,TNBC is associated with PD-L1 co-expression |
| 2019 | Asghar et al[28] | Pakistani (n = 100) | IDO expression in TNBC may suggest its role in disease pathogenesis |
| 2019 | Onseti et al[26] | Belgian (n = 202) | Kynurenine/tryptophan ratio in plasma might differentiate breast cancer patients from healthy controls |
| 2019 | Asghar et al[35] | Pakistani (n = 100) | IDO expression is associated with COX-2 expression in breast cancer patients |
| 2019 | Zhao et al[40] | Chinese (n = 53) | IDO expression and activity is linked with advanced breast cancer and poor response to neoadjuvant chemotherapy |
| 2020 | Wei et al[41] | Chinese (n = 77) | IDO and tumour infiltrating immune cells can help to evaluate the prognosis of breast cancer patient |
Soliman et al[24] also examined IDO expression in breast cancer patients (n = 203). IDO overexpression was detected in ER+ tumours but not ER- tumours. Overall survival was better in ER+ patients with high IDO expression. This study provided a new prospective for ongoing clinical trials of IDO inhibitors in metastatic breast cancer. They proposed further studies to understand the complex role of IDO in the natural progression of breast cancer at different stages of the disease[24]. Another study published by Dewi et al[25] demonstrated that increased IDO expression in ER- breast cancer might influence its malignant phenotype and result in a poor prognosis[25].
The kynurenine to tryptophan (Kyn/Trp) ratio is used to measure IDO enzymatic activity. Onesti et al[26] measured IDO activity in breast cancer patients (n = 202) with all subtypes and healthy controls (n = 146). They reported that the Kyn/Trp ratio might differentiate breast cancer patients from healthy controls[26]. A similar study conducted by Lyon et al[27] compared the levels of tryptophan degradation in women with or without breast cancer and observed an increased Kyn/Trp ratio in women with breast cancer. Keeping in view the multifactorial role of IDO, the authors suggested that further research was necessary to determine the relationships among these important biological factors and neuropsychiatric symptoms in women with breast cancer[27].
Due to advancements in early diagnosis and treatment of breast cancer, the overall survival of patients has significantly improved over the years. Nevertheless, triple- negative breast cancer (TNBC) is a more aggressive tumour than other breast cancers[28]. Our recent data showed high IDO expression in TNBC patients. Furthermore, we observed that high IDO expression was significantly correlated with decreased overall survival[28]. Dill et al[29] also observed IDO expression among high-grade TNBC. In addition, they determined that IDO expression was associated with PD-L1 co-expression. They suggested clinical trials to assess the effectiveness of IDO inhibition relative to IDO expression as well as its role when combined with anti-programmed cell death protein 1 (PD-1)/PD-L1 immunotherapy[29]. Another study by Kim et al[30] was conducted to evaluate the clinical and pathological characteristics of an IDO-expressing TNBC subset, and the authors observed that IDO positivity was associated with the basal-like phenotype. They also suggested the role of IDO blockade in the treatment of TNBC patients[30].
Among the molecular subtypes of breast cancer, basal-like breast carcinoma (BLBC) has the poorest outcomes[31]. Jacquemier et al[31] determined that IDO was overexpressed at the transcriptional and translational levels in a subset of TNBC. They elaborated that IDO overexpression was correlated with morphological medullary features and had autonomous prognostic significance in BLBC. Medullary breast carcinoma (MBC) had a better prognosis than non-MBC, but IDO was overexpressed at the mRNA level in BLBC and MBC compared with non-MBC. IDO expression is thus correlated with tumour infiltrating lymphocytes, which are present in both MBC and BLBC. IDO overexpression in BLBC with a favourable prognosis may be due to kynurenines, which have the capacity to induce apoptosis in lymphocytes as well as tumour cells[31]. Another study revealed that IDO was mostly expressed in the TNBC subtype. The same authors also observed IDO expression in breast cancer and circulating microvesicles from breast cancer patients with advanced stages[32].
IDO inhibitors have therapeutic significance when given in combination with chemotherapeutic agents for breast cancer treatment[33]. An IDO inhibitor (1-methyl-DL-tryptophan) in combination with paclitaxel may be a new therapeutic strategy for breast cancer[33]. Several studies support this hypothesis; for instance, Ye et al[34] investigated the association between IDO and PD-1 in the tumour microenvironment and in tumour-draining lymph nodes in breast cancer patients. They observed a positive association between the expression of IDO and PD-1. The team also proposed that inhibiting both of these pathways might act as a novel therapeutic strategy in breast cancer treatment[34]. Another study published by Asghar et al[35] reported that high IDO expression was correlated with high cyclooxygenase-2 expression in breast cancer patients. It was suggested that the simultaneous targeting of cyclooxygenase-2 and IDO may have potential for treatment of breast cancer[35]. Carvajal-Hausdorf et al[36] observed that the IDO protein was expressed in hormone receptor-positive breast cancer. Furthermore, IDO was negatively correlated with B-cell infiltration in tumours, and high IDO expression was associated with poor overall survival. The authors proposed that IDO quantification has the potential to differentiate a population that might obtain an advantage from IDO-1 blockade[36].
IDO has not only therapeutic significance but also prognostic significance. Bi et al[37] observed the co-expression of IDO and epidermal growth factor receptor (EGFR) in breast cancer and suggested that IDO and EGFR may serve as potential biomarkers for breast cancer prognosis and treatment[37]. Another study conducted by Li et al[38] in 2017, aimed to investigate the co-expression of IDO and interleukin-6 in breast cancer patients prior to neoadjuvant chemotherapy. They observed that IDO and interleukin-6 expression was related to advanced breast cancer and a poor response to neoadjuvant therapy[38]. In 2018, Li et al[39] further explored whether tumour-infiltrating T-regs, myeloid-derived suppressor cells and IDO expression may be used as prognostic markers for the outcome of neoadjuvant chemotherapy[39]. Additionally, Zhao et al[40] observed that high IDO expression and activity were associated with advanced disease, a poor prognosis and chemoresistance in breast cancer[40]. In 2020, Wei et al[41] identified tumour infiltrating immune cells, and IDO and PDL-1 expression in breast cancer patients. The authors proposed that IDO in combination with tumour infiltrating immune cells might help to assess the prognosis of patients with breast cancer[41].
IDO is involved in the regulation of the immune system. Upregulated IDO is associated with a poor prognosis in various cancers[42-44], but in the case of BLBC, high IDO expression indicates a favourable prognosis[31]. Another study revealed improved overall survival among ER+ breast cancer patients with high IDO expression[24]. However, several studies carried out regarding IDO involvement in breast cancer showed that IDO overexpression was associated with breast tumour growth and metastasis[4,21,45,46]. In view of the contradictory findings, further studies are therefore required to understand the complex role of IDO in breast cancer. Identification of more efficient and less toxic IDO inhibitors is urgent. Currently, two IDO inhibitors are in a clinical development stage as immunotherapeutic agents in breast cancer treatment. These inhibitors are Indoximod (NLG2101) developed by NewLink Genetics[47] and INCB024360 developed by Incyte[48,49]. Two therapies have been exclusively designed to treat HER2-positive breast cancer in combination with the AD.p53 DC vaccine and docetaxel (NCT01042535 and NCT01792050 respectively)[50]. IDO inhibitors as adjuvant therapeutic agents may have clinical implications in breast cancer[33]. Targeted IDO inhibition using nanoparticles may provide a better outcome. Tryptophan-2,3-dioxygenase (TDO) has biochemical activity similar to that of IDO[51,52]. Apart from IDO, another isoform IDO-2 has also been discovered[53,54]. Both IDO-2 and TDO are involved in the degradation of tryptophan[55]. Future studies should focus on the role of IDO-2 and TDO in breast cancer.
The therapeutic implications of IDO are unquestionable but its potential as a prognostic biomarker may have significant outcomes.
Manuscript source: Unsolicited manuscript
Specialty type: Immunology
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Perez CA, Raiter A S-Editor: Zhang L L-Editor: A P-Editor: Xing YX
| 1. | Standish LJ, Sweet ES, Novack J, Wenner CA, Bridge C, Nelson A, Martzen M, Torkelson C. Breast cancer and the immune system. J Soc Integr Oncol. 2008;6:158-168. [PubMed] [Cited in This Article: ] |
| 2. | DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res 2007; 9:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 485] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 3. | Campbell MJ, Scott J, Maecker HT, Park JW, Esserman LJ. Immune dysfunction and micrometastases in women with breast cancer. Breast Cancer Res Treat. 2005;91:163-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Mansfield AS, Heikkila PS, Vaara AT, von Smitten KA, Vakkila JM, Leidenius MH. Simultaneous Foxp3 and IDO expression is associated with sentinel lymph node metastases in breast cancer. BMC Cancer. 2009;9:231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 808] [Cited by in F6Publishing: 820] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 6. | Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363-1372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1172] [Cited by in F6Publishing: 1182] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 7. | Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1666] [Cited by in F6Publishing: 1695] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 8. | Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24:242-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 570] [Cited by in F6Publishing: 543] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 9. | Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 310] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 10. | Schröcksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. 2006;364:82-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 382] [Cited by in F6Publishing: 395] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 11. | Nakamura T, Shima T, Saeki A, Hidaka T, Nakashima A, Takikawa O, Saito S. Expression of indoleamine 2, 3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer. Cancer Sci. 2007;98:874-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Moffett JR, Espey MG, Namboodiri MA. Antibodies to quinolinic acid and the determination of its cellular distribution within the rat immune system. Cell Tissue Res. 1994;278:461-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Yoshida R, Park SW, Yasui H, Takikawa O. Tryptophan degradation in transplanted tumor cells undergoing rejection. J Immunol. 1988;141:2819-2823. [PubMed] [Cited in This Article: ] |
| 14. | Malina HZ, Martin XD. Indoleamine 2,3-dioxygenase: antioxidant enzyme in the human eye. Graefes Arch Clin Exp Ophthalmol. 1996;234:457-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596-3599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 573] [Cited by in F6Publishing: 553] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 16. | Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL Jr, Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867-1870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 767] [Cited by in F6Publishing: 736] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 17. | Mellor AL, Munn DH. Immunology at the maternal-fetal interface: lessons for T cell tolerance and suppression. Annu Rev Immunol. 2000;18:367-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 216] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8693] [Cited by in F6Publishing: 9313] [Article Influence: 776.1] [Reference Citation Analysis (0)] |
| 19. | Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269-1274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1642] [Cited by in F6Publishing: 1665] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 20. | Sadun RE, Sachsman SM, Chen X, Christenson KW, Morris WZ, Hu P, Epstein AL. Immune signatures of murine and human cancers reveal unique mechanisms of tumor escape and new targets for cancer immunotherapy. Clin Cancer Res. 2007;13:4016-4025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Yu J, Sun J, Wang SE, Li H, Cao S, Cong Y, Liu J, Ren X. Upregulated expression of indoleamine 2, 3-dioxygenase in primary breast cancer correlates with increase of infiltrated regulatory T cells in situ and lymph node metastasis. Clin Dev Immunol. 2011;2011:469135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu W, Shen C, Liu J, Ren X. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190:3783-3797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 424] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 23. | Wei L, Zhu S, Li M, Li F, Wei F, Liu J, Ren X. High Indoleamine 2,3-Dioxygenase Is Correlated With Microvessel Density and Worse Prognosis in Breast Cancer. Front Immunol. 2018;9:724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Soliman H, Rawal B, Fulp J, Lee JH, Lopez A, Bui MM, Khalil F, Antonia S, Yfantis HG, Lee DH, Dorsey TH, Ambs S. Analysis of indoleamine 2-3 dioxygenase (IDO1) expression in breast cancer tissue by immunohistochemistry. Cancer Immunol Immunother. 2013;62:829-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Dewi DL, Mohapatra SR, Blanco Cabañes S, Adam I, Somarribas Patterson LF, Berdel B, Kahloon M, Thürmann L, Loth S, Heilmann K, Weichenhan D, Mücke O, Heiland I, Wimberger P, Kuhlmann JD, Kellner KH, Schott S, Plass C, Platten M, Gerhäuser C, Trump S, Opitz CA. Suppression of indoleamine-2,3-dioxygenase 1 expression by promoter hypermethylation in ER-positive breast cancer. Oncoimmunology. 2017;6:e1274477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Onesti CE, Boemer F, Josse C, Leduc S, Bours V, Jerusalem G. Tryptophan catabolism increases in breast cancer patients compared to healthy controls without affecting the cancer outcome or response to chemotherapy. J Transl Med. 2019;17:239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Lyon DE, Walter JM, Starkweather AR, Schubert CM, McCain NL. Tryptophan degradation in women with breast cancer: a pilot study. BMC Res Notes. 2011;4:156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Asghar K, Loya A, Rana IA, Tahseen M, Ishaq M, Farooq A, Bakar MA, Masood I. Indoleamine 2,3-dioxygenase expression and overall survival in patients diagnosed with breast cancer in Pakistan. Cancer Manag Res. 2019;11:475-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Dill EA, Dillon PM, Bullock TN, Mills AM. IDO expression in breast cancer: an assessment of 281 primary and metastatic cases with comparison to PD-L1. Mod Pathol. 2018;31:1513-1522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 30. | Kim S, Park S, Cho MS, Lim W, Moon BI, Sung SH. Strong Correlation of Indoleamine 2,3-Dioxygenase 1 Expression with Basal-Like Phenotype and Increased Lymphocytic Infiltration in Triple-Negative Breast Cancer. J Cancer. 2017;8:124-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Jacquemier J, Bertucci F, Finetti P, Esterni B, Charafe-Jauffret E, Thibult ML, Houvenaeghel G, Van den Eynde B, Birnbaum D, Olive D, Xerri L. High expression of indoleamine 2,3-dioxygenase in the tumour is associated with medullary features and favourable outcome in basal-like breast carcinoma. Int J Cancer. 2012;130:96-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Isla Larrain MT, Rabassa ME, Lacunza E, Barbera A, Cretón A, Segal-Eiras A, Croce MV. IDO is highly expressed in breast cancer and breast cancer-derived circulating microvesicles and associated to aggressive types of tumors by in silico analysis. Tumour Biol. 2014;35:6511-6519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Salvadori ML, da Cunha Bianchi PK, Gebrim LH, Silva RS, Kfoury JR Jr. Effect of the association of 1-methyl-DL-tryptophan with paclitaxel on the expression of indoleamine 2,3-dioxygenase in cultured cancer cells from patients with breast cancer. Med Oncol. 2015;32:248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Ye Q, Wang C, Xian J, Zhang M, Cao Y. Expression of programmed cell death protein 1 (PD-1) and indoleamine 2,3-dioxygenase (IDO) in the tumor microenvironment and in tumor-draining lymph nodes of breast cancer. Hum Pathol. 2018;75:81-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Asghar K, Loya A, Rana IA, Abu Bakar M, Farooq A, Tahseen M, Ishaq M, Rashid MU. Association between Cyclooxygenase-2 and Indoleamine 2,3-Dioxygenase Expression in Breast Cancer Patients from Pakistan. Asian Pac J Cancer Prev. 2019;20:3521-3525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Carvajal-Hausdorf DE, Mani N, Velcheti V, Schalper KA, Rimm DL. Objective measurement and clinical significance of IDO1 protein in hormone receptor-positive breast cancer. J Immunother Cancer. 2017;5:81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Bi WW, Zhang WH, Yin GH, Luo H, Wang SQ, Wang H, Li C, Yan WQ, Nie DZ. Analysis of indoleamine 2-3 dioxygenase (IDO) and EGFR co-expression in breast cancer tissue by immunohistochemistry. Asian Pac J Cancer Prev. 2014;15:5535-5538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Li F, Wei L, Li S, Liu J. Indoleamine-2,3-dioxygenase and Interleukin-6 associated with tumor response to neoadjuvant chemotherapy in breast cancer. Oncotarget. 2017;8:107844-107858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Li F, Zhao Y, Wei L, Li S, Liu J. Tumor-infiltrating Treg, MDSC, and IDO expression associated with outcomes of neoadjuvant chemotherapy of breast cancer. Cancer Biol Ther. 2018;19:695-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 40. | Zhao Y, Wei L, Liu J, Li F. Chemoresistance was correlated with elevated expression and activity of indoleamine 2,3-dioxygenase in breast cancer. Cancer Chemother Pharmacol. 2020;85:77-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Wei L, Wu N, Wei F, Li F, Zhang Y, Liu J, Ren X. Prognosis significance of indoleamine 2, 3-dioxygenase, programmed death ligand-1 and tumor-infiltrating immune cells in microenvironment of breast cancer. Int Immunopharmacol. 2020;84:106506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Gao YF, Peng RQ, Li J, Ding Y, Zhang X, Wu XJ, Pan ZZ, Wan DS, Zeng YX, Zhang XS. The paradoxical patterns of expression of indoleamine 2,3-dioxygenase in colon cancer. J Transl Med. 2009;7:71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Inaba T, Ino K, Kajiyama H, Yamamoto E, Shibata K, Nawa A, Nagasaka T, Akimoto H, Takikawa O, Kikkawa F. Role of the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the progression of ovarian carcinoma. Gynecol Oncol. 2009;115:185-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 44. | Nelson BH. IDO and outcomes in ovarian cancer. Gynecol Oncol. 2009;115:179-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Sun J, Yu J, Li H, Yang L, Wei F, Yu W, Liu J, Ren X. Upregulated expression of indoleamine 2, 3-dioxygenase in CHO cells induces apoptosis of competent T cells and increases proportion of Treg cells. J Exp Clin Cancer Res. 2011;30:82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Levina V, Su Y, Gorelik E. Immunological and nonimmunological effects of indoleamine 2,3-dioxygenase on breast tumor growth and spontaneous metastasis formation. Clin Dev Immunol. 2012;2012:173029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN, Link CJ, Prendergast GC. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology. 2012;1:1460-1468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 283] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 48. | Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K, Leffet L, Hansbury MJ, Thomas B, Rupar M, Waeltz P, Bowman KJ, Polam P, Sparks RB, Yue EW, Li Y, Wynn R, Fridman JS, Burn TC, Combs AP, Newton RC, Scherle PA. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115:3520-3530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 408] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 49. | Koblish HK, Hansbury MJ, Bowman KJ, Yang G, Neilan CL, Haley PJ, Burn TC, Waeltz P, Sparks RB, Yue EW, Combs AP, Scherle PA, Vaddi K, Fridman JS. Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Mol Cancer Ther. 2010;9:489-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 50. | Moon YW, Hajjar J, Hwu P, Naing A. Targeting the indoleamine 2,3-dioxygenase pathway in cancer. J Immunother Cancer. 2015;3:51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 238] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 51. | Thackray SJ, Mowat CG, Chapman SK. Exploring the mechanism of tryptophan 2,3-dioxygenase. Biochem Soc Trans. 2008;36:1120-1123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 52. | Rafice SA, Chauhan N, Efimov I, Basran J, Raven EL. Oxidation of L-tryptophan in biology: a comparison between tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase. Biochem Soc Trans. 2009;37:408-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Ball HJ, Yuasa HJ, Austin CJ, Weiser S, Hunt NH. Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int J Biochem Cell Biol. 2009;41:467-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 192] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 54. | Löb S, Königsrainer A, Zieker D, Brücher BL, Rammensee HG, Opelz G, Terness P. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother. 2009;58:153-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 240] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 55. | Fatokun AA, Hunt NH, Ball HJ. Indoleamine 2,3-dioxygenase 2 (IDO2) and the kynurenine pathway: characteristics and potential roles in health and disease. Amino Acids. 2013;45:1319-1329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |